Astaxanthin Induces Apoptosis in MCF-7 Cells through a p53-Dependent Pathway
Abstract
1. Introduction
2. Results
2.1. Roles of AXT in MCF-7 Viability and Apoptosis
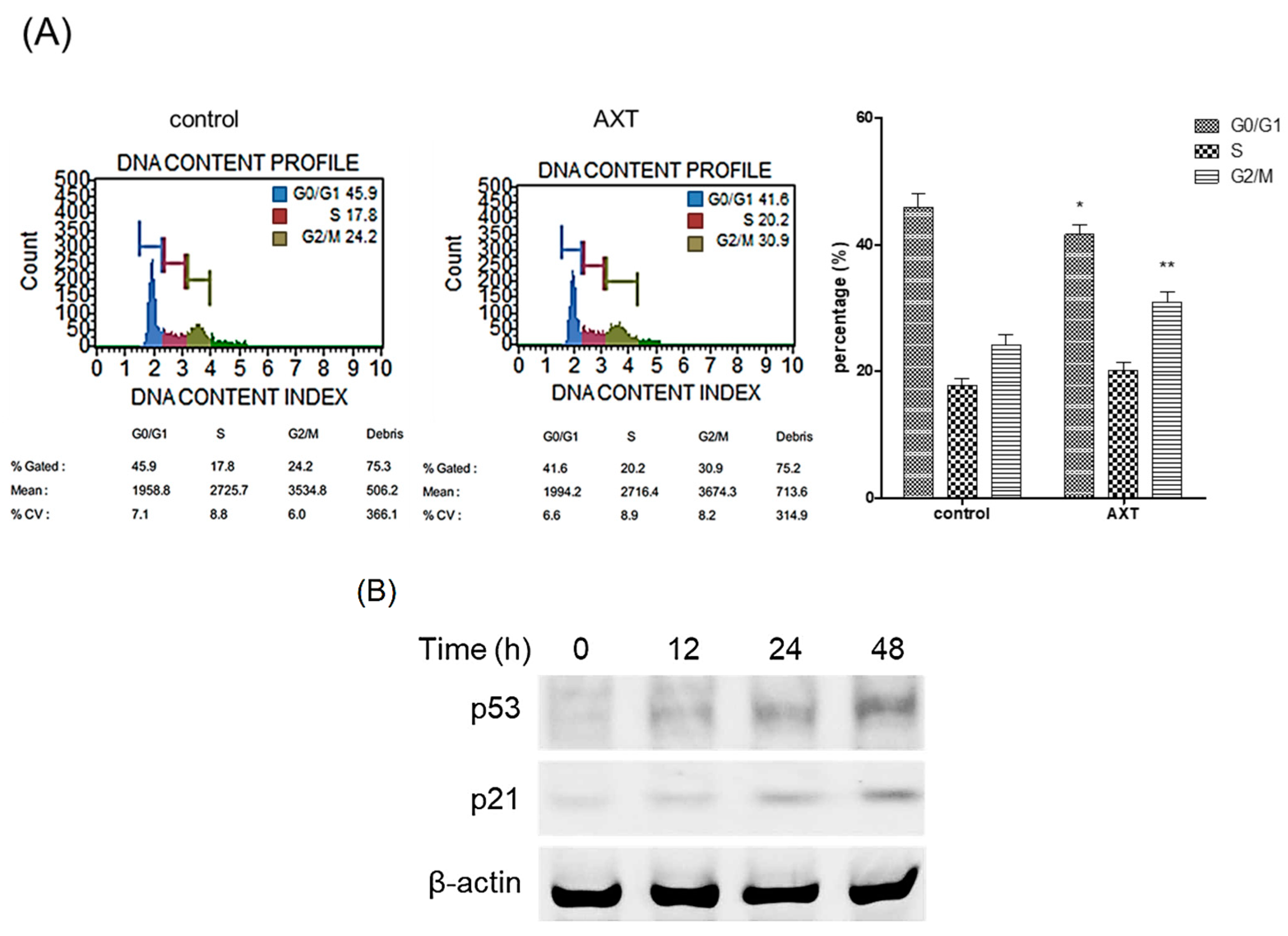
2.2. Roles of AXT in Cell Cycle and the Expression of Tumor Suppressor Proteins
2.3. Effects of AXT on Caspase-3 Activity
3. Materials and Methods
3.1. Cell and Reagents
3.2. Western Blot
3.3. Cell Viability Assay
3.4. Analysis of Caspase-3 Activity
3.5. Cell Cycle Analysis
3.6. Cell Apoptosis Analysis
3.7. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ohgami, K.; Shiratori, K.; Jin, X.H.; Ilieva, I.; Koyama, Y.; Yazawa, K.; Yoshida, K.; Kase, S.; Ohno, S. Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-kappaB signaling pathway. Exp. Eye Res. 2006, 82, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Izumi-Nagai, K.; Nagai, N.; Ohgami, K.; Satofuka, S.; Ozawa, Y.; Tsubota, K.; Ohno, S.; Oike, Y.; Ishida, S. Inhibition of choroidal neovascularization with an anti-inflammatory carotenoid astaxanthin. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Campoio, T.R.; Oliveira, F.A.; Otton, R. Oxidative stress in human lymphocytes treated with fatty acid mixture: Role of carotenoid astaxanthin. Toxicol. Vitr. 2011, 25, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Hosokawa, M.; Mikami, N.; Miyashita, K.; Tanaka, T. Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cytokines. Chem. Biol. Interact. 2011, 193, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ishiki, M.; Nishida, Y.; Ishibashi, H.; Wada, T.; Fujisaka, S.; Takikawa, A.; Urakaze, M.; Sasaoka, T.; Usui, I.; Tobe, K. Impact of divergent effects of astaxanthin on Insulin Signaling in L6 cells. Endocrinology 2013, 154, 2600–2612. [Google Scholar] [CrossRef] [PubMed]
- Kuerbitz, S.J.; Plunkett, B.S.; Walsh, W.V.; Kastan, M.B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc. Natl. Acad. Sci. USA 1992, 89, 7491–7495. [Google Scholar] [CrossRef] [PubMed]
- Debbas, M.; White, E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes. Dev. 1993, 7, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.W.; Ruley, H.E.; Jacks, T.; Housman, D.E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 1993, 74, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Bae, I.; Kastan, M.B.; Fornace, A.J., Jr. The p53-dependent gamma-ray response of GADD45. Cancer Res. 1994, 54, 2755–2760. [Google Scholar] [PubMed]
- Smith, M.L.; Chen, I.T.; Zhan, Q.; Bae, I.; Chen, C.Y.; Gilmer, T.M.; Kastan, M.B.; O’Connor, P.M.; Fornace, A.J., Jr. Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science 1994, 266, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Mummenbrauer, T.; Janus, F.; Muller, B.; Wiesmuller, L.; Deppert, W.; Grosse, F. p53 Protein exhibits 3′-to-5′ exonuclease activity. Cell 1996, 85, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.D.; Xavier, J.M.; Steer, C.J.; Rodrigues, C.M. Targeting the p53 pathway of apoptosis. Curr. Pharm. Des. 2010, 16, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.T.; Kim, M.S.; Kim, Y.S.; An, W.G. Astaxanthin Reduces Stemness Markers in BT20 and T47D Breast Cancer Stem Cells by Inhibiting Expression of Pontin and Mutant p53. Mar. Drugs 2020, 18, 577. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, K.J.; Jiang, S.M.; Fu, L.; Shi, Y.; Tan, R.B.; Cui, J.; Zhou, Y. p53: A Key Protein That Regulates Pulmonary Fibrosis. Oxidative Med. Cell. Longev. 2020, 2020, 6635794. [Google Scholar] [CrossRef] [PubMed]
- Karimian, A.; Mir Mohammadrezaei, F.; Hajizadeh Moghadam, A.; Bahadori, M.H.; Ghorbani-Anarkooli, M.; Asadi, A.; Abdolmaleki, A. Effect of astaxanthin and melatonin on cell viability and DNA damage in human breast cancer cell lines. Acta Histochem. 2022, 124, 151832. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Cho, H.-r.; Son, Y. Astaxanthin Induces Apoptosis in MCF-7 Cells through a p53-Dependent Pathway. Int. J. Mol. Sci. 2024, 25, 7111. https://doi.org/10.3390/ijms25137111
Kim K, Cho H-r, Son Y. Astaxanthin Induces Apoptosis in MCF-7 Cells through a p53-Dependent Pathway. International Journal of Molecular Sciences. 2024; 25(13):7111. https://doi.org/10.3390/ijms25137111
Chicago/Turabian StyleKim, Koanhoi, Hyok-rae Cho, and Yonghae Son. 2024. "Astaxanthin Induces Apoptosis in MCF-7 Cells through a p53-Dependent Pathway" International Journal of Molecular Sciences 25, no. 13: 7111. https://doi.org/10.3390/ijms25137111
APA StyleKim, K., Cho, H.-r., & Son, Y. (2024). Astaxanthin Induces Apoptosis in MCF-7 Cells through a p53-Dependent Pathway. International Journal of Molecular Sciences, 25(13), 7111. https://doi.org/10.3390/ijms25137111





