Effects of Chronic Exposure to Low Doses of Rotenone on Dopaminergic and Cholinergic Neurons in the CNS of Hemigrapsus sanguineus
Abstract
1. Introduction
2. Results
2.1. Effects of Rotenone Exposure on Motor Behavior and Survival
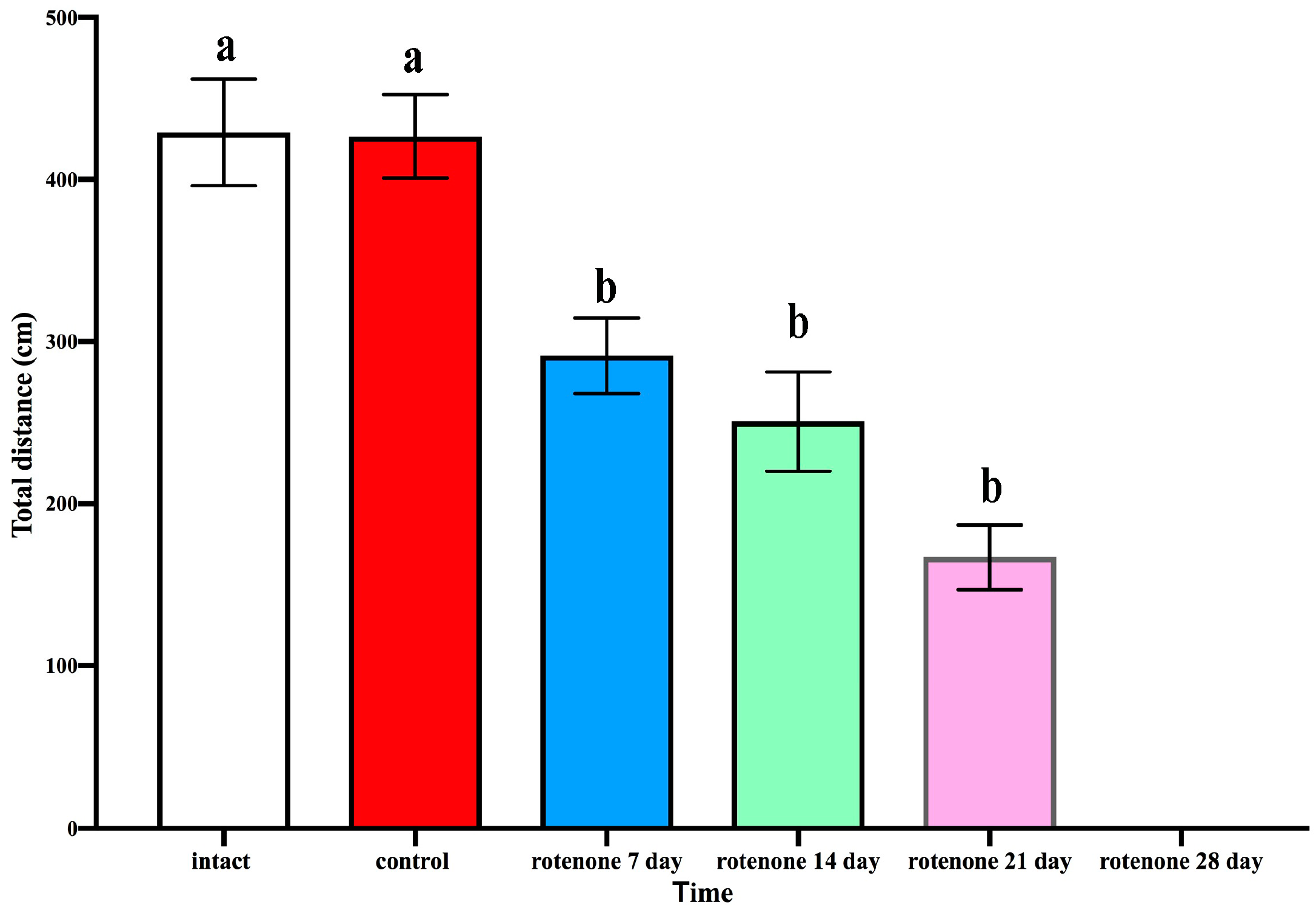
2.1.1. Open Field Test
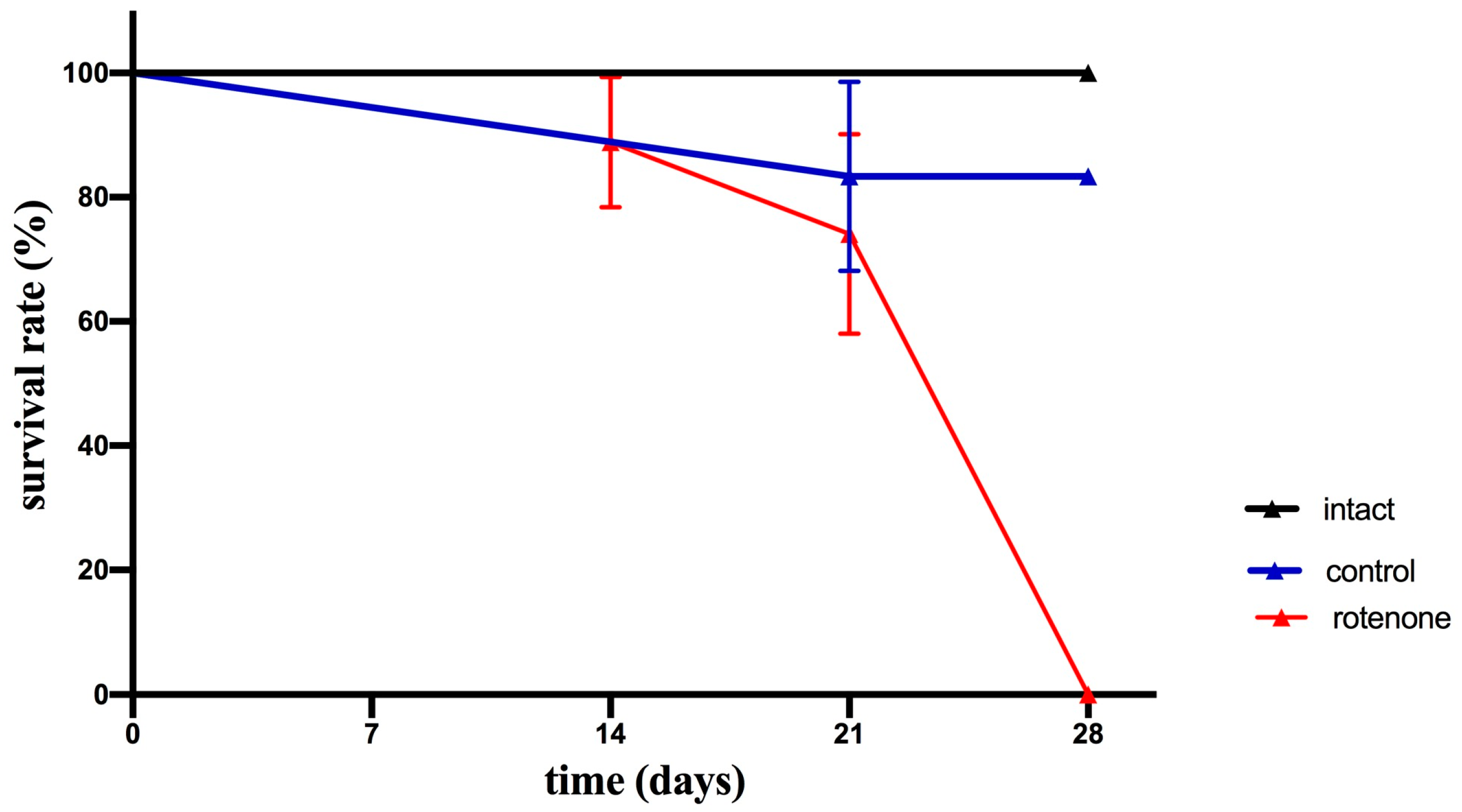
2.1.2. Survival Rate
2.2. Effect of Chronic Exposure to Low Doses of Rotenone on Dopaminergic and Cholinergic Immunoreactivity in the CNS of Hemigrapsus sanguineus
2.2.1. TH-lir after Chronic Exposure to Low Doses of Rotenone
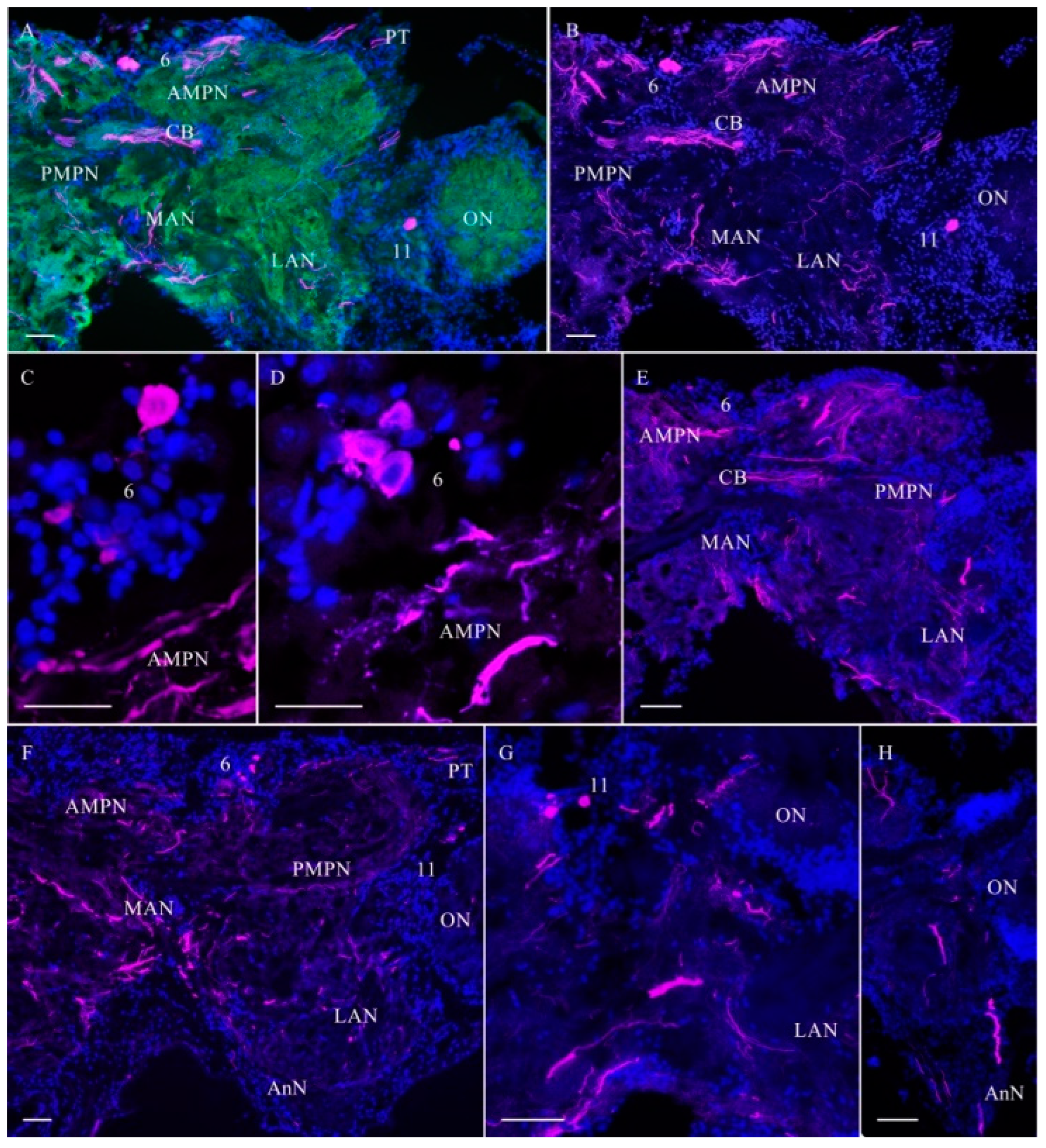
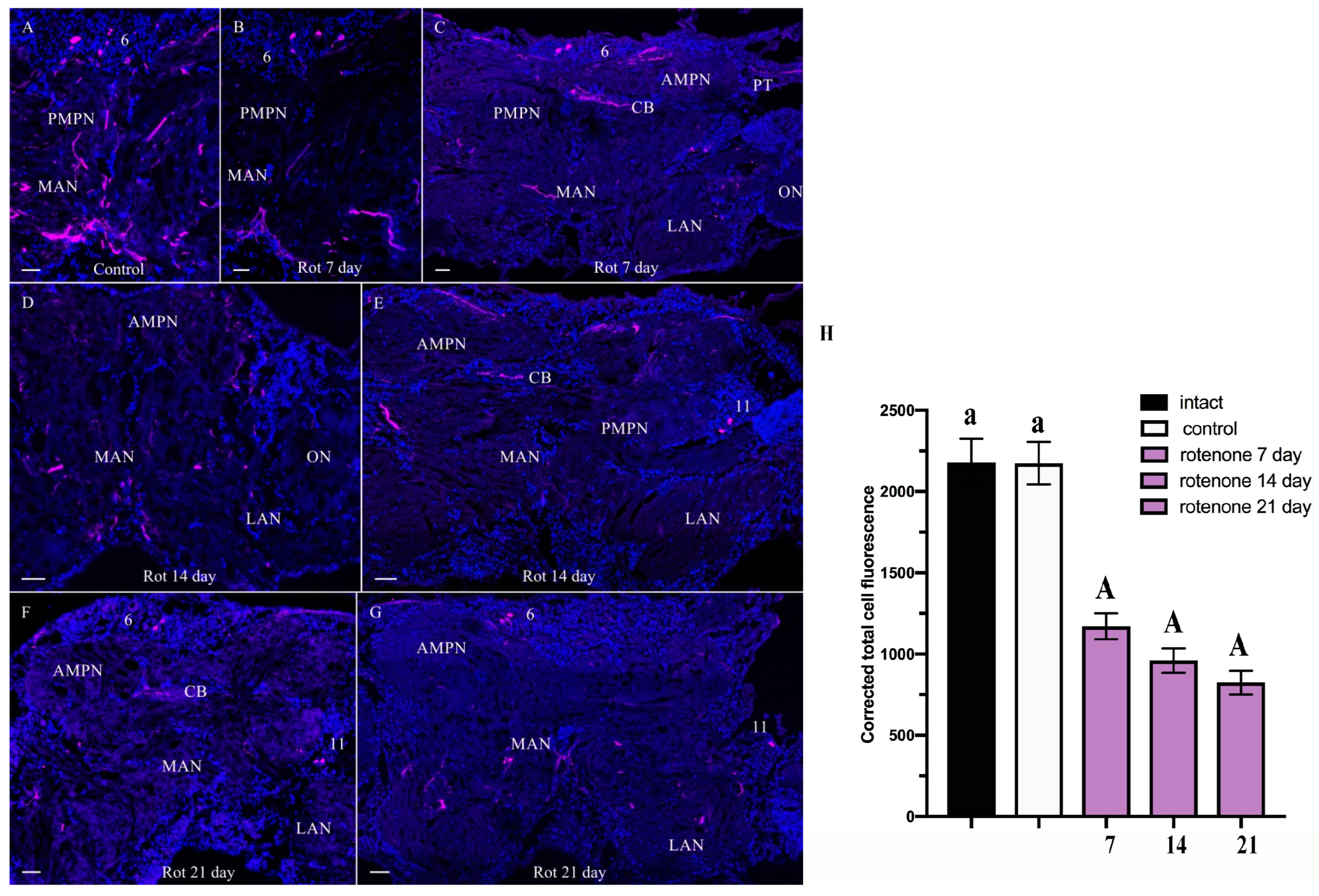
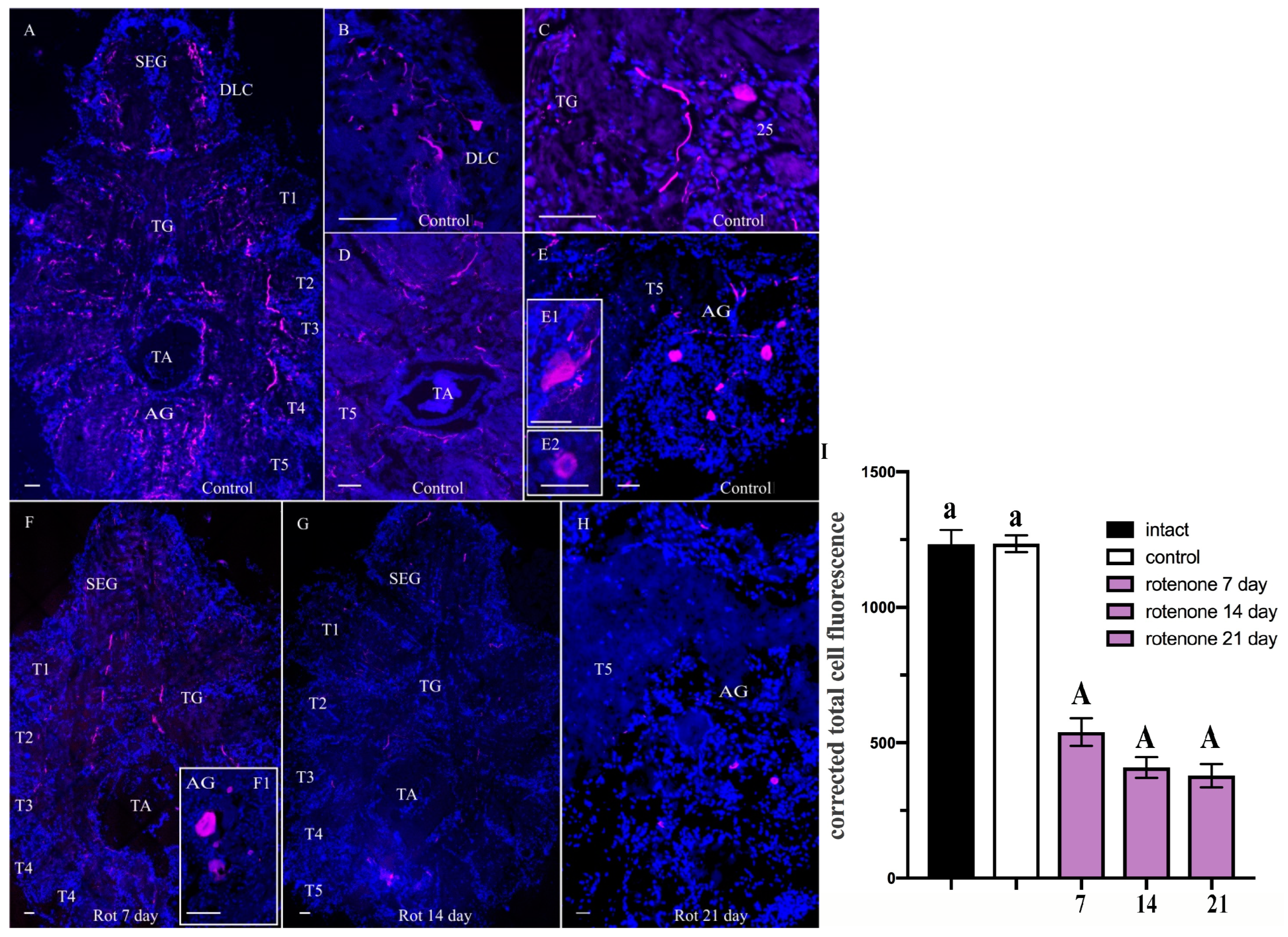
2.2.2. ChAT-lir after Chronic Exposure to Low Doses of Rotenone

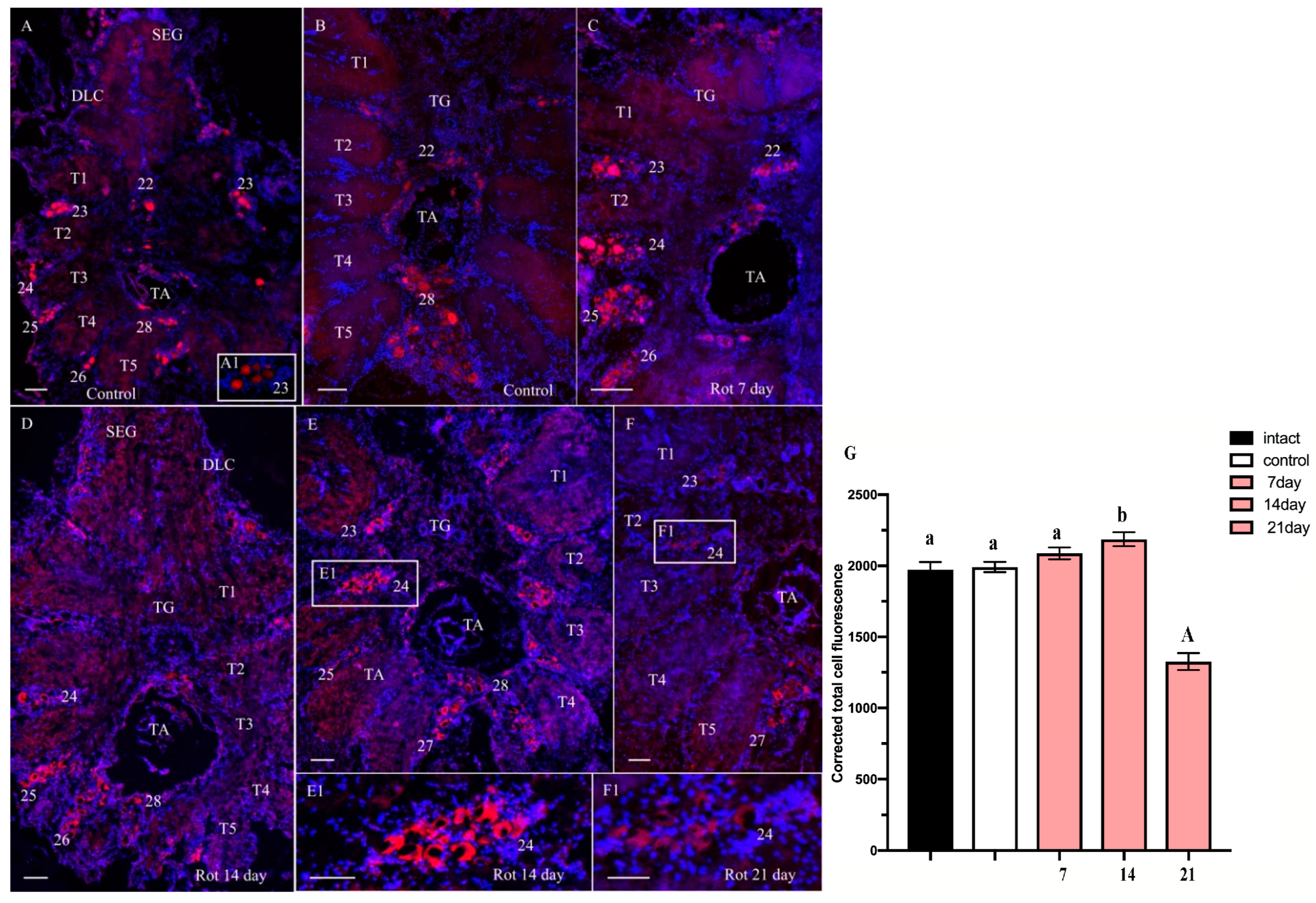
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Behavioral Assessment
Open Field Test
4.3. Antibodies
4.4. Immunocytochemistry
4.5. Microscopy and Imaging
4.6. Quantification and Statistical Analysis
4.7. Neuroanatomical Nomenclature
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ling, N. Rotenone—A review of Its Use for Fisheries Management; Department of Conservation Science Publishing and Service Unit: Wellington, New Zealand, 2002. [Google Scholar]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Coulom, H.; Birman, S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 2004, 24, 10993–10998. [Google Scholar] [CrossRef] [PubMed]
- Testa, C.M.; Sherer, T.B.; Greenamyre, J.T. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res. Mol. Brain Res. 2005, 134, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Subaraja, M.; Vanisree, A.J. Aberrant neurotransmissional mRNAs in cerebral ganglions of rotenone-exposed Lumbricus terrestris exhibiting motor dysfunction and altered cognitive behavior. Environ. Sci. Pollut. Res. Int. 2019, 26, 14461–14472. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular pathophysiology of Parkinson’s disease. Ann. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef]
- Betarbet, R.; Canet-Aviles, R.M.; Sherer, T.B.; Mastroberardino, P.G.; McLendon, C.; Kim, J.H.; Lund, S.; Na, H.M.; Taylor, G.; Bence, N.F.; et al. Intersecting pathways to neurodegeneration in Parkinson’s disease: Effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol. Dis. 2006, 22, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Aosaki, T.; Miura, M.; Suzuki, T.; Nishimura, K.; Masuda, M. Acetylcholine-dopamine balance hypothesis in the striatum: An update. Geriatr. Gerontol. Int. 2010, 10, 148–157. [Google Scholar] [CrossRef]
- Pisani, A.; Bernardi, G.; Ding, J.; Surmeier, D.J. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007, 30, 545–553. [Google Scholar] [CrossRef]
- McKinley, J.W.; Shi, Z.; Kawikova, I.; Hur, M.; Bamford, I.J.; Sudarsana Devi, S.P.; Vahedipour, A.; Darvas, M.; Bamford, N.S. Dopamine Deficiency Reduces Striatal Cholinergic Interneuron Function in Models of Parkinson’s Disease. Neuron 2019, 103, 1056–1072. [Google Scholar] [CrossRef]
- Amalric, M.; Pattij, T.; Sotiropoulos, I.; Silva, J.M.; Sousa, N.; Ztaou, S.; Chiamulera, C.; Wahlberg, L.U.; Emerich, D.F.; Paolone, G. Where Dopaminergic and Cholinergic Systems Interact: A Gateway for Tuning Neurodegenerative Disorders. Front. Behav. Neurosci. 2021, 15, 661973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Cragg, S.J. Revisiting dopamine-acetylcholine imbalance in Parkinson’s disease: Glutamate co-transmission as an exciting partner in crime. Neuron 2021, 109, 1070–1071. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Nielsen, B.E.; Boxer, E.E.; Aoto, J.; Ford, C.P. Loss of nigral excitation of cholinergic interneurons contributes to parkinsonian motor impairments. Neuron 2021, 109, 1137–1149.e5. [Google Scholar] [CrossRef] [PubMed]
- Vehovszky, Á.; Szabó, H.; Acs, A.; Győri, J.; Farkas, A. Effects of rotenone and other mitochondrial complex I inhibitors on the brine shrimp Artemia. Acta Biol. Hung. 2010, 61, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. Emerging therapeutic roles for NAD (+) metabolism in mitochondrial and age-related disorders. Clin. Transl. Med. 2016, 5, 25. [Google Scholar] [CrossRef]
- Fricker, R.A.; Green, E.L.; Jenkins, S.I.; Griffin, S.M. The influence of nicotinamide on health and disease in the central nervous system. Int. J. Tryptophan Res. 2018, 11, 1178646918776658. [Google Scholar] [CrossRef] [PubMed]
- Meulener, M.; Whitworth, A.J.; Armstrong-Gold, C.E.; Rizzu, P.; Heutink, P.; Wes, P.D.; Pallanck, L.J.; Bonini, N.M. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr. Biol. 2005, 15, 1572–1577. [Google Scholar] [CrossRef]
- Penney, E.B.; Mccabe, B.D. Parkinson’s disease: Insights from invertebrates. In Parkinson’s Disease: Molecular and Therapeutic Insights from Model Systems; Nass, R., Przedborski, S., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 323–333. [Google Scholar]
- Correa, C.L.; de Andrade Gomes, C.A.B.; Mello, A.A.; Nogueira, N.D.S.; Medeiros, T.B.; de Barros, C.M.; Allodi, S. Use of invertebrates to model chemically induced parkinsonism-symptoms. Biochem. Soc. Trans. 2023, 51, 435–445. [Google Scholar] [CrossRef]
- Barrière, G.; Mellen, N.; Cazalets, J.R. Neuromodulation of the locomotor network by dopamine in the isolated spinal cord of newborn rat. Eur. J. Neurosci. 2004, 19, 1325–1335. [Google Scholar] [CrossRef]
- Puhl, J.G.; Mesce, K.A. Dopamine Activates the Motor Pattern for Crawling in the Medicinal Leech. J. Neurosci. 2008, 28, 4192–4200. [Google Scholar] [CrossRef]
- Miles, G.B.; Sillar, K.T. Neuromodulation of vertebrate locomotor control networks. Physiology 2011, 26, 393–411. [Google Scholar] [CrossRef]
- Zeng, X.S.; Geng, W.S.; Jia, J.J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 1759091418777438. [Google Scholar] [CrossRef] [PubMed]
- Dalu, T.; Wasserman, R.J.; Jordaan, M.; Froneman, W.P.; Weyl, O.L. An Assessment of the Effect of Rotenone on Selected Non-Target Aquatic Fauna. PLoS ONE 2015, 10, e0142140. [Google Scholar] [CrossRef] [PubMed]
- Ogunsanya, T.; Durborow, R.M.; Webster, C.D.; Tidwell, J.H.; Coyle, S.; Thompson, K.; Huang, L. Toxicity of rotenone to giant river freshwater prawn Macrobrachium rosenbergii. N. Am. J. Aquac. 2011, 73, 159–163. [Google Scholar] [CrossRef]
- Passantino, A.; Elwood, R.W.; Coluccio, P. Why Protect Decapod Crustaceans Used as Models in Biomedical Research and in Ecotoxicology? Ethical and Legislative Considerations. Animals 2021, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Deidda, I.; Russo, R.; Bonaventura, R.; Costa, C.; Zito, F.; Lampiasi, N. Neurotoxicity in Marine Invertebrates: An Update. Biology 2021, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Büschges, A.; Scholz, H.; El Manira, A. New moves in motor control. Curr. Biol. 2011, 21, R513–R524. [Google Scholar] [CrossRef] [PubMed]
- Mulloney, B.; Smarandache-Wellmann, C. Neurobiology of the crustacean swimmeret system. Prog. Neurobiol. 2012, 96, 242–267. [Google Scholar] [CrossRef]
- Cournil, I.; Helluy, S.M.; Beltz, B.S. Dopamine in the lobster Homarus gammarus. I. Comparative analysis of dopamine and tyrosine hydroxylase immunoreactivities in the nervous system of the juvenile. J. Comp. Neurol. 1994, 344, 455–469. [Google Scholar] [CrossRef]
- Tierney, A.J.; Kim, T.; Abrams, R. Dopamine in crayfish and other crustaceans: Distribution in the central nervous system and physiological functions. Microsc. Res. Tech. 2003, 60, 325–335. [Google Scholar] [CrossRef]
- Tinikul, Y.; Poljaroen, J.; Kornthong, N.; Chotwiwatthanakun, C.; Anuracpreeda, P.; Poomtong, T.; Hanna, P.J.; Sobhon, P. Distribution and changes of serotonin and dopamine levels in the central nervous system and ovary of the Pacific white shrimp, Litopenaeus vannamei, during ovarian maturation cycle. Cell Tissue Res. 2011, 345, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Khornchatri, K.; Kornthong, N.; Saetan, J.; Tinikul, Y.; Chotwiwatthanakun, C.; Cummins, S.F.; Hanna, P.J.; Sobhon, P. Distribution of serotonin and dopamine in the central nervous system of the female mud crab, Scylla olivacea (Herbst). Acta Histochem. 2015, 117, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Beltz, B.S. Distribution and functional anatomy of amine-containing neurons in decapod crustaceans. Microsc. Res. Tech. 1999, 44, 105–120. [Google Scholar] [CrossRef]
- Mercier, A.J.; Orchard, I.; Schmoeckel, A. Catecholaminergic neurons sup-plying the hindgut of the crayfish Procambarus clarkii. Can. J. Zool. 1991, 69, 2778–2785. [Google Scholar] [CrossRef]
- Sandeman, D.; Sandeman, R.; Derby, C.; Schmidt, M. Morphology of the brain of crayfish, crabs, and spiny lobsters: A common nomenclature for homologous structures. Biol. Bull. 1992, 183, 304–326. [Google Scholar] [CrossRef]
- Honkanen, A.; Adden, A.; da Silva Freitas, J.; Heinze, S. The insect central complex and the neural basis of navigational strategies. J. Exp. Biol. 2019, 222, jeb188854. [Google Scholar] [CrossRef]
- Utting, M.; Agricola, H.; Sandeman, R.; Sandeman, D. Central complex in the brain of crayfish and its possible homology with that of insects. J. Comp. Neurol. 2000, 416, 245–261. [Google Scholar] [CrossRef]
- Polanska, M.; Yasuda, A.; Harzsch, S. Immunolocalisation of crustacean-SIFamide in the median brain and eyestalk neuropils of the marbled crayfish. Cell Tissue Res. 2007, 330, 331–344. [Google Scholar] [CrossRef]
- Martinez, E.A.; Murray, M.; Leung, M.K.; Stefano, G.B. Evidence for dopaminergic and opiod involvement in the regulation of locomotor activity in the land crab Gecarcinus lateralis. Comp. Biochem. Phys. Part C 1988, 90, 89–93. [Google Scholar] [CrossRef]
- Vidal-Gadea, A.G.; Pierce-Shimomura, J.T. Conserved role of dopamine in the modulation of behavior. Commun. Integr. Biol. 2012, 5, 440–447. [Google Scholar] [CrossRef]
- Schultz, W. Updating dopamine reward signals. Curr. Opin. Neurobiol. 2013, 23, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Barthe, J.Y.; Mons, N.; Cattaert, D.; Geffard, M.; Clarac, F. Dopamine and motor activity in the lobster Homarus gammarus. Brain Res. 1989, 497, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Chang, Q.A.; Tong, C.W.; Leurgans, S.E.; Lipton, J.W.; Carvey, P.M. Rotenone potentiates dopamine neuron loss in animals exposed to lipopolysaccharide prenatally. Exp. Neurol. 2004, 190, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Schmidt, W.J. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav. Brain Res. 2002, 136, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Isooka, N.; Imafuku, F.; Sun, J.; Kikuoka, R.; Furukawa, C.; Asanuma, M. Chronic Systemic Exposure to Low-Dose Rotenone Induced Central and Peripheral Neuropathology and Motor Deficits in Mice: Reproducible Animal Model of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3254. [Google Scholar] [CrossRef] [PubMed]
- Fieber, L.A. Neurotransmitters and Neuropeptides of Invertebrates. In The Oxford Handbook of Invertebrate Neurobiology; Oxford University Press: Oxford, UK, 2017; pp. 284–300. [Google Scholar] [CrossRef]
- Sam, C.; Bordoni, B. Physiology, Acetylcholine; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Cattaert, D.; Araque, A.; Buno, W.; Clarac, F. Nicotinic and muscarinic activation of motoneurons in the crayfish locomotor network. J. Neurophysiol. 1994, 72, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Braun, G.; Mulloney, B. Cholinergic modulation of the swimmeret motor system in crayfish. J. Neurophysiol. 1993, 70, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Braun, G.; Mulloney, B. Coordination in the crayfish swimmeret system: Differential excitation causes changes in intersegmental phase. J. Neurophysiol. 1995, 73, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Mulloney, B. A Test of the Excitability-Gradient Hypothesis in the Swimmeret System of Crayfish. J. Neurosci. 1997, 17, 1860–1868. [Google Scholar] [CrossRef]
- Mulloney, B.; Hall, W.M. Not by spikes alone: Responses of coordinating neurons and the swimmeret system to local differences in excitation. J. Neurophysiol. 2007, 97, 436–450. [Google Scholar] [CrossRef]
- Schneider, A.C.; Seichter, H.A.; Neupert, S.; Hochhaus, A.M.; Smarandache-Wellmann, C.R. Profiling neurotransmitters in a crustacean neural circuit for locomotion. PLoS ONE 2018, 13, e0197781. [Google Scholar] [CrossRef] [PubMed]
- Mineur, Y.S.; Mose, T.N.; Vanopdenbosch, L.; Etherington, I.M.; Ogbejesi, C.; Islam, A.; Pineda, C.M.; Crouse, R.B.; Zhou, W.; Thompson, D.C.; et al. Hippocampal acetylcholine modulates stress-related behaviors independent of specific cholinergic inputs. Mol. Psychiatry 2022, 27, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Hoglinger, G.U.; Feger, J.; Prigent, A.; Michel, P.P.; Parain, K.; Champy, P.; Ruberg, M.; Oertel, W.H.; Hirsch, E.C. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J. Neurochem. 2003, 84, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, C.; Humpel, C. Rotenone induces cell death of cholinergic neurons in an organotypic co-culture brain slice model. Neurochem Res. 2009, 34, 2147–2153. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.D.; Davison, C.A.; Aschner, M.; Bowman, A.B. From the Cover: Manganese and Rotenone-Induced Oxidative Stress Signatures Differ in iPSC-Derived Human Dopamine Neurons. Toxicol. Sci. 2017, 159, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef] [PubMed]
- Greenamyre, J.T.; Betarbet, R.; Sherer, T.B. The rotenone model of Parkinson’s disease: Genes, environment and mitochondria. Park. Relat Disord. 2003, 9, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, R.J.; Schulz, J.B.; Kowall, N.W.; Beal, M.F. Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Res. 1997, 753, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Hong, J.S.; Zhang, W.; Liu, B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J. Neurosci. 2002, 22, 782–790. [Google Scholar] [CrossRef]
- Beal, M.F. Experimental models of Parkinson’s disease. Nat. Rev. Neurosci. 2001, 2, 325–334. [Google Scholar] [CrossRef]
- Klappenbach, M.; Maldonado, H.; Locatelli, F.; Kaczer, L. Opposite actions of dopamine on aversive and appetitive memories in the crab. Learn. Mem. 2012, 19, 73–83. [Google Scholar] [CrossRef]
- Maza, F.J.; Sztarker, J.; Cozzarin, M.E.; Lepore, M.G.; Delorenzi, A. A crabs’ high-order brain center resolved as a mushroom body-like structure. J. Comp. Neurol. 2021, 529, 501–523. [Google Scholar] [CrossRef]
- Wood, D.E.; Derby, C.D. Distribution of dopamine-like immunoreactivity suggests a role for dopamine in the courtship display behavior of the blue crab Callinectes sapidus. Cell Tissue Res. 1996, 285, 321–330. [Google Scholar] [CrossRef]
- Salvaterra, P.M.; Kitamoto, T. Drosophila cholinergic neurons and processes visualized with Gal4/UAS-GFP. Brain Res. Gene Expr. Patterns 2001, 1, 73–82. [Google Scholar] [CrossRef]
- Harzsch, S.; Hansson, B. Brain architecture in the terrestrial hermit crab Coenobita clypeatus (Anomura, Coenobitidae), a crustacean with a good aerial sense of smell. BMC Neurosci. 2008, 9, 58. [Google Scholar] [CrossRef]
- Krieger, J.; Sombke, A.; Seefluth, F.; Kenning, M.; Hansson, B.S.; Harzsch, S. Comparative brain architecture of the European shore crab Carcinus maenas (Brachyura) and the common hermit crab Pagurus bernhardus (Anomura) with notes on other marine hermit crabs. Cell Tissue Res. 2012, 348, 47–69. [Google Scholar] [CrossRef]
- Dyachuk, V.A.; Maiorova, M.A.; Odintsova, N.A. Identification of β integrin-like- and fibronectin-like proteins in the bivalve mollusk Mytilus trossulus. Dev. Growth Differ. 2015, 57, 515–528. [Google Scholar] [CrossRef]
- Yancheva, V.; Georgieva, E.; Velcheva, I.; Iliev, I.; Stoyanova, S.; Vasileva, T.; Bivolarski, V.; Todorova-Bambaldokova, D.; Zulkipli, N.; Antal, L.; et al. Assessment of the exposure of two pesticides on common carp (Cyprinus carpio Linnaeus, 1758): Are the prolonged biomarker responses adaptive or destructive? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 261, 109446. [Google Scholar] [CrossRef]
- Georgieva, E.; Yancheva, V.; Stoyanova, S.; Velcheva, I.; Iliev, I.; Vasileva, T.; Bivolarski, V.; Petkova, E.; László, B.; Nyeste, K.; et al. Which Is More Toxic? Evaluation of the Short-Term Toxic Effects of Chlorpyrifos and Cypermethrin on Selected Biomarkers in Common Carp (Cyprinus carpio, Linnaeus 1758). Toxics 2021, 9, 125. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org (accessed on 25 November 2019).
- Harrington, D.P.; Fleming, T.R. A class of rank test procedures for censored survival data. Biometrika 1982, 69, 553–566. [Google Scholar] [CrossRef]
- Sándor, D.; Gyöngy, M.; Nyeste, K.; Czeglédi, I.; Székely, C.; Buchmann, K.; Cech, G. Digenean Holostephanus (Trematoda: Digenea: Cyathocotylidae) metacercariae in common carp (Cyprinus carpio Linnaeus, 1758) muscle: Zoonotic potential and sensitivity to physico-chemical treatments. J. Helminthol. 2020, 94, e117. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Richter, S.; Loesel, R.; Purschke, G.; Schmidt-Rhaesa, A.; Scholtz, G.; Stach, T.; Vogt, L.; Wanninger, A.; Brenneis, G.; Doring, C.; et al. Invertebrate neurophylogeny: Suggested terms and definitions for a neuroanatomical glossary. Front. Zool. 2010, 7, 29. [Google Scholar] [CrossRef]
- Sosa, M.A.; Spitzer, N.; Edwads, D.H.; Baro, D.J. A crustacean serotonin receptor: Cloning and distribution in the thoracic ganglia of crayfish and freshwater prawn. J. Comp. Neurol. 2004, 47, 526–537. [Google Scholar] [CrossRef]
- Stewart, M.J.; Stewart, P.; Sroyraya, M.; Soonklang, N.; Cummins, S.F.; Hanna, P.J.; Duan, W.; Sobhon, P. Cloning of the crustacean hyperglycemic hormone and evidence for molt-inhibiting hormone within the central nervous system of the blue crab Portunus pelagicus. Comp. Biochem. Physiol. A 2013, 164, 276–290. [Google Scholar] [CrossRef]
- Saetan, J.; Senarai, T.; Tamtin, M.; Weerachatyanukul, W.; Chavadej, J.; Hanna, P.J.; Parhar, I.; Sobhon, P.; Sretarugsa, P. Histological organization of the central nervous system and distribution of a gonadotropin-releasing hormone-like peptide in the blue crab, Portunus pelagicus. Cell Tissue Res. 2013, 353, 493–510. [Google Scholar] [CrossRef]
| Group | N | Observed | Expected | (0 − E)2/E | (0 − E)2/V |
|---|---|---|---|---|---|
| Control | 23 | 1 | 1.950 | 0.463 | 0.798 |
| Intact | 24 | 0 | 2.199 | 2.199 | 4.130 |
| Rotenone | 11 | 4 | 0.851 | 11.655 | 14.744 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsyuba, E.; Dyachuk, V. Effects of Chronic Exposure to Low Doses of Rotenone on Dopaminergic and Cholinergic Neurons in the CNS of Hemigrapsus sanguineus. Int. J. Mol. Sci. 2024, 25, 7159. https://doi.org/10.3390/ijms25137159
Kotsyuba E, Dyachuk V. Effects of Chronic Exposure to Low Doses of Rotenone on Dopaminergic and Cholinergic Neurons in the CNS of Hemigrapsus sanguineus. International Journal of Molecular Sciences. 2024; 25(13):7159. https://doi.org/10.3390/ijms25137159
Chicago/Turabian StyleKotsyuba, Elena, and Vyacheslav Dyachuk. 2024. "Effects of Chronic Exposure to Low Doses of Rotenone on Dopaminergic and Cholinergic Neurons in the CNS of Hemigrapsus sanguineus" International Journal of Molecular Sciences 25, no. 13: 7159. https://doi.org/10.3390/ijms25137159
APA StyleKotsyuba, E., & Dyachuk, V. (2024). Effects of Chronic Exposure to Low Doses of Rotenone on Dopaminergic and Cholinergic Neurons in the CNS of Hemigrapsus sanguineus. International Journal of Molecular Sciences, 25(13), 7159. https://doi.org/10.3390/ijms25137159





