CHI3L1 as a Prognostic Biomarker and Therapeutic Target in Glioma
Abstract
:1. Introduction
2. Results
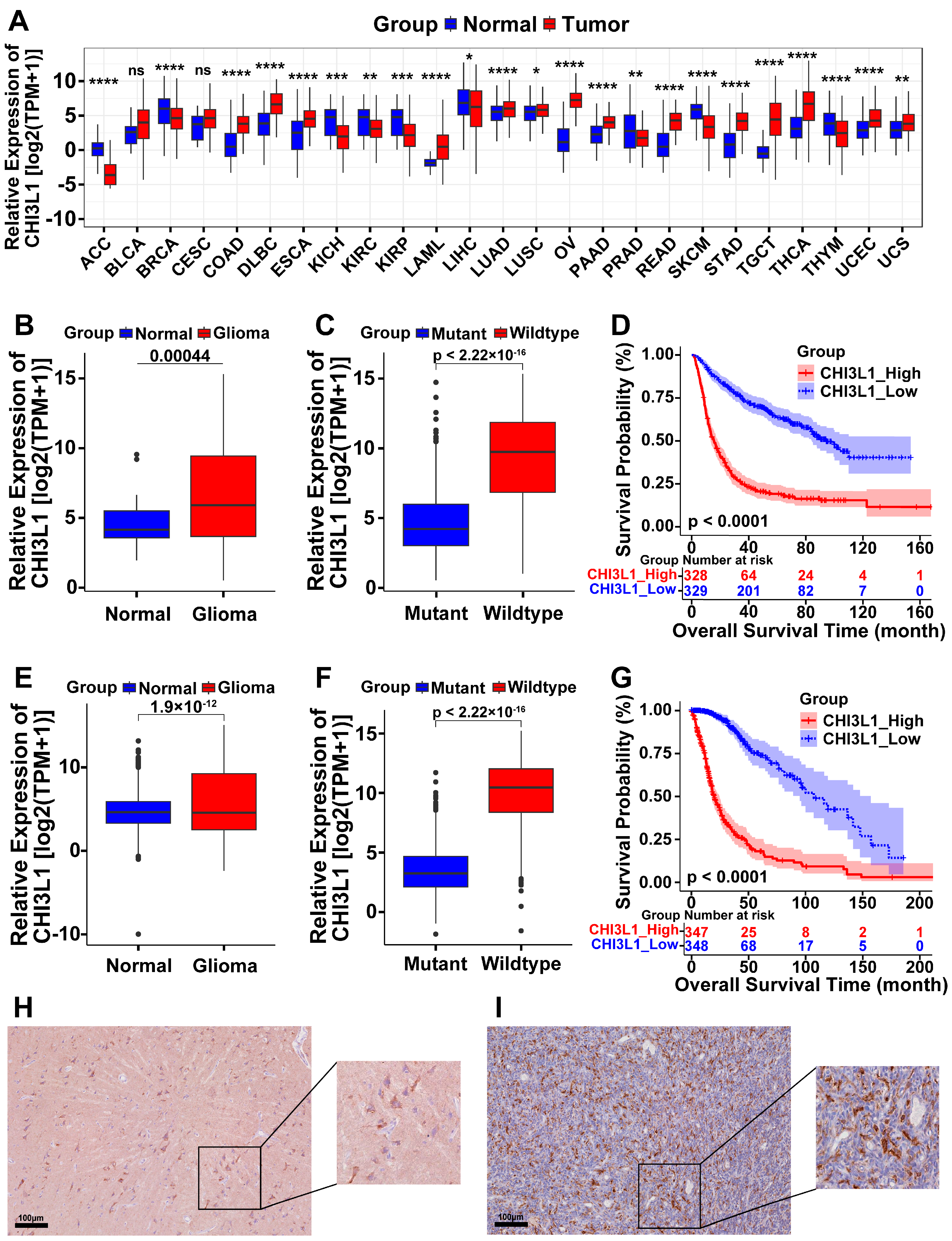
2.1. High Expression of CHI3L1 in Pan-Cancer and Glioma
2.2. CHI3L1 Is Involved in Oxidative Stress in Glioma
2.3. Identification of an 11-Gene Risk Index Associated with Oxidative Stress

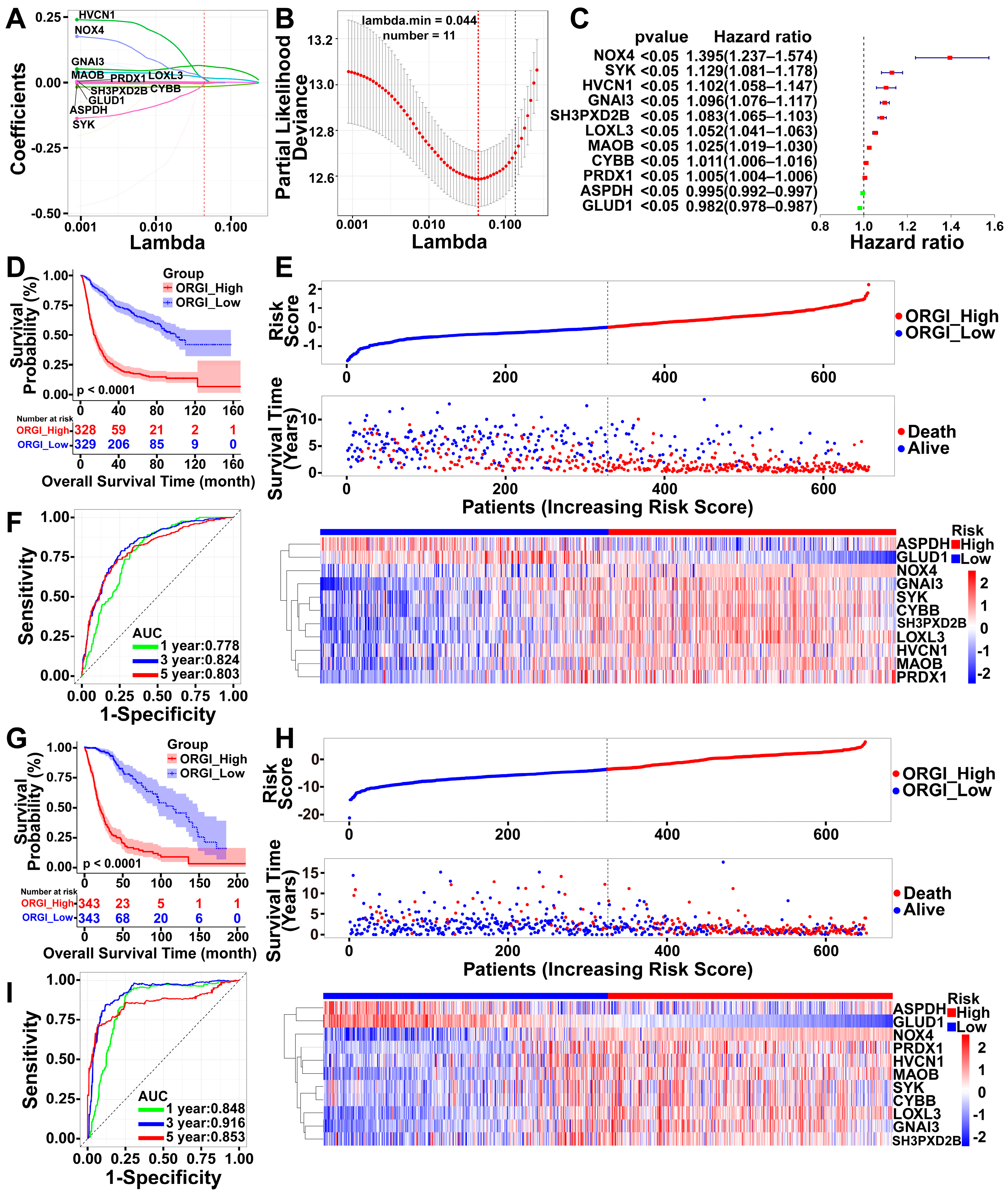
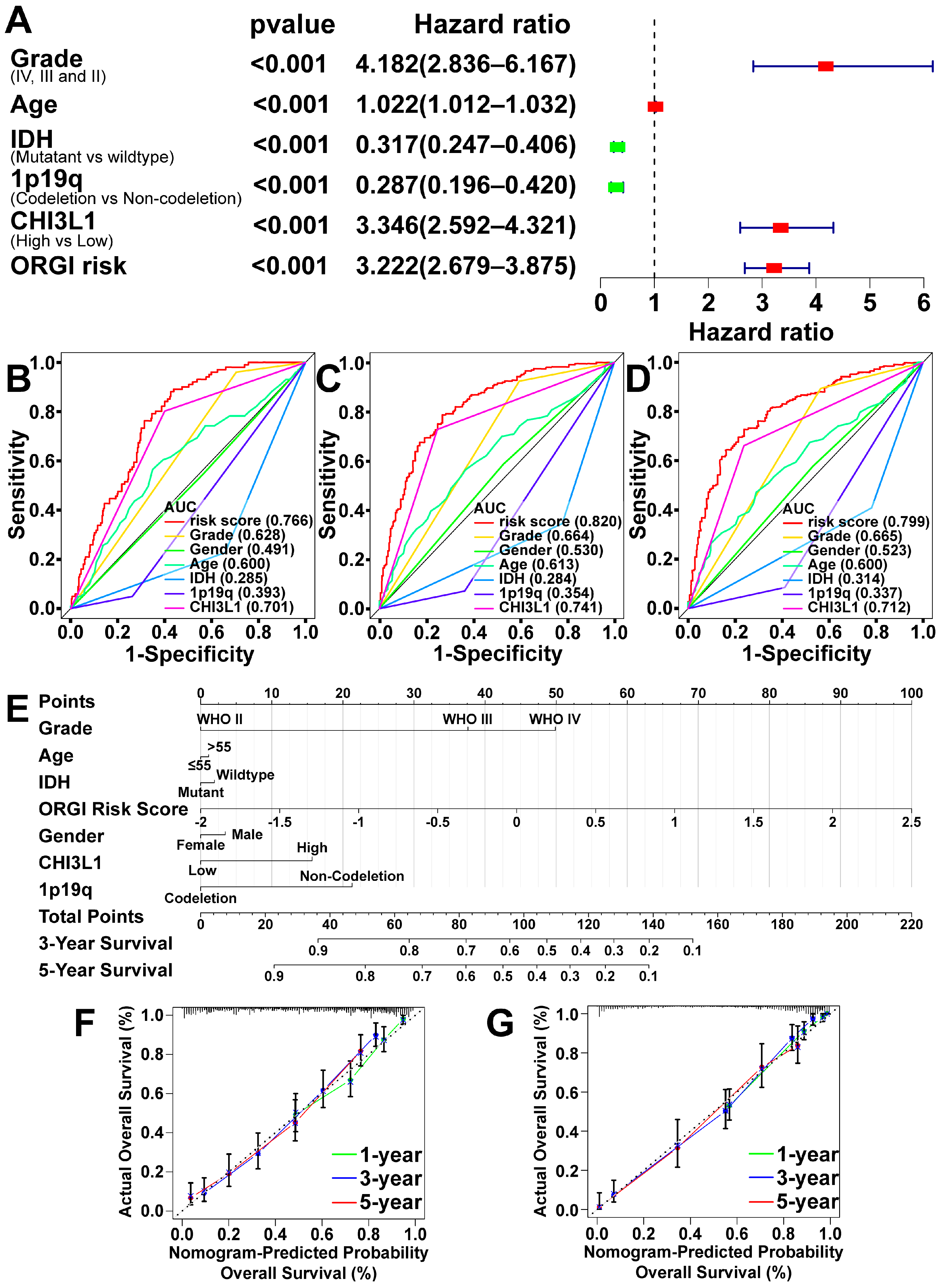
2.4. Prognostic Value of CHI3L1 and ORGI for Glioma Patients
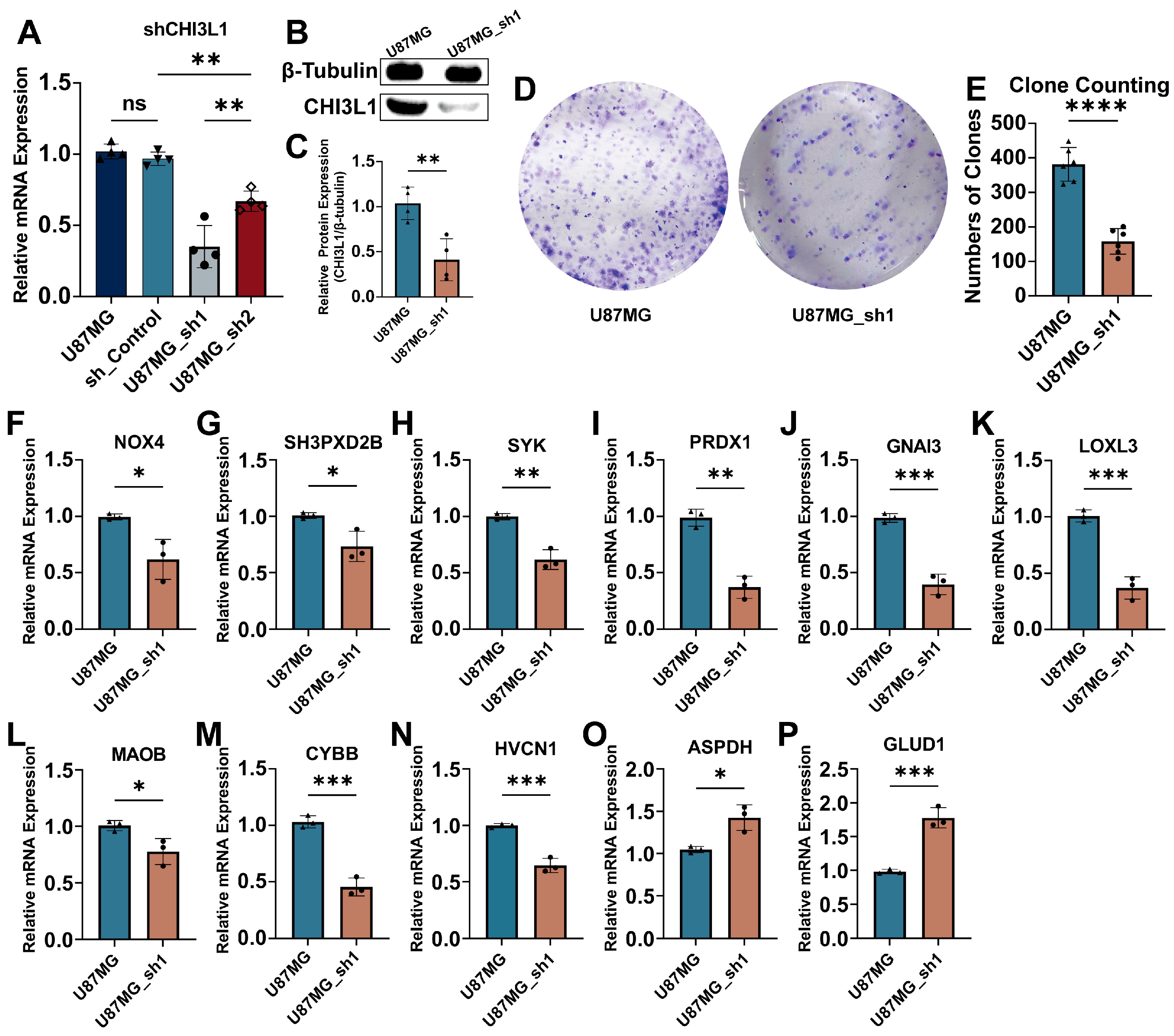
2.5. Knockdown of CHI3L1 Inhibited Proliferation of Glioblastoma Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. Deferential Expression and Survival Analysis
4.3. Functions and Pathway Enrichment Analysis
4.4. Index of Oxidative Stress-Related Genes (ORGI) and Prognostic Value
4.5. Establishment and Validation of ORGI Prognostic Model
4.6. Clinical Case Validation
4.7. Cell Culture
4.8. Real-Time Quantitative PCR (qPCR)
4.9. CHI3L1 Knockdown In Vitro
4.10. Western Blotting
4.11. Colony Formation Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22, iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Huse, J.T. 2016 World Health Organization Classification of Central Nervous System Tumors. Continuum 2017, 23, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. Population-Based Studies on Incidence, Survival Rates, and Genetic Alterations in Astrocytic and Oligodendroglial Gliomas. J. Neuropathol. Exp. Neurol. 2005, 64, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Dirven, L.; Aaronson, N.K.; Heimans, J.J.; Taphoorn, M.J. Health-related quality of life in high-grade glioma patients. Chin. J. Cancer 2014, 33, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Przybylowski, C.J.; Hervey-Jumper, S.L.; Sanai, N. Surgical strategy for insular glioma. J. Neurooncol. 2021, 151, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 2017, 14, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.J.; He, C.H.; Takyar, S.; Elias, J.A. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef]
- Johansen, J.S.; Williamson, M.K.; Rice, J.S.; Price, P.A. Identification of proteins secreted by human osteoblastic cells in culture. J. Bone Miner. Res. 1992, 7, 501–512. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Marrocco, F.; Limatola, C. Microglial cells: Sensors for neuronal activity and microbiota-derived molecules. Front. Immunol. 2022, 13, 1011129. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.E.; Yeo, I.J.; Son, D.J.; Yun, J.; Han, S.B.; Hong, J.T. Anti-Chi3L1 antibody suppresses lung tumor growth and metastasis through inhibition of M2 polarization. Mol. Oncol. 2022, 16, 2214–2234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Wang, Q.; Zhang, X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J. Hematol. Oncol. 2017, 10, 36. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Mamidi, S.; Gratchev, A.; Kremmer, E.; Schmuttermaier, C.; Krusell, L.; Haus, G.; Utikal, J.; Schledzewski, K.; Scholtze, J.; et al. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood 2006, 107, 3221–3228. [Google Scholar] [CrossRef] [PubMed]
- Libreros, S.; Garcia-Areas, R.; Keating, P.; Gazaniga, N.; Robinson, P.; Humbles, A.; Iragavarapu-Charyulu, V.L. Allergen induced pulmonary inflammation enhances mammary tumor growth and metastasis: Role of CHI3L1. J. Leukoc. Biol. 2015, 97, 929–940. [Google Scholar] [CrossRef]
- Libreros, S.; Garcia-Areas, R.; Shibata, Y.; Carrio, R.; Torroella-Kouri, M.; Iragavarapu-Charyulu, V. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: Decreased tumor metastasis in a breast cancer model. Int. J. Cancer 2012, 131, 377–386. [Google Scholar] [CrossRef]
- Kavsan, V.M.; Baklaushev, V.P.; Balynska, O.V.; Iershov, A.V.; Areshkov, P.O.; Yusubalieva, G.M.; Grinenko, N.P.; Victorov, I.V.; Rymar, V.I.; Sanson, M.; et al. Gene Encoding Chitinase 3-Like 1 Protein (CHI3L1) is a Putative Oncogene. Int. J. Biomed. Sci. 2011, 7, 230–237. [Google Scholar] [CrossRef]
- Baldacci, F.; Lista, S.; Cavedo, E.; Bonuccelli, U.; Hampel, H. Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer’s disease and other neurodegenerative diseases. Expert. Rev. Proteom. 2017, 14, 285–299. [Google Scholar] [CrossRef]
- Létuvé, S.; Kozhich, A.; Arouche, N.; Grandsaigne, M.; Reed, J.; Dombret, M.C.; Kiener, P.A.; Aubier, M.; Coyle, A.J.; Pretolani, M. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J. Immunol. 2008, 181, 5167–5173. [Google Scholar] [CrossRef]
- Libreros, S.; Iragavarapu-Charyulu, V. YKL-40/CHI3L1 drives inflammation on the road of tumor progression. J. Leukoc. Biol. 2015, 98, 931–936. [Google Scholar] [CrossRef]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef] [PubMed]
- García, J.G.; Ansorena, E.; Izal, I.; Zalba, G.; de Miguel, C.; Milagro, F.I. Structure, regulation, and physiological functions of NADPH oxidase 5 (NOX5). J. Physiol. Biochem. 2023, 79, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Wu, Q.; Chen, Y.; Deng, Y.; Yang, Z.; Zhang, L.; Liu, B. Tumoral NOX4 recruits M2 tumor-associated macrophages via ROS/PI3K signaling-dependent various cytokine production to promote NSCLC growth. Redox Biol. 2019, 22, 101116. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.L.; Yang, L.; Fu, S.W.; Lin, W.F.; Gao, Y.J.; Chen, H.Y.; Ge, Z.Z. Overexpression of NOX4 predicts poor prognosis and promotes tumor progression in human colorectal cancer. Oncotarget 2017, 8, 33586–33600. [Google Scholar] [CrossRef]
- Bi, Y.; Lei, X.; Chai, N.; Linghu, E. NOX4: A potential therapeutic target for pancreatic cancer and its mechanism. J. Transl. Med. 2021, 19, 515. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Johansson, J.; Nazir, F.H.; Hellstrand, K.; Martner, A. Role of NOX2-Derived Reactive Oxygen Species in NK Cell-Mediated Control of Murine Melanoma Metastasis. Cancer Immunol. Res. 2017, 5, 804–811. [Google Scholar] [CrossRef]
- Gianni, D.; Diaz, B.; Taulet, N.; Fowler, B.; Courtneidge, S.A.; Bokoch, G.M. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci. Signal 2009, 2, ra54. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Li, Y.L.; Jiang, H.; Yin, X.H.; Jin, X.L. PRDX1 Influences The Occurrence and Progression of Liver Cancer by Inhibiting Mitochondrial Apoptosis Pathway. Cell J. 2022, 24, 657–664. [Google Scholar] [CrossRef]
- Kim, J.H.; Bogner, P.N.; Baek, S.H.; Ramnath, N.; Liang, P.; Kim, H.R.; Andrews, C.; Park, Y.M. Up-regulation of peroxiredoxin 1 in lung cancer and its implication as a prognostic and therapeutic target. Clin. Cancer Res. 2008, 14, 2326–2333. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, F.; Lu, M.; Liu, Z.; Wang, M.; Cao, J.; Ke, X.; Yi, M. DDX43 recruits TRIF or IPS-1 as an adaptor and activates the IFN-β pathway in Nile tilapia (Oreochromis niloticus). Mol. Immunol. 2022, 143, 7–16. [Google Scholar] [CrossRef]
- Pu, T.; Wang, J.; Wei, J.; Zeng, A.; Zhang, J.; Chen, J.; Yin, L.; Li, J.; Lin, T.P.; Melamed, J.; et al. Stromal-derived MAOB promotes prostate cancer growth and progression. Sci. Adv. 2024, 10, eadi4935. [Google Scholar] [CrossRef]
- Zhan, M.; Ding, Y.; Huang, S.; Liu, Y.; Xiao, J.; Yu, H.; Lu, L.; Wang, X. Lysyl oxidase-like 3 restrains mitochondrial ferroptosis to promote liver cancer chemoresistance by stabilizing dihydroorotate dehydrogenase. Nat. Commun. 2023, 14, 3123. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, S.; Du, M.; Chu, H.; Wang, M.; Zhang, Z. Novel CpG-SNPs in the gastric acid secretion pathway GNAI3 and susceptibility to gastric cancer. Gene 2020, 736, 144447. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Gupta, S.; Bakshi, A.; Kaur, H.; Jain, A.; Senapati, S.; Baghel, M.S. Targeting mitochondria in the regulation of neurodegenerative diseases: A comprehensive review. J. Neurosci. Res. 2022, 100, 1845–1861. [Google Scholar] [CrossRef]
- Li, J.Y.; Yao, Y.M.; Tian, Y.P. Ferroptosis: A Trigger of Proinflammatory State Progression to Immunogenicity in Necroinflammatory Disease. Front. Immunol. 2021, 12, 701163. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
| Gene Symbol | Sequences |
|---|---|
| CHI3L1 | Forward sequence (5′→3′) GTGAAGGCGTCTCAAACAGG Reverse sequence (5′→3′) GAAGCGGTCAAGGGCATCT |
| GAPDH | Forward sequence (5′→3′) GGAGCGAGATCCCTCCAAAAT Reverse sequence (5′→3′) GGCTGTTGTCATACTTCTCATGG |
| NOX4 | Forward sequence (5′→3′) CAGATGTTGGGGCTAGGATTG Reverse sequence (5′→3′) GAGTGTTCGGCACATGGGTA |
| SYK | Forward sequence (5′→3′) CATGGAAAAATCTCTCGGGAAGA Reverse sequence (5′→3′) GTCGATGCGATAGTGCAGCA |
| HVCN1 | Forward sequence (5′→3′) CACCCACACCAGTCTCAGG Reverse sequence (5′→3′) TGTCGGGCTGGATGATCTTCA |
| GNAI3 | Forward sequence (5′→3′) ATCGACCGCAACTTACGGG Reverse sequence (5′→3′) AGTCAATCTTTAGCCGTCCCA |
| SH3PXD2B | Forward sequence (5′→3′) TGGAGGTGAAGGTGCTAGAC Reverse sequence (5′→3′) CTGTAGCGCCGGTAAATGG |
| LOXL3 | Forward sequence (5′→3′) GATACAGCGAGCTGGTGAATG Reverse sequence (5′→3′) CATCCTCATCGTGCGTACAGT |
| MAOB | Forward sequence (5′→3′) GGCGGCATCTCAGGTATGG Reverse sequence (5′→3′) GGTCTCCAATCCTAGCTCCTTG |
| CYBB | Forward sequence (5′→3′) ACCGGGTTTATGATATTCCACCT Reverse sequence (5′→3′) GATTTCGACAGACTGGCAAGA |
| PRDX1 | Forward sequence (5′→3′) CCACGGAGATCATTGCTTTCA Reverse sequence (5′→3′) AGGTGTATTGACCCATGCTAGAT |
| ASPDH | Forward sequence (5′→3′) GCACTGTGCTCTACGAAGG Reverse sequence (5′→3′) ATCACCCCATCGAAGCCCA |
| GLUD1 | Forward sequence (5′→3′) ACAGTCCAAAGAATGCAGTCAC Reverse sequence (5′→3′) CAGGTGAGTAGGGGCCATTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhao, D.; Tan, H.; Lan, J.; Bao, Y. CHI3L1 as a Prognostic Biomarker and Therapeutic Target in Glioma. Int. J. Mol. Sci. 2024, 25, 7094. https://doi.org/10.3390/ijms25137094
Zhou J, Zhao D, Tan H, Lan J, Bao Y. CHI3L1 as a Prognostic Biomarker and Therapeutic Target in Glioma. International Journal of Molecular Sciences. 2024; 25(13):7094. https://doi.org/10.3390/ijms25137094
Chicago/Turabian StyleZhou, Jue, Dongxu Zhao, Haoyuan Tan, Jin Lan, and Yinghui Bao. 2024. "CHI3L1 as a Prognostic Biomarker and Therapeutic Target in Glioma" International Journal of Molecular Sciences 25, no. 13: 7094. https://doi.org/10.3390/ijms25137094
APA StyleZhou, J., Zhao, D., Tan, H., Lan, J., & Bao, Y. (2024). CHI3L1 as a Prognostic Biomarker and Therapeutic Target in Glioma. International Journal of Molecular Sciences, 25(13), 7094. https://doi.org/10.3390/ijms25137094






