Plantago major and Plantago lanceolata Exhibit Antioxidant and Borrelia burgdorferi Inhibiting Activities
Abstract
1. Introduction
1.1. Lyme Disease
1.2. Ethnomedicinal Use and Therapeutic Properties of the Plantago Species
1.3. Phytochemical Screening of Plantago Extracts
1.4. Specific Antibacterial Potential of Plantago Extracts
2. Results and Discussion
2.1. Antioxidative Activity and Colorimetric Analyses
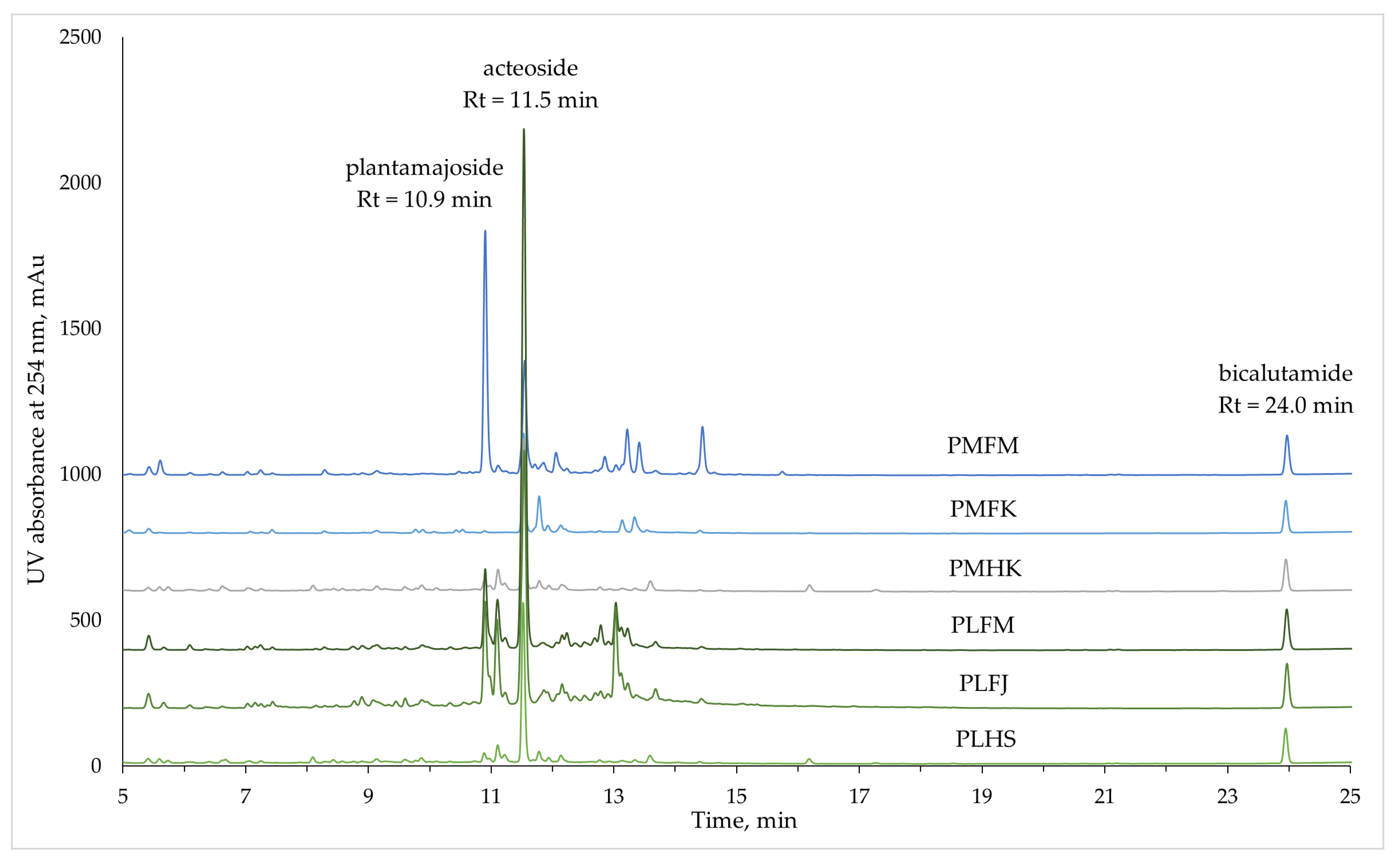
2.2. Identification and Quantification of Non-Volatile Phytochemicals
2.3. Identification and Quantification of Volatile Phytochemicals
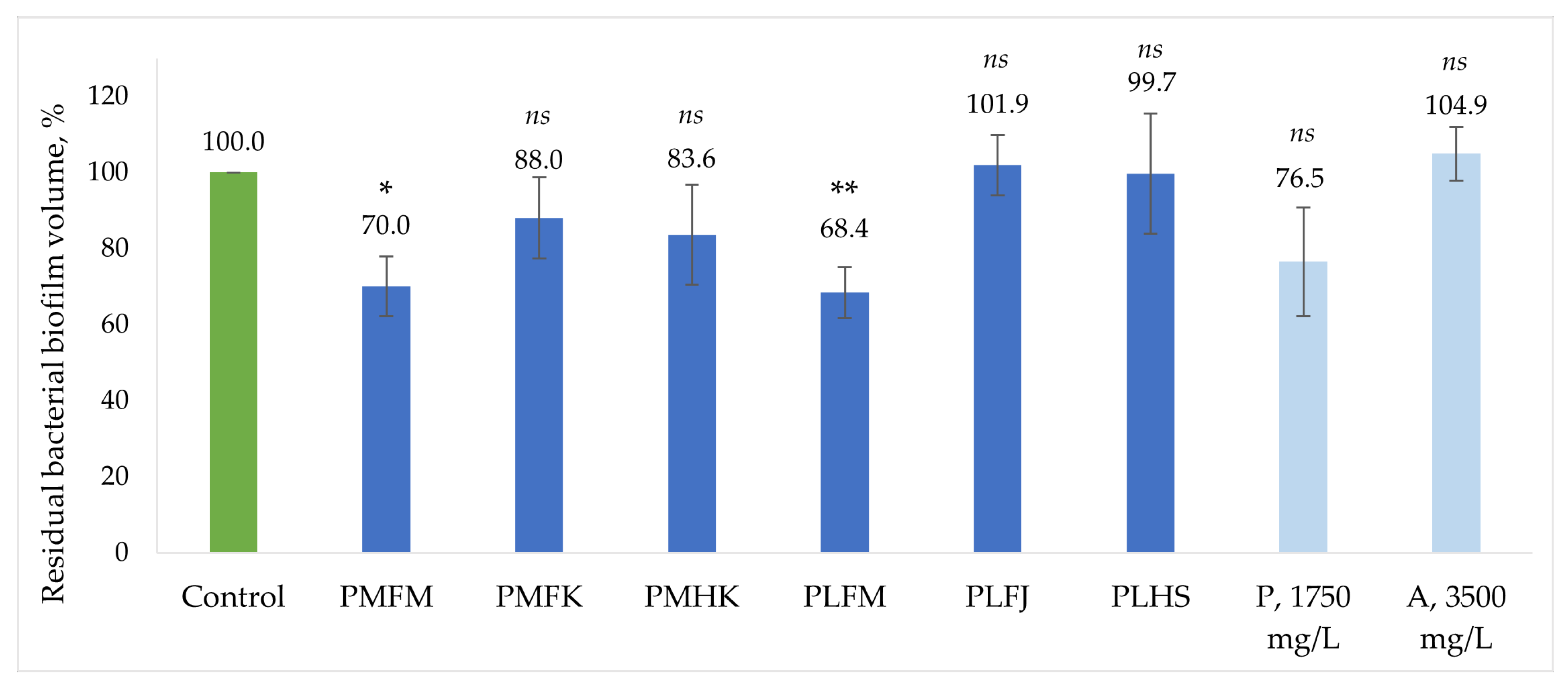
2.4. Evaluation of B. burgdorferi Inhibiting Activity
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Plant Extracts
3.3. Colorimetric Analyses
3.4. Antioxidative Activity Measurements
3.5. HPLC-DAD-MS Analyses
3.6. HS-SPME-GC-MS Analyses
3.7. Antibacterial Activity Evaluation
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radolf, J.D.; Strle, K.; Lemieux, J.E.; Strle, F. Lyme Disease in Humans. Curr. Issues Mol. Biol. 2021, 42, 333. [Google Scholar] [CrossRef]
- Meriläinen, L.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Morphological and Biochemical Features of Borrelia Burgdorferi Pleomorphic Forms. Microbiology 2015, 161, 516. [Google Scholar] [CrossRef] [PubMed]
- Pitrak, D.; Nguyen, C.T.; Cifu, A.S. Diagnosis of Lyme Disease. J. Am. Med. Assoc. 2022, 327, 676–677. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme Borreliosis. Nat. Rev. Dis. Prim. 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Melia, M.T.; Auwaerter, P.G. Time for a Different Approach to Lyme Disease and Long-Term Symptoms. N. Engl. J. Med. 2016, 374, 1277–1278. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Girschick, H.J. Lyme Borreliosis: From Infection to Autoimmunity. Clin. Microbiol. Infect. 2004, 10, 598–614. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; D’Agosto, G.; Pontone, M.; Trento, E.; Gallo, M.T.; Prignano, G.; Pimpinelli, F.; Toma, L.; et al. The Emerging Role of Microbial Biofilm in Lyme Neuroborreliosis. Front. Neurol. 2018, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Al-Robaiy, S.; Dihazi, H.; Kacza, J.; Seeger, J.; Schiller, J.; Huster, D.; Knauer, J.; Straubinger, R.K. Metamorphosis of Borrelia Burgdorferi Organisms—RNA, Lipid and Protein Composition in Context with the Spirochetes’ Shape. J. Basic Microbiol. 2010, 50 (Suppl. S1), S5–S17. [Google Scholar] [CrossRef] [PubMed]
- Kersten, A.; Poitschek, C.; Rauch, S.; Aberer, E. Effects of Penicillin, Ceftriaxone, and Doxycycline on Morphology of Borrelia Burgdorferi. Antimicrob. Agents Chemother. 1995, 39, 1127. [Google Scholar] [CrossRef][Green Version]
- Aberer, E.; Duray, P.H. Morphology of Borrelia Burgdorferi: Structural Patterns of Cultured Borreliae in Relation to Staining Methods. J. Clin. Microbiol. 1991, 29, 764. [Google Scholar] [CrossRef]
- Sapi, E.; Bastian, S.L.; Mpoy, C.M.; Scott, S.; Rattelle, A.; Pabbati, N.; Poruri, A.; Burugu, D.; Theophilus, P.A.S.; Pham, T.V.; et al. Characterization of Biofilm Formation by Borrelia Burgdorferi In Vitro. PLoS ONE 2012, 7, 48277. [Google Scholar] [CrossRef]
- Justice, S.S.; Hunstad, D.A.; Cegelski, L.; Hultgren, S.J. Morphological Plasticity as a Bacterial Survival Strategy. Nat. Rev. Microbiol. 2008, 6, 162–168. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Saar-Reismaa, P.; Bragina, O.; Kuhtinskaja, M.; Reile, I.; Laanet, P.-R.; Kulp, M.; Vaher, M. Extraction and Fractionation of Bioactives from Dipsacus Fullonum L. Leaves and Evaluation of Their Anti-Borrelia Activity. Pharmaceuticals 2022, 15, 87. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. The Medicinal Potential of Plants from the Genus Plantago (Plantaginaceae). Ind. Crops Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The Traditional Uses, Chemical Constituents and Biological Activities of Plantago Major L. A Review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Fons, F.; Gargadennec, A.; Gueiffier, A.; Roussel, J.L.; Andary, C. Effects of Cinnamic Acid on Polyphenol Production in Plantago lanceolata. Phytochemistry 1998, 49, 697–702. [Google Scholar] [CrossRef]
- Adams, M.; Berset, C.; Kessler, M.; Hamburger, M. Medicinal Herbs for the Treatment of Rheumatic Disorders—A Survey of European Herbals from the 16th and 17th Century. J. Ethnopharmacol. 2009, 121, 343–359. [Google Scholar] [CrossRef]
- Oloumi, M.M.; Vosough, D.; Derakhshanfar, A.; Nematollahi, M.H. The Healing Potential of Plantago lanceolata Ointment on Collagenase-Induced Tendinitis in Burros (Equus asinus). J. Equine Vet. Sci. 2011, 31, 470–474. [Google Scholar] [CrossRef]
- Rønsted, N.; Göbel, E.; Franzyk, H.; Jensen, S.R.; Olsen, C.E. Chemotaxonomy of Plantago. Iridoid Glucosides and Caffeoyl Phenylethanoid Glycosides. Phytochemistry 2000, 55, 337–348. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.; Zamboni, P.; Mahajan, R. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in Vitro Studies on Austria’s Folk Medicine—An Unexplored Lore in Vitro Anti-Inflammatory Activities of 71 Austrian Traditional Herbal Drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef]
- Gomez-Flores, R.; Calderon, C.L.; Scheibel, L.W.; Tamez-Guerra, P.; Rodriguez-Padilla, C.; Tamez-Guerra, R.; Weber, R.J. Immunoenhancing Properties of Plantago Major Leaf Extract. Phyther. Res. 2000, 14, 617–622. [Google Scholar] [CrossRef]
- Beara, I.N.; Orčić, D.Z.; Lesjak, M.M.; Mimica-Dukić, N.M.; Peković, B.A.; Popović, M.R. Liquid Chromatography/Tandem Mass Spectrometry Study of Anti-Inflammatory Activity of Plantain (Plantago L.) Species. J. Pharm. Biomed. Anal. 2010, 52, 701–706. [Google Scholar] [CrossRef]
- Vigo, E.; Cepeda, A.; Gualillo, O.; Perez-Fernandez, R. In-Vitro Anti-Inflammatory Activity of Pinus Sylvestris and Plantago lanceolata Extracts: Effect on Inducible NOS, COX-1, COX-2 and Their Products in J774A.1 Murine Macrophages. J. Pharm. Pharmacol. 2005, 57, 383–391. [Google Scholar] [CrossRef]
- Amini, M.; Kherad, M.; Mehrabani, D.; Azarpira, N.; Panjehshahin, M.R.; Tanideh, N. Effect of Plantago Major on Burn Wound Healing in Rat. J. Appl. Anim. Res. 2010, 37, 53–56. [Google Scholar] [CrossRef]
- Krasnov, M.S.; Yamskova, V.P.; Margasyuk, D.V.; Kulikova, O.G.; Il’ina, A.P.; Rybakova, E.Y.; Yamskov, I.A. Study of a New Group of Bioregulators Isolated from the Greater Plantain (Plantago Major L.). Appl. Biochem. Microbiol. 2011, 47, 128–135. [Google Scholar] [CrossRef]
- Thomé, R.G.; dos Santos, H.B.; dos Santos, F.V.; Da Silva Oliveira, R.J.; de Camargos, L.F.; Pereira, M.N.; Longatti, T.R.; Souto, C.M.; Franco, C.S.; De Oliveira Aquino Schüffner, R.; et al. Evaluation of Healing Wound and Genotoxicity Potentials from Extracts Hydroalcoholic of Plantago Major and Siparuna Guianensis. Exp. Biol. Med. 2012, 237, 1379–1386. [Google Scholar] [CrossRef]
- Kováč, I.; ɰurkáč, J.; Hollý, M.; Jakubčová, K.; Peržeová, V.; Mučaji, P.; Švajdlenka, E.; Sabol, F.; Legáth, J.; Belák, J.; et al. Plantago lanceolata L. Water Extract Induces Transition of Fibroblasts into Myofibroblasts and Increases Tensile Strength of Healing Skin Wounds. J. Pharm. Pharmacol. 2014, 67, 117–125. [Google Scholar] [CrossRef]
- Janković, T.; Menković, N.; Zdunić, G.; Beara, I.; Balog, K.; Šavikin, K.; Mimica-Dukić, N. Quantitative Determination of Aucubin in Seven Plantago Species Using HPLC, HPTLC, and LC-ESI-MS Methods. Anal. Lett. 2010, 43, 2487–2495. [Google Scholar] [CrossRef]
- Jurišić Grubešić, R.; Srečnik, G.; Kremer, D.; Vuković Rodríguez, J.; Nikolić, T.; Vladimir-Knežević, S. Simultaneous RP-HPLC-DAD Separation, and Determination of Flavonoids and Phenolic Acids in Plantago L. Species. Chem. Biodivers. 2013, 10, 1305–1316. [Google Scholar] [CrossRef]
- Qi, M.; Xiong, A.; Geng, F.; Yang, L.; Wang, Z. A Novel Strategy for Target Profiling Analysis of Bioactive Phenylethanoid Glycosides in Plantago Medicinal Plants Using Ultra-Performance Liquid Chromatography Coupled with Tandem Quadrupole Mass Spectrometry. J. Sep. Sci. 2012, 35, 1470–1478. [Google Scholar] [CrossRef]
- Gonda, S.; Nguyen, N.M.; Batta, G.; Gyémánt, G.; Máthé, C.; Vasas, G. Determination of Phenylethanoid Glycosides and Iridoid Glycosides from Therapeutically Used Plantago Species by CE-MEKC. Electrophoresis 2013, 34, 2577–2584. [Google Scholar] [CrossRef]
- Li, Y.; Gan, L.; Li, G.Q.; Deng, L.; Zhang, X.; Deng, Y. Pharmacokinetics of Plantamajoside and Acteoside from Plantago asiatica in Rats by Liquid Chromatography–Mass Spectrometry. J. Pharm. Biomed. Anal. 2014, 89, 251–256. [Google Scholar] [CrossRef]
- Turgumbayeva, A.; Zhakipbekov, K.; Shimirova, Z.; Akhelova, S.; Amirkhanova, A.; Koilybayeva, M.; Seitimova, G.; Abdambayev, D. Study of Phytochemical Compounds of Plantago Major Leaves Grown in Kazakhstan. Pharmacia 2022, 69, 1019–1026. [Google Scholar] [CrossRef]
- Rahamouz-Haghighi, S.; Bagheri, K.; Sharafi, A. Antibacterial Activities and Chemical Compounds of Plantago lanceolata (Ribwort Plantain) and Plantago Major (Broadleaf Plantain) Leaf Extracts. Pharm. Biomed. Res. 2023, 9, 183–200. [Google Scholar] [CrossRef]
- Laanet, P.-R.; Saar-Reismaa, P.; Jõul, P.; Bragina, O.; Vaher, M. Phytochemical Screening and Antioxidant Activity of Selected Estonian Galium Species. Molecules 2023, 28, 2867. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Nadeem, S.; Komal, S.; Naqvi, S.A.A.; Mubarik, M.S.; Qureshi, S.Y.; Ahmad, S.; Abbas, A.; Zahid, M.; Khan, N.-U.-H.; et al. Antioxidants: Natural Antibiotics. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Gonçalves, A.S.C.; Leitão, M.M.; Simões, M.; Borges, A. The Action of Phytochemicals in Biofilm Control. Nat. Prod. Rep. 2023, 40, 595–627. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Dalar, A.; Türker, M.; Konczak, I. Antioxidant Capacity and Phenolic Constituents of Malva Neglecta Wallr. and Plantago lanceolata L. from Eastern Anatolia Region of Turkey. J. Herb. Med. 2012, 2, 42–51. [Google Scholar] [CrossRef]
- Amakura, Y.; Yoshimura, A.; Yoshimura, M.; Yoshida, T. Isolation and Characterization of Phenolic Antioxidants from Plantago Herb. Molecules 2012, 17, 5459–5466. [Google Scholar] [CrossRef]
- Xiao, Y.; Ren, Q.; Wu, L. The Pharmacokinetic Property and Pharmacological Activity of Acteoside: A Review. Biomed. Pharmacother. 2022, 153, 113296. [Google Scholar] [CrossRef]
- Bajer, T.; Janda, V.; Bajerová, P.; Kremr, D.; Eisner, A.; Ventura, K. Chemical Composition of Essential Oils from Plantago lanceolata L. Leaves Extracted by Hydrodistillation. J. Food Sci. Technol. 2016, 53, 1576. [Google Scholar] [CrossRef]
- Hashiesh, H.M.; Sharma, C.; Goyal, S.N.; Sadek, B.; Jha, N.K.; Al Kaabi, J.; Ojha, S. A Focused Review on CB2 Receptor-Selective Pharmacological Properties and Therapeutic Potential of β-Caryophyllene, a Dietary Cannabinoid. Biomed. Pharmacother. 2021, 140, 111639. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-Limonene: A Multifunctional Compound with Potent Therapeutic Effects. J. Food Biochem. 2021, 45, 13566. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chinese Star Anise and Anise, Magic Herbs in Traditional Chinese Medicine and Modern Pharmaceutical Science. Asian J. Med. Biol. Res. 2019, 5, 162–179. [Google Scholar] [CrossRef]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Inhibition of Bacterial Biofilm Formation. In Bacterial Biofilms; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Rover, M.R.; Brown, R.C. Quantification of Total Phenols in Bio-Oil Using the Folin-Ciocalteu Method. J. Anal. Appl. Pyrolysis 2013, 104, 366–371. [Google Scholar] [CrossRef]
- Pharmacognosy: Fundamentals, Applications and Strategies; Badal, S., Delgoda, R., Eds.; Elsevier, Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Janković, T.; Zdunić, G.; Beara, I.; Balog, K.; Pljevljakušić, D.; Stešević, D.; Šavikin, K. Comparative Study of Some Polyphenols in Plantago Species. Biochem. Syst. Ecol. 2012, 42, 69–74. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. A Fluorometric Method for Measurement of Oxygen Radical-Scavenging Activity of Water-Soluble Antioxidants. Anal. Biochem. 2000, 284, 93–98. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An Optimized SYBR Green I/PI Assay for Rapid Viability Assessment and Antibiotic Susceptibility Testing for Borrelia Burgdorferi. PLoS ONE 2014, 9, 111809. [Google Scholar] [CrossRef]
- Woolard, K.J.; Sandala, J.L.; Melander, R.J.; Gunn, J.S.; Melander, C. Development of Small Molecules That Work Cooperatively with Ciprofloxacin to Clear Salmonella Biofilms in a Chronic Gallbladder Carriage Model. Eur. J. Med. Chem. 2022, 232, 114203. [Google Scholar] [CrossRef] [PubMed]


| Abbreviated Sample Name | Species Name | Part of the Plant Used | Place of Origin |
|---|---|---|---|
| PMFM | Plantago major Great plantain | Plantaginis majoris folium Great plantain leaves | Matsalu, Estonia |
| PMFK | Kubja, Estonia | ||
| PMHK | Plantaginis majoris herba Great plantain herb | Kubja, Estonia | |
| PLFM | Plantago lanceolata Ribworth plantain | Plantaginis lanceolatae folium Ribworth plantain leaves | Matsalu, Estonia |
| PLFJ | Jälgimäe, Estonia | ||
| PLHS | Plantaginis lanceolatae herba Ribworth plantain herb | Salus, Germany |
| Plantago Sample | Antioxidant Activity, mg TE 1/g (mmol TE/g) | Total Polyphenolic Content, mg GAE 2/g | Total Flavonoid Content, mg QE 3/g | Total Iridoid Content, mg AE 4/g |
|---|---|---|---|---|
| PMFM | 377.8 ± 11.2 (1.51 ± 0.04) | 32.7 ± 2.4 | 8.3 ± 0.2 | 5.5 ± 0.9 |
| PMFK | 152.7 ± 23.2 (0.61 ± 0.09) | 19.7 ± 0.7 | 5.9 ± 0.6 | 2.3 ± 0.3 |
| PMHK | 300.8 ± 64.5 (1.20 ± 0.26) | 25.2 ± 1.7 | 6.1 ± 0.4 | 11.4 ± 0.9 |
| PLFM | 459.2 ± 78.9 (1.83 ± 0.32) | 55.5 ± 4.8 | 12.4 ± 1.2 | 23.4 ± 4.6 |
| PLFJ | 369.2 ± 85.9 (1.48 ± 0.34) | 45.9 ± 0.8 | 14.0 ± 0.7 | 10.4 ± 2.1 |
| PLHS | 246.3 ± 60.3 (0.98 ± 0.24) | 23.4 ± 1.5 | 4.4 ± 0.3 | 14.3 ± 1.4 |
| Peak no. | Rt, min | UV Absorption Spectra | Standard Compound for Quantification | Concentration in the Extract, mg/L | Mass Spectra | Tentative Identification | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max(s), nm | Min(s), nm | PMFM | PMFK | PMHK | PLFM | PLFJ | PLHS | Molecular Ion [M-H]− | MS/MS Fragments | ||||
| 1 | 5.41 | 214, 252, 292 | 234, 276 | Apigenin | 8.1 ± 0.8 | <LOQ 1 | <LOQ | 16.1 ± 0.2 | 14.7 ± 1.2 | <LOQ | 807.8 | 329.0, 477.0, 644.9 | |
| 2 | 5.60 | 234 | 212 | Luteolin | 15.3 ± 1.0 | n.d. 2 | <LOQ | n.d. | n.d. | <LOQ | 747.6 | 373.0 | Geniposidic acid dimer |
| 3 | 8.89 | 216, 324 | 262 | Apigenin | n.d. | n.d. | n.d. | <LOQ | 10.6 ± 0.6 | n.d. | 878.6 | 373.1, 521.1, 637.1, 717.2, 799.1 | Geniposidic acid derivative |
| 4 | 9.07 | 216, 300, 326 | 262, 304 | Apigenin | n.d. | n.d. | n.d. | n.d. | 9.9 ± 0.7 | n.d. | 735.2 | 544.9, 573.2, 636.1, 699.0, 732.9 | |
| 5 | 10.89 | 218, 328 | 262 | Plantamajoside | 1693.0 ± 65.6 | <LOQ | 100.3 ± 5.8 | 562.2 ± 35.1 | 681.1 ± 12.0 | 72.4 ± 4.6 | 639.5 | 477.3 | Plantamajoside 3 |
| 6 | 10.97 | 212, 272, 332 | 260, 282 | Acteoside | n.d. | n.d. | <LOQ | <LOQ | 98.3 ± 5.8 | n.d. | 639.6 | 477.2 | Plantamajoside isomer |
| 7 | 11.09 | 248, 268, 348 | 242, 262, 284 | Luteolin | 11.8 ± 0.4 | n.d. | n.d. | 54.4 ± 3.2 | 93.6 ± 11.2 | n.d. | 681.5 | 351.1, 547.0, 663.0 | |
| 8 | 11.11 | 268, 340 | 254, 294 | Apigenin | n.d. | n.d. | 50.4 ± 4.8 | n.d. | n.d. | 22.2 ± 0.6 | 637.4 | 285.8, 461.1 | Kaempferol diglucuronide |
| 9 | 11.22 | 218, 328 | 262 | Plantamajoside | <LOQ | n.d. | 100.0 ± 10.2 | 88.7 ± 6.3 | 126.4 ± 11.8 | 77.4 ± 6.9 | 755.6 | 461.9, 593.3, 623.1 | Acteoside derivative |
| 10 | 11.52 | 218, 330 | 264 | Acteoside | 807.9 ± 26.2 | 773.7 ± 69.0 | 1150.3 ± 29.8 | 3309.0 ± 249.8 | 1652.6 ± 163.5 | 1004.3 ± 72.0 | 623.5 | 461.2 | Acteoside 3 |
| 11 | 11.71 | 216, 284, 334 | 256, 302 | Apigenin | 10.4 ± 0.5 | n.d. | n.d. | n.d. | n.d. | n.d. | 639.6 | 477.2 | Plantamajoside isomer |
| 12 | 11.77 | 214, 266, 342 | 262, 282 | Luteolin | n.d. | n.d. | 25.9 ± 1.9 | n.d. | n.d. | 14.4 ± 0.9 | 639.7 | 315.1, 477.2 | Plantamajoside isomer |
| 13 | 11.78 | 254, 266, 348 | 262, 294 | Luteolin | n.d. | 88.6 ± 7.9 | n.d. | n.d. | n.d. | n.d. | 923.7 | 285.0, 461.1 | Kaempferol glucuronide |
| 14 | 11.84 | 218, 328 | 262 | Plantamajoside | 145.2 ± 5.7 | n.d. | n.d. | 169.8 ± 11.5 | n.d. | n.d. | 640.0 | 160.9, 314.9, 477.2, 653.2 | Plantamajoside isomer |
| 15 | 11.85 | 216, 328 | 262 | Plantamajoside | n.d. | n.d. | n.d. | n.d. | 181.4 ± 17.8 | n.d. | 551.1 | 533.0 | |
| 16 | 11.91 | 216, 328 | 262 | Plantamajoside | n.d. | n.d. | n.d. | n.d. | 86.6 ± 6.2 | n.d. | 872.8 | 625.0, 718.7, 768.9, 827.6 | |
| 17 | 11.92 | 216, 284, 342 | 262, 304 | Apigenin | n.d. | 21.9 ± 2.2 | n.d. | n.d. | n.d. | n.d. | 565.7 | 168.9, 322.7, 423.2, 506.0, 528.8, 547.0 | |
| 18 | 12.05 | 214, 274, 338 | 262, 298 | Apigenin | 34.9 ± 1.1 | n.d. | n.d. | n.d. | n.d. | n.d. | 550.1 | 531.1 | |
| 19 | 12.08 | 218, 328 | 262 | Plantamajoside | n.d. | n.d. | n.d. | <LOQ | 87.6 ± 8.9 | n.d. | 756.2 | 531.5, 593.4, 623.2 | |
| 20 | 12.14 | 218, 328 | 262 | Plantamajoside | n.d. | 137.4 ± 12.9 | 112.5 ± 9.4 | 94.7 ± 7.9 | 144.8 ± 12.4 | 66.2 ± 5.0 | 623.5 | 315.4, 461.2 | Acteoside isomer |
| 21 | 12.22 | 216, 328 | 264 | Acteoside | <LOQ | n.d. | n.d. | 83.5 ± 6.5 | 61.2 ± 6.8 | n.d. | 623.5 | 461.2 | Acteoside isomer |
| 22 | 12.35 | 218, 332 | 264 | Plantamajoside | <LOQ | n.d. | n.d. | <LOQ | 84.1 ± 8.7 | n.d. | 844.2 | 513.3, 681.2, 799.3, 825.2 | |
| 23 | 12.51 | 216, 332 | 264 | Acteoside | <LOQ | n.d. | n.d. | <LOQ | 80.5 ± 8.0 | n.d. | 887.8 | 557.9 | |
| 24 | 12.69 | 218, 328 | 264 | Acteoside | <LOQ | n.d. | n.d. | 69.0 ± 8.9 | 83.1 ± 8.4 | n.d. | 772.1 | 463.2, 595.1, 639.1, 694.1, 729.3, 753.1 | |
| 25 | 12.78 | 218, 328 | 264 | Acteoside | n.d. | n.d. | n.d. | n.d. | 86.5 ± 14.5 | n.d. | 736.3 | 637.1, 687.0 | |
| 26 | 12.79 | 218, 330 | 264 | Acteoside | n.d. | n.d. | n.d. | 146.8 ± 17.5 | n.d. | n.d. | 637.7 | 461.3, 491.1 | |
| 27 | 12.85 | 216, 282, 334 | 252, 302 | Apigenin | 26.6 ± 4.8 | n.d. | n.d. | n.d. | n.d. | n.d. | 563.8 | 270.8, 336.9, 402.8, 493.1, 519.1, 544.6 | Apigenin derivative |
| 28 | 12.90 | 218, 326 | 264 | Chlorogenic acid | n.d. | n.d. | n.d. | n.d. | 42.2 ± 4.3 | n.d. | 807.0 | 623.1, 767.2, 785.3 | |
| 29 | 13.03 | 246, 270, 336 | 264, 282 | Luteolin | 10.6 ± 0.5 | n.d. | n.d. | 50.2 ± 1.3 | 98.4 ± 3.1 | n.d. | 857.7 | 527.3, 663.1 | |
| 30 | 13.11 | 248, 270, 334 | 264, 282 | Luteolin | n.d. | n.d. | n.d. | 23.3 ± 4.6 | 34.6 ± 7.8 | n.d. | 857.7 | 527.3, 663.1 | |
| 31 | 13.13 | 218, 266, 334 | 250, 290 | Apigenin | n.d. | 35.4 ± 3.0 | n.d. | n.d. | n.d. | n.d. | 638.3 | 461.1, 475.1 | |
| 32 | 13.21 | 212, 274, 334 | 248, 298 | Apigenin | 66.0 ± 5.8 | n.d. | n.d. | n.d. | n.d. | n.d. | 923.9 | 461.2 | |
| 33 | 13.22 | 248, 270, 338 | 264, 280 | Luteolin | n.d. | n.d. | <LOQ | 26.5 ± 4.9 | 29.0 ± 5.7 | <LOQ | 736.1 | 637.1, 691.0 | |
| 34 | 13.33 | 214, 250, 266, 344 | 244, 262, 294 | Luteolin | n.d. | 43.0 ± 3.6 | n.d. | n.d. | n.d. | n.d. | 548.1 | 475.0, 512.8, 621.1 | |
| 35 | 13.36 | 216, 328 | 266 | Acteoside | n.d. | n.d. | n.d. | <LOQ | 77.3 ± 8.5 | <LOQ | 770.1 | 593.1, 623.0, 667.1, 764.2 | |
| 36 | 13.41 | 214, 274, 334 | 248, 298 | Apigenin | 41.0 ± 0.9 | n.d. | n.d. | n.d. | n.d. | n.d. | 547.6 | 387.0, 426.6, 454.8, 485.0, 530.1 | |
| 37 | 13.58 | 218, 284, 334 | 254, 314 | Apigenin | n.d. | n.d. | 23.3 ± 2.9 | n.d. | n.d. | 11.6 ± 3.2 | 531.1 | 308.8, 353.1, 483.0 | |
| 38 | 13.67 | 220, 328 | 272 | Plantamajoside | n.d. | n.d. | n.d. | <LOQ | 210.3 ± 39.9 | n.d. | 912.0 | 411.1, 455.1, 869.4 | |
| 39 | 14.44 | 214, 274, 332 | 248, 298 | Apigenin | 63.0 ± 3.5 | n.d. | n.d. | n.d. | n.d. | n.d. | 580.0 | 265.6, 355.0, 518.7 | |
| 40 | 16.18 | 220, 266, 346 | 262, 282 | Luteolin | n.d. | n.d. | 10.0 ± 1.0 | n.d. | n.d. | <LOQ | 285.3 | 107.0, 150.8, 174.9, 198.8, 214.7, 242.9 | Luteolin |
| Total Concentration of Phytochemicals in the Extracts, mg/L | 2934.0 ± 103.4 | 1100.0 ± 42.7 | 1572.9 ± 57.9 | 4694.1 ± 211.3 | 4074.8 ± 150.3 | 1268.5 ± 83.1 | |||||||
| Name of the Compound | Rt, min | PMFM, % | PMFK, % | PMHK, % | PLFM, % | PLFJ, % | PLHS, % |
|---|---|---|---|---|---|---|---|
| Isovaleric acid | 7.18 | <1.0 | <1.0 | 3.0 | 1.8 | 2.3 | 1.1 |
| α-Methylbutyric acid | 7.50 | <1.0 | <1.0 | 1.4 | <1.0 | - | <1.0 |
| Pentanoic acid | 8.06 | <1.0 | <1.0 | <1.0 | <1.0 | 1.1 | <1.0 |
| Butyrolactone | 8.76 | 1.0 | <1.0 | 2.4 | <1.0 | <1.0 | 2.1 |
| Hexanoic acid | 10.81 | 1.6 | 4.4 | 2.4 | 2.1 | 3.5 | 5.1 |
| D-Limonene | 12.15 | 6.9 | 6.0 | 2.3 | 10.3 | 11.6 | 4.3 |
| Fenchone | 13.91 | - | - | - | <1.0 | <1.0 | 1.1 |
| Linalool | 14.17 | 1.0 | 2.5 | 2.5 | 2.3 | 2.8 | 2.4 |
| Isomenthone | 15.79 | - | 1.0 | <1.0 | 1.0 | 1.3 | 1.7 |
| Menthol | 16.33 | - | 1.0 | 1.1 | <1.0 | <1.0 | 2.8 |
| α-Terpineol | 16.84 | <1.0 | 1.2 | 1.3 | 1.0 | 1.0 | 1.2 |
| Thymol methyl ether | 17.97 | 1.4 | <1.0 | 1.4 | <1.0 | - | <1.0 |
| Carvone | 18.30 | <1.0 | 4.6 | 4.0 | 4.0 | 4.0 | 4.4 |
| Anethole | 19.42 | 1.2 | 5.1 | 4.0 | 6.0 | 5.2 | 7.4 |
| Thymol | 19.52 | <1.0 | 1.8 | 1.0 | 1.9 | 1.6 | 2.3 |
| β-Caryophyllene | 22.67 | 38.9 | 22.0 | 22.6 | 23.3 | 23.1 | 20.7 |
| cis-α-Bergamotene | 22.77 | 1.0 | - | - | - | - | - |
| trans-α-Bergamotene | 23.30 | 3.3 | 2.4 | 2.2 | 2.4 | 2.4 | 2.2 |
| cis-β-Farnesene | 23.71 | 2.7 | 1.7 | 1.6 | 1.7 | 1.6 | 1.6 |
| α-Caryophyllene | 23.85 | 9.1 | 6.0 | 6.1 | 5.9 | 5.5 | 5.6 |
| Aromandendrene | 24.33 | <1.0 | <1.0 | 1.3 | 1.4 | - | <1.0 |
| β-Ionone | 24.51 | 1.9 | 1.9 | 2.1 | 1.7 | 2.0 | 2.2 |
| β-Selinene | 24.63 | 4.2 | 3.5 | 3.4 | 3.5 | 2.8 | 2.9 |
| α-Selinene | 24.84 | 2.1 | 2.3 | 1.9 | 2.1 | 1.3 | 1.0 |
| β-Bisabolene | 25.03 | 2.3 | 3.5 | 3.3 | 3.1 | 2.5 | 3.0 |
| δ-Cadinene | 25.43 | 1.0 | 1.8 | 1.9 | 1.7 | 1.4 | 1.8 |
| Dihydroactinidiolide | 25.66 | 1.3 | <1.0 | 2.0 | <1.0 | 1.7 | 1.9 |
| Caryophyllene oxide | 26.33 | 1.3 | 1.3 | 3.2 | <1.0 | 1.1 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laanet, P.-R.; Bragina, O.; Jõul, P.; Vaher, M. Plantago major and Plantago lanceolata Exhibit Antioxidant and Borrelia burgdorferi Inhibiting Activities. Int. J. Mol. Sci. 2024, 25, 7112. https://doi.org/10.3390/ijms25137112
Laanet P-R, Bragina O, Jõul P, Vaher M. Plantago major and Plantago lanceolata Exhibit Antioxidant and Borrelia burgdorferi Inhibiting Activities. International Journal of Molecular Sciences. 2024; 25(13):7112. https://doi.org/10.3390/ijms25137112
Chicago/Turabian StyleLaanet, Pille-Riin, Olga Bragina, Piia Jõul, and Merike Vaher. 2024. "Plantago major and Plantago lanceolata Exhibit Antioxidant and Borrelia burgdorferi Inhibiting Activities" International Journal of Molecular Sciences 25, no. 13: 7112. https://doi.org/10.3390/ijms25137112
APA StyleLaanet, P.-R., Bragina, O., Jõul, P., & Vaher, M. (2024). Plantago major and Plantago lanceolata Exhibit Antioxidant and Borrelia burgdorferi Inhibiting Activities. International Journal of Molecular Sciences, 25(13), 7112. https://doi.org/10.3390/ijms25137112







