Narrative Review of the Current and Future Perspectives of Phycobiliproteins’ Applications in the Food Industry: From Natural Colors to Alternative Proteins
Abstract
1. Introduction
2. Methods
3. Phycobiliproteins as Food Colors
4. Phycobiliproteins as Alternative Proteins and Food Fortifiers
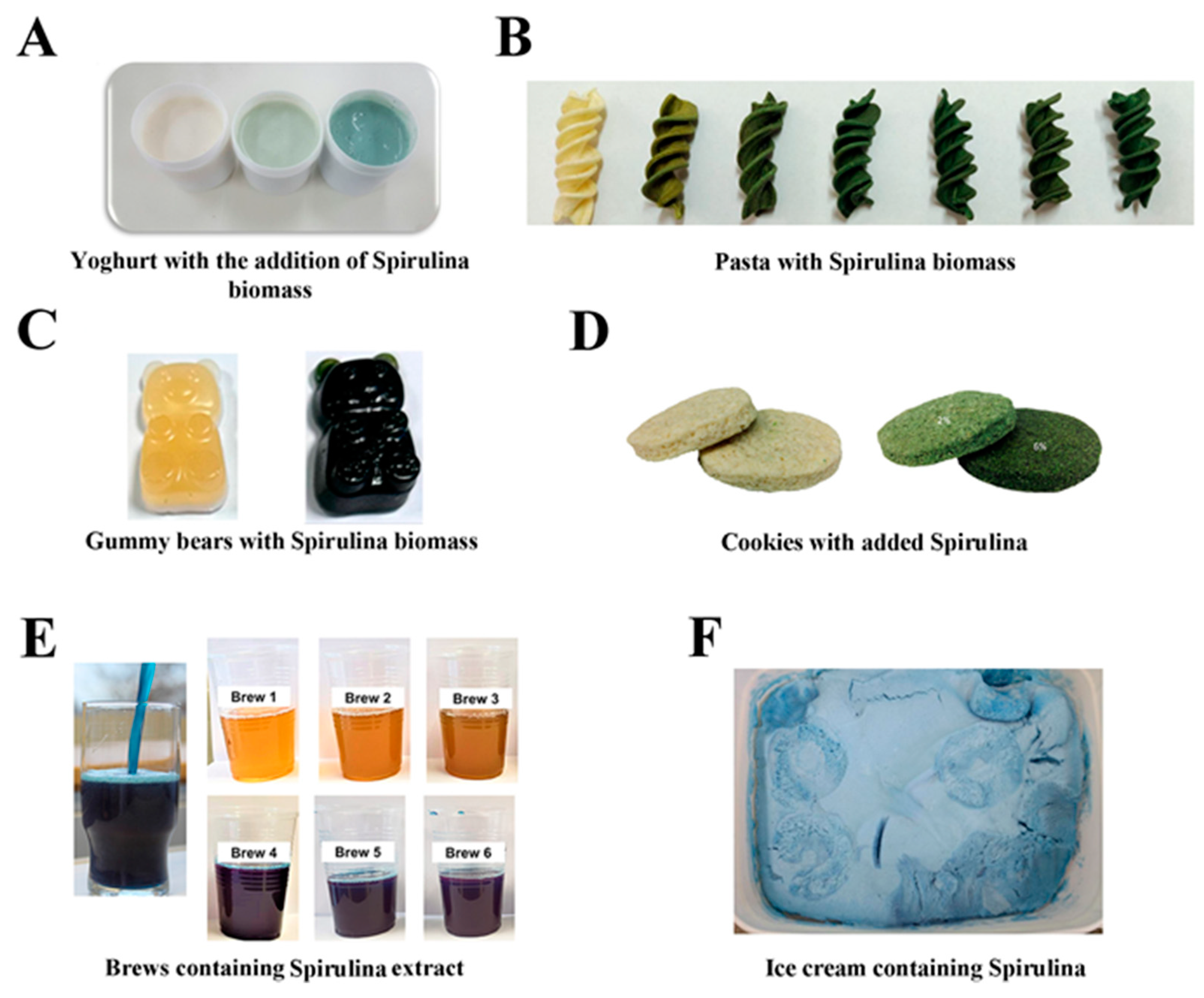
4.1. Phycobiliprotein-Based Food Products
4.2. Fortification of the Commonly Used Foods with Phycobiliproteins
| Protein Sample | Concentration (w/w) | Food Type | Major Food Product Characteristics | Reference |
|---|---|---|---|---|
| Phycoerythrin-rich water extract (Porphyridium cruentum) | 0.00015–0.00029% | Commercial beverages (e.g., gin and wine) | The pink color was stable during 11 days of storage; well accepted by a semi-trained panelist | [26] |
| C-PC from Spirulina (Arthrospira platensis) | 0.025% | Ice cream | Stable color during the 6 months; increased antioxidant activity after in vitro simulated digestion | [108] |
| PBPs from cyanobacteria Nostoc sp. | 0.03–0.14% | Skim milk | Satisfactory sensory characteristics | [118] |
| Encapsulated R-phycoerythrin (Kappaphycus alvarezii) | 0.1% | Ice cream | Better rheology; pink color intensity increased during 90 days of storage; enhanced antioxidant activity | [119] |
| C-PC from Spirulina (Arthrospira platensis) | 0.1–0.2% | Ice cream | Smoother and softer texture; sugar (25%) and fat (50%) content reduction; no significant influence on consumer acceptance | [120] |
| Porphyridium cruentum spray-dried biomass | 0.1–0.3% | Ice cream | Increased consistency index | [121] |
| C-Phycocyanin extract | 0.18–0.32% | Soft beverage | Improved product’s antioxidant activity; good sensorial attributes | [122] |
| Spirulina (Arthrospira platensis) biomass | 0.25% | Craft beer | Increased total polyphenols, tannins, and antioxidant power; cytoprotective properties towards the oxidative damage | [123] |
| Spirulina (Arthrospira platensis) powder | 0.25–1% | Yogurt | Better water holding capacity and lower whey syneresis (28 days of storage); improved antioxidant activity; lower firmness but better elasticity; acceleration of the end of fermentation; acceptable sensory characteristics only at 0.25% | [124] |
| C-Phycocyanin | 0.3% | Biscuit | High oxidative stability during 30 days of storage; satisfactory all main sensory characteristics (e.g., odor, flavor, texture, and overall acceptability) | [125] |
| C-PC from Spirulina (Arthrospira platensis) | 0.3–0.4% | Ice cream | Emulsifying and stabilizing activity; lower consumer acceptance | [126] |
| Spirulina (Arthrospira platensis) powder | 0.4–1.2% | Bread | Higher moisture content; lower hardness; highest consumer acceptance with 0.8%; higher antioxidant activity | [127] |
| Spirulina (Arthrospira platensis) microencapsulated in alginate | 0.5% | Yogurt | Improved viscosity stability during storage; better consumer acceptance | [103] |
| Spirulina (Arthrospira platensis) powder | 0.5–1.5% | Feta-type cheese | A higher number of lactic acid bacteria after 60 days of storage; softer texture and sensory characteristics (at 0.5 and 1%) | [128] |
| C-PC from Spirulina (Arthrospira platensis) | 0.5–2% | Cow’s milk | Increased solid non-fat content; enhanced antioxidant activity; improved sensory characteristics | [129] |
| Spirulina maxima biomass | 0.5–2% | Pasta | The color was relatively stable after cooking, with increased firmness. Higher consumer acceptance scores | [130] |
| Spirulina (Arthrospira platensis) powder | 0.5–3% | Processed cheese | Decrease in adhesiveness, cohesiveness, springiness, chewiness; increase in hardness and gumminess; deterioration in the overall sensory acceptability | [131] |
| Spirulina (Arthrospira platensis) powder | 0.63–2.5% | Fresh noodles | Increased antioxidant capacity; increased hardness, cohesiveness, springiness, gumminess, and chewiness; the highest consumer acceptance with 1.25% | [132] |
| Spirulina (Arthrospira platensis) powder | 1% | Yogurt | Higher antioxidant activity and number of lactic acid bacteria; higher water holding capacity and viscosity; lower syneresis; decreased consumer acceptance | [133] |
| Spirulina (Arthrospira platensis) biomass and wheat germ | 1% (both) | Pear–cantaloupe-based beverage | Increased antioxidant capacity, total phenol, and flavonoid content; good organoleptic score | [134] |
| Spirulina (Arthrospira platensis) powder | 1.5–3.5% | Yogurt spread | Increased viscosity and spreadability; lower consumer acceptance with a higher Spirulina concentration | [135] |
| Spirulina (Arthrospira platensis) powder | 1.6% | Baguette bread | Decreased hardness and gumminess; lower sensory score | [136] |
| Spirulina (Arthrospira platensis) powder | 1–15% | Gluten-free fresh pasta | Higher antioxidant activity, without affecting product cooking and texture quality properties; a favorable sensory evaluation | [137] |
| Spirulina (Arthrospira platensis) biomass | 1–2% | Cookie | Harder and darker product with increased protein content; questionable sensory quality | [138] |
| Spirulina (Arthrospira platensis) extract | 1–5% | Chinese-style pork-sausage | Small changes in pH; inhibition of lipid oxidation; 2.5 and 5% retarded the decrease in sensory acceptability (storage at 4 °C) | [139] |
| Spirulina (Arthrospira platensis) biomass | 2 or 6% | Cookie | Color and texture stability over 8 weeks; higher protein and total phenolic content and in vitro antioxidant capacity; without in vitro digestibility changes | [106] |
| Spirulina (Arthrospira platensis) biomass | 2% | 3D-printed cookie dough | All formulations were suitable for extrusion and microbiologically stable; stable texture after 30 days of storage; improved antioxidant properties and color stability after extract encapsulation in alginate microbeads | [140] |
| Spirulina (Arthrospira platensis) powder | 2.5–10% | Pasta | Increased rheological parameters, color, and cooking quality; decreased dough stability; sensory acceptable up to 5% | [141] |
| Nano-liposomes containing PBPs from Gracilaria gracilis | 2.5–5% | Carp burger | Lower oxidative spoilage and microbial deterioration; no significant loss of overall consumer acceptability (18 days of refrigerated storage) | [142] |
| Spirulina (Arthrospira platensis F&M-C256) biomass | 2–10% | Sourdough bread | Higher antioxidant activity; highest consumer acceptance with 2% | [143] |
| Spirulina (Arthrospira platensis) biomass | 2–15% | Pasta | Lower firmness, cut force, and consistency; higher stickiness; highest consumer acceptance for 12.5% | [104] |
| C-PC from Spirulina (Arthrospira platensis) | 2–8% | Yogurt | Decreased syneresis; increased firmness and viscosity; higher pH and color stability; no pathogen growth during 21 days of storage; overall acceptability not affected at 4% | [144] |
| Microencapsulated Spirulina (Arthrospira platensis) in alginate | 3% | Pasta | Protection of antioxidant potential; higher firmness; acceptable sensory characteristics | [117] |
| Spirulina (Arthrospira platensis) powder | 4–6.5% | Dry noodles | Lower cooking loss; higher elongation and tensile strength; highest consumer acceptance with 6% | [145] |
| Spirulina (Arthrospira platensis) powder | 5% | Beer | Slightly altered fermentation parameters; typical beer-like product character; odor and taste alteration compromising the consumer acceptance | [107] |
| Microencapsulated Spirulina sp. LEB-18 in maltodextrin and soy lecithin | 5–8.75% | Chocolate milk | Increased antioxidant activity; good suspension stability and low hygroscopicity; questionable consumer acceptance. | [146] |
4.3. Challenges for Proper Phycobiliproteins Utilization as Alternative Proteins
4.3.1. Cultivation of Cyanobacteria/Algae as Sources of Phycobiliproteins
4.3.2. Isolation and Purification of Phycobiliproteins from Cyanobacterial/Algal Biomass
4.3.3. Economic Aspects of Phycobiliproteins Production
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Dagnino-Leone, J.; Figueroa, C.P.; Castañeda, M.L.; Youlton, A.D.; Vallejos-Almirall, A.; Agurto-Muñoz, A.; Pavón Pérez, J.; Agurto-Muñoz, C. Phycobiliproteins: Structural aspects, functional characteristics, and biotechnological perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 1506–1527. [Google Scholar] [CrossRef]
- Stadnichuk, I.N.; Tropin, I.V. Phycobiliproteins: Structure, functions and biotechnological applications. Appl. Biochem. Microbiol. 2017, 53, 1–10. [Google Scholar] [CrossRef]
- Brejc, K.; Ficner, R.; Huber, R.; Steinbacher, S. Isolation, crystallization, crystal structure analysis and refinement of allophycocyanin from the cyanobacterium Spirulina platensis at 2.3 Å resolution. J. Mol. Biol. 1995, 249, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Glazer, A.N. Light guides. J. Biol. Chem. 1989, 264, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Scheer, H.; Zhao, K.-H. Biliprotein maturation: The chromophore attachment. Mol. Microbiol. 2008, 68, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Adir, N.; Dobrovetsky, Y.; Lerner, N. Structure of c-phycocyanin from the thermophilic cyanobacterium Synechococcus vulcanus at 2.5 Å: Structural implications for thermal stability in phycobilisome assembly 1 1Edited by R. Huber. J. Mol. Biol. 2001, 313, 71–81. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Kumar, D.; Pathak, J.; Sinha, R.P. Phycobiliproteins and Their Commercial Significance. In Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2019; pp. 207–216. [Google Scholar]
- Ma, J.; Hu, J.; Sha, X.; Meng, D.; Yang, R. Phycobiliproteins, the pigment-protein complex form of natural food colorants and bioactive ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 2999–3017. [Google Scholar] [CrossRef] [PubMed]
- Kovaleski, G.; Kholany, M.; Dias, L.M.S.; Correia, S.F.H.; Ferreira, R.A.S.; Coutinho, J.A.P.; Ventura, S.P.M. Extraction and purification of phycobiliproteins from algae and their applications. Front. Chem. 2022, 10, 1065355. [Google Scholar] [CrossRef]
- Li, W.; Su, H.-N.; Pu, Y.; Chen, J.; Liu, L.-N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Tounsi, L.; Ben Hlima, H.; Hentati, F.; Hentati, O.; Derbel, H.; Michaud, P.; Abdelkafi, S. Microalgae: A Promising Source of Bioactive Phycobiliproteins. Mar. Drugs 2023, 21, 440. [Google Scholar] [CrossRef]
- Puzorjov, A.; McCormick, A.J. Phycobiliproteins from extreme environments and their potential applications. J. Exp. Bot. 2020, 71, 3827–3842. [Google Scholar] [CrossRef] [PubMed]
- Minic, S.L.; Stanic-Vucinic, D.; Mihailovic, J.; Krstic, M.; Nikolic, M.R.; Cirkovic Velickovic, T. Digestion by pepsin releases biologically active chromopeptides from C-phycocyanin, a blue-colored biliprotein of microalga Spirulina. J. Proteomics 2016, 147, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-Z.; Li, W.-J.; Zhang, J.-J.; Liu, Z.-Y.; Li, Y.; Liu, C.; Qin, S. The Inhibitory Effect of Phycocyanin Peptide on Pulmonary Fibrosis In Vitro. Mar. Drugs 2022, 20, 696. [Google Scholar] [CrossRef]
- Fernandes, R.; Campos, J.; Serra, M.; Fidalgo, J.; Almeida, H.; Casas, A.; Toubarro, D.; Barros, A.I.R.N.A. Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals 2023, 16, 592. [Google Scholar] [CrossRef] [PubMed]
- Rawiwan, P.; Peng, Y.; Paramayuda, I.G.P.B.; Quek, S.Y. Red seaweed: A promising alternative protein source for global food sustainability. Trends Food Sci. Technol. 2022, 123, 37–56. [Google Scholar] [CrossRef]
- Taufikurahman, T.; Ilhamsyah, D.P.A.; Rosanti, S.; Ardiansyah, M.A. Preliminary Design of Phycocyanin Production from Spirulina platensis Using Anaerobically Digested Dairy Manure Wastewater. IOP Conf. Ser. Earth Environ. Sci. 2020, 520, 012007. [Google Scholar] [CrossRef]
- FAO Global Seaweeds and Microalgae Production, 1950–2019. Available online: https://www.fao.org/3/cb4579en/cb4579en.pdf (accessed on 12 January 2024).
- Zhang, L.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global seaweed farming and processing in the past 20 years. Food Prod. Process. Nutr. 2022, 4, 23. [Google Scholar] [CrossRef]
- Li, P.; Sheng, L.; Ye, Y.; Wang, J.; Geng, S.; Ning, D.; Sun, X. Allergenicity of alternative proteins: Research hotspots, new findings, evaluation strategies, regulatory status, and future trends: A bibliometric analysis. Crit. Rev. Food Sci. Nutr. 2024, 1–12. [Google Scholar] [CrossRef]
- Petrus, M.; Culerrier, R.; Campistron, M.; Barre, A.; Rougé, P. First case report of anaphylaxis to spirulin: Identification of phycocyanin as responsible allergen. Allergy 2010, 65, 924–925. [Google Scholar] [CrossRef]
- Le, T.-M.; Knulst, A.C.; Röckmann, H. Anaphylaxis to Spirulina confirmed by skin prick test with ingredients of Spirulina tablets. Food Chem. Toxicol. 2014, 74, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Nemoto-Kawamura, C.; Hirahashi, T.; Nagai, T.; Yamada, H.; Katoh, T.; Hayashi, O. Phycocyanin Enhances Secretary IgA Antibody Response and Suppresses Allergic IgE Antibody Response in Mice Immunized with Antigen-Entrapped Biodegradable Microparticles. J. Nutr. Sci. Vitaminol. 2004, 50, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Cao, M.; Pan, T.; Yang, Y.; Mao, H.; Sun, L.; Liu, G. Anti-allergic activity of R-phycocyanin from Porphyra haitanensis in antigen-sensitized mice and mast cells. Int. Immunopharmacol. 2015, 25, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Carmona, R.; Murillo, M.C.; Lafarga, T.; Bermejo, R. Assessment of the potential of microalgae-derived phycoerythrin as a natural colorant in beverages. J. Appl. Phycol. 2022, 34, 3025–3034. [Google Scholar] [CrossRef]
- Lins, M.; Zandonadi, R.P.; Raposo, A.; Ginani, V.C. Food waste on foodservice: An overview through the perspective of sustainable dimensions. Foods 2021, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Landim Neves, M.I.; Silva, E.K.; Meireles, M.A.A. Natural blue food colorants: Consumer acceptance, current alternatives, trends, challenges, and future strategies. Trends Food Sci. Technol. 2021, 112, 163–173. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Spence, C. Background colour & its impact on food perception & behaviour. Food Qual. Prefer. 2018, 68, 156–166. [Google Scholar] [CrossRef]
- Santiago-Santos, M.C.; Ponce-Noyola, T.; Olvera-Ramírez, R.; Ortega-López, J.; Cañizares-Villanueva, R.O. Extraction and purification of phycocyanin from Calothrix sp. Process Biochem. 2004, 39, 2047–2052. [Google Scholar] [CrossRef]
- FDA Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices. Available online: https://www.fda.gov/industry/color-additive-inventories/summary-color-additives-use-united-states-foods-drugs-cosmetics-and-medical-devices#table1A (accessed on 12 November 2023).
- Adjali, A.; Clarot, I.; Chen, Z.; Marchioni, E.; Boudier, A. Physicochemical degradation of phycocyanin and means to improve its stability: A short review. J. Pharm. Anal. 2022, 12, 406–414. [Google Scholar] [CrossRef]
- Patil, G.; Raghavarao, K.S.M.S. Aqueous two phase extraction for purification of C-phycocyanin. Biochem. Eng. J. 2007, 34, 156–164. [Google Scholar] [CrossRef]
- García, A.B.; Longo, E.; Bermejo, R. The application of a phycocyanin extract obtained from Arthrospira platensis as a blue natural colorant in beverages. J. Appl. Phycol. 2021, 33, 3059–3070. [Google Scholar] [CrossRef]
- Jespersen, L.; Strømdahl, L.D.; Olsen, K.; Skibsted, L.H. Heat and light stability of three natural blue colorants for use in confectionery and beverages. Eur. Food Res. Technol. 2005, 220, 261–266. [Google Scholar] [CrossRef]
- Liu, L.-N.; Su, H.-N.; Yan, S.-G.; Shao, S.-M.; Xie, B.-B.; Chen, X.-L.; Zhang, X.-Y.; Zhou, B.-C.; Zhang, Y.-Z. Probing the pH sensitivity of R-phycoerythrin: Investigations of active conformational and functional variation. Biochim. Biophys. Acta-Bioenerg. 2009, 1787, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Munier, M.; Jubeau, S.; Wijaya, A.; Morançais, M.; Dumay, J.; Marchal, L.; Jaouen, P.; Fleurence, J. Physicochemical factors affecting the stability of two pigments: R-phycoerythrin of Grateloupia turuturu and B-phycoerythrin of Porphyridium cruentum. Food Chem. 2014, 150, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Simovic, A.; Combet, S.; Cirkovic Velickovic, T.; Nikolic, M.; Minic, S. Probing the stability of the food colourant R-phycoerythrin from dried Nori flakes. Food Chem. 2022, 374, 131780. [Google Scholar] [CrossRef] [PubMed]
- Veličković, L.; Simović, A.; Gligorijević, N.; Thureau, A.; Obradović, M.; Vasović, T.; Sotiroudis, G.; Zoumpanioti, M.; Brûlet, A.; Ćirković Veličković, T.; et al. Exploring and strengthening the potential of R-phycocyanin from Nori flakes as a food colourant. Food Chem. 2023, 426, 136669. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.R.; Hauer, C.; Stack, R.F.; Eisele, L.E.; MacColl, R. Thermophilic C-phycocyanin: Effect of temperature, monomer stability, and structure. Biochim. Biophys. Acta-Bioenerg. 1997, 1321, 157–164. [Google Scholar] [CrossRef]
- Ferraro, G.; Imbimbo, P.; Marseglia, A.; Illiano, A.; Fontanarosa, C.; Amoresano, A.; Olivieri, G.; Pollio, A.; Monti, D.M.; Merlino, A. A thermophilic C-phycocyanin with unprecedented biophysical and biochemical properties. Int. J. Biol. Macromol. 2020, 150, 38–51. [Google Scholar] [CrossRef]
- Faieta, M.; Neri, L.; Sacchetti, G.; Di Michele, A.; Pittia, P. Role of saccharides on thermal stability of phycocyanin in aqueous solutions. Food Res. Int. 2020, 132, 109093. [Google Scholar] [CrossRef]
- Nowruzi, B.; Konur, O.; Anvar, S.A.A. The Stability of the Phycobiliproteins in the Adverse Environmental Conditions Relevant to the Food Storage. Food Bioprocess Technol. 2022, 15, 2646–2663. [Google Scholar] [CrossRef]
- Yuan, B.; Li, Z.; Shan, H.; Dashnyam, B.; Xu, X.; McClements, D.J.; Zhang, B.; Tan, M.; Wang, Z.; Cao, C. A review of recent strategies to improve the physical stability of phycocyanin. Curr. Res. Food Sci. 2022, 5, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; Granchi, L.; Tredici, M.R. Vegetable oils protect phycocyanin from thermal degradation during cooking of spirulina-based “crostini”. LWT 2021, 138, 110776. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Antelo, F.S.; Costa, J.A.V.; Kalil, S.J. Thermal degradation kinetics of the phycocyanin from Spirulina platensis. Biochem. Eng. J. 2008, 41, 43–47. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Figueira, F.D.S.; Silveira, J.T.D.; Morais, M.G.D.; Costa, J.A.V.; Kalil, S.J. Improvement of Thermal Stability of C-Phycocyanin by Nanofiber and Preservative Agents. J. Food Process. Preserv. 2016, 40, 1264–1269. [Google Scholar] [CrossRef]
- Martelli, G.; Folli, C.; Visai, L.; Daglia, M.; Ferrari, D. Thermal stability improvement of blue colorant C-Phycocyanin from Spirulina platensis for food industry applications. Process Biochem. 2014, 49, 154–159. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Abbaspourrad, A. Improvement of the colloidal stability of phycocyanin in acidified conditions using whey protein-phycocyanin interactions. Food Hydrocoll. 2020, 105, 105747. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bhayani, K.; Ghosh, T.; Bajaj, S.; Trivedi, N.; Mishra, S. Stability of Phycobiliproteins Using Natural Preservative ε- Polylysine (ε-PL). Ferment. Technol. 2018, 7, 1000149. [Google Scholar] [CrossRef]
- Lee, C.-W.; Bae, G.Y.; Bae, S.-H.; Suh, H.J.; Jo, K. Increased thermal stability of phycocyanin extracted from Spirulina platensis by cysteine addition during enzyme extraction. Food Sci. Technol. 2022, 42, e15021. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Hu, J.; Wang, Z.; Meng, D.; Li, H.; Zhou, Z.; Yang, R. The structural characterization and color stabilization of the pigment protein-phycoerythrin glycosylated with oligochitosan. Food Hydrocoll. 2023, 136, 108241. [Google Scholar] [CrossRef]
- Buecker, S.; Grossmann, L.; Loeffler, M.; Leeb, E.; Weiss, J. Influence of storage temperature on the stability of heat treated phycocyanin-λ-carrageenan complexes in liquid formulations. Green Chem. 2022, 24, 4174–4185. [Google Scholar] [CrossRef]
- Buecker, S.; Grossmann, L.; Loeffler, M.; Leeb, E.; Weiss, J. Thermal and acidic denaturation of phycocyanin from Arthrospira platensis: Effects of complexation with λ-carrageenan on blue color stability. Food Chem. 2022, 380, 132157. [Google Scholar] [CrossRef] [PubMed]
- Gligorijević, N.; Jovanović, Z.; Cvijetić, I.; Šunderić, M.; Veličković, L.; Katrlík, J.; Holazová, A.; Nikolić, M.; Minić, S. Investigation of the Potential of Selected Food-Derived Antioxidants to Bind and Stabilise the Bioactive Blue Protein C-Phycocyanin from Cyanobacteria Spirulina. Int. J. Mol. Sci. 2023, 25, 229. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.; Ali, S.A.; Hifney, A.F. Enhancement of phycocyanin productivity and thermostability from Arthrospira platensis using organic acids. Microb. Cell Fact. 2023, 22, 248. [Google Scholar] [CrossRef]
- Huo, Y.; Hou, X.; Yu, Y.; Wen, X.; Ding, Y.; Li, Y.; Wang, Z. Improving the Thermal and Oxidative Stability of Food-Grade Phycocyanin from Arthrospira platensis by Addition of Saccharides and Sugar Alcohols. Foods 2022, 11, 1752. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Abbaspourrad, A. Improved thermal stability of phycocyanin under acidic conditions by forming soluble complexes with polysaccharides. Food Hydrocoll. 2021, 119, 106852. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, L.; Wang, Q.; Zhang, Y.; Sun, Y.; Zhang, H.; Wang, Z.; Zhou, Z.; Yang, R. Self-Assembly of Phycoerythrin with Oligochitosan by Electrostatic Interaction for Stabilization of Phycoerythrin. J. Agric. Food Chem. 2021, 69, 12818–12827. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Nath, P.C.; Vanitha, K.; Tiwari, O.N.; Bandyopadhyay, T.K.; Bhunia, B. Effects of different monosaccharides on thermal stability of phycobiliproteins from Oscillatoria sp. (BTA-170): Analysis of kinetics, thermodynamics, colour and antioxidant properties. Food Biosci. 2021, 44, 101354. [Google Scholar] [CrossRef]
- Wang, F.; Yu, X.; Cui, Y.; Xu, L.; Huo, S.; Ding, Z.; Hu, Q.; Xie, W.; Xiao, H.; Zhang, D. Efficient extraction of phycobiliproteins from dry biomass of Spirulina platensis using sodium chloride as extraction enhancer. Food Chem. 2023, 406, 135005. [Google Scholar] [CrossRef]
- Yang, R.; Ma, T.; Shi, L.; Wang, Q.; Zhang, L.; Zhang, F.; Wang, Z.; Zhou, Z. The formation of phycocyanin-EGCG complex for improving the color protection stability exposing to light. Food Chem. 2022, 370, 130985. [Google Scholar] [CrossRef]
- Yoshida, C.; Murakami, M.; Niwa, A.; Takeya, M.; Osanai, T. Efficient extraction and preservation of thermotolerant phycocyanins from red alga Cyanidioschyzon merolae. J. Biosci. Bioeng. 2021, 131, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Z.; Dadmohammadi, Y.; Li, Y.; Jaiswal, A.; Abbaspourrad, A. Whey protein improves the stability of C-phycocyanin in acidified conditions during light storage. Food Chem. 2021, 344, 128642. [Google Scholar] [CrossRef] [PubMed]
- Bharmoria, P.; Correia, S.F.H.; Martins, M.; Hernández-Rodríguez, M.A.; Ventura, S.P.M.; Ferreira, R.A.S.; Carlos, L.D.; Coutinho, J.A.P. Protein Cohabitation: Improving the Photochemical Stability of R-Phycoerythrin in the Solid State. J. Phys. Chem. Lett. 2020, 11, 6249–6255. [Google Scholar] [CrossRef] [PubMed]
- Buecker, S.; Gibis, M.; Bartmann, L.; Bussler, S.; Weiss, J. Improving the colloidal stability of pectin–phycocyanin complexes by increasing the mixing ratio. J. Food Sci. 2024, 89, 1086–1097. [Google Scholar] [CrossRef]
- Chandralekha, A.; Prashanth, H.S.; Tavanandi, H.; Raghavarao, K.S.M.S. A novel method for double encapsulation of C-phycocyanin using aqueous two phase systems for extension of shelf life. J. Food Sci. Technol. 2021, 58, 1750–1763. [Google Scholar] [CrossRef] [PubMed]
- Faieta, M.; Neri, L.; Di Michele, A.; Di Mattia, C.D.; Pittia, P. High hydrostatic pressure treatment of Arthrospira (Spirulina) platensis extracts and the baroprotective effect of sugars on phycobiliproteins. Innov. Food Sci. Emerg. Technol. 2021, 70, 102693. [Google Scholar] [CrossRef]
- İlter, I.; Koç, M.; Demirel, Z.; Conk Dalay, M.; Kaymak Ertekin, F. Improving the stability of phycocyanin by spray dried microencapsulation. J. Food Process. Preserv. 2021, 45, e15646. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Abbaspourrad, A. Improved pH stability, heat stability, and functionality of phycocyanin after PEGylation. Int. J. Biol. Macromol. 2022, 222, 1758–1767. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Perales Romero, E.; Bermejo, R.; Jordán-Núñez, J.; Viqueira, V.; Pérez, J. Using Laminar Nanoclays for Phycocyanin and Phycoerythrin Stabilization as New Natural Hybrid Pigments from Microalgae Extraction. Appl. Sci. 2021, 11, 11992. [Google Scholar] [CrossRef]
- Munawaroh, H.S.H.; Gumilar, G.G.; Alifia, C.R.; Marthania, M.; Stellasary, B.; Yuliani, G.; Wulandari, A.P.; Kurniawan, I.; Hidayat, R.; Ningrum, A.; et al. Photostabilization of phycocyanin from Spirulina platensis modified by formaldehyde. Process Biochem. 2020, 94, 297–304. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Enhanced Microencapsulation of C-Phycocyanin from Arthrospira by Freeze-Drying with Different Wall Materials. Food Technol. Biotechnol. 2020, 58, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Lakshmibalasubramaniam, S.; Nayak, B.; Tripp, C.; Kar, A.; Sappati, P.K. Improved stability of phycobiliprotein within liposome stabilized by polyethylene glycol adsorbed cellulose nanocrystals. Int. J. Biol. Macromol. 2020, 163, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cho, S.; Dadmohammadi, Y.; Li, Y.; Abbaspourrad, A. Improvement of the storage stability of C-phycocyanin in beverages by high-pressure processing. Food Hydrocoll. 2021, 110, 106055. [Google Scholar] [CrossRef]
- Azari, A.; Ghaboos, S.H.H.; Moghadam, V.E.; Jafari, S.M. Influence of chitosan coating on the physicochemical and antioxidant properties of phycocyanin-loaded nanoliposomes. Algal Res. 2023, 72, 103120. [Google Scholar] [CrossRef]
- Gustiningtyas, A.; Setyaningsih, I.; Hardiningtyas, S.D.; Susila, A.A.R. Improvement stability of phycocyanin from Spirulina platensis encapsulated by water soluble chitosan nanoparticles. IOP Conf. Ser. Earth Environ. Sci. 2020, 414, 012005. [Google Scholar] [CrossRef]
- Cao, J.; Wang, J.; Wang, S.; Xu, X. Porphyra Species: A Mini-Review of Its Pharmacological and Nutritional Properties. J. Med. Food 2016, 19, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Silveira, S.T.; Burkert, J.F.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef]
- Tibbettes, S.M.; MacPherson, M.J.; Park, K.C.; Melanson, R.J.; Patelakis, S.J.J. Composition and apparent digestibility coefficients of essential nutrients and energy of cyanobacterium meal produced from Spirulina (Arthrospira platensis) for freshwater-phase Atlantic salmon (Salmo salar L.) pre-smolts. Algal Res. 2023, 70, 103017. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, X.; Li, D.; Wang, L.; Wang, Y. Effects of κ-Carrageenan and Guar Gum on the Rheological Properties and Microstructure of Phycocyanin Gel. Foods 2022, 11, 734. [Google Scholar] [CrossRef]
- Qiao, B.-W.; Liu, X.-T.; Wang, C.-X.; Song, S.; Ai, C.-Q.; Fu, Y.-H. Preparation, Characterization, and Antioxidant Properties of Phycocyanin Complexes Based on Sodium Alginate and Lysozyme. Front. Nutr. 2022, 9, 890942. [Google Scholar] [CrossRef] [PubMed]
- Alavi, N.; Golmakani, M.-T.; Hosseini, S.M.H. Fabrication and characterization of phycocyanin-alginate-pregelatinized corn starch composite gel beads: Effects of carriers on kinetic stability of phycocyanin. Int. J. Biol. Macromol. 2022, 218, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, H.N.; Nayak, C.A. Enhanced stability of C-phycocyanin colorant by extrusion encapsulation. J. Food Sci. Technol. 2019, 56, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Castro-Varela, P.; Rubilar, M.; Martínez-Férez, A.; Fuentes-Ríos, D.; López-Romero, J.M.; Alarcón, C.; Abdala-Díaz, R.; Figueroa, F.L. R-phycoerythrin alginate/shellac beads by external gelation: Process optimization and the effects of gastrointestinal digestion for nutraceutical applications. Algal Res. 2024, 79, 103473. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Opretzka, L.C.F.; Almeida, L.S.; Cardoso, L.G.; da Silva, J.B.A.; de Souza, C.O.; Villarreal, C.F.; Druzian, J.I. Preparation and characterization of C-phycocyanin coated with STMP/STPP cross-linked starches from different botanical sources. Int. J. Biol. Macromol. 2020, 159, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yin, Z.; Sun, W.; Zhong, Q.; Zhang, Y.; Zeng, M. Microalgae play a structuring role in food: Effect of Spirulina platensis on the rheological, gelling characteristics, and mechanical properties of soy protein isolate hydrogel. Food Hydrocoll. 2023, 136, 108244. [Google Scholar] [CrossRef]
- Wang, H.; Ouyang, Z.; Hu, L.; Cheng, Y.; Zhu, J.; Ma, L.; Zhang, Y. Self-assembly of gelatin and phycocyanin for stabilizing thixotropic emulsions and its effect on 3D printing. Food Chem. 2022, 397, 133725. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, H.; Su, W.; Song, Y.; Zaky, A.A.; Abd El-Aty, A.M.; Tan, M. Co-delivery of hydrophobic astaxanthin and hydrophilic phycocyanin by a pH-sensitive water-in-oil-in-water double emulsion-filled gellan gum hydrogel. Food Hydrocoll. 2022, 131, 107810. [Google Scholar] [CrossRef]
- Zhong, Y.; Sun, S.; Dai, T.; Zhang, H.; Wu, J.; Gong, E.S. Phycocyanin-chitosan complex stabilized emulsion: Preparation, characteristics, digestibility, and stability. Int. J. Biol. Macromol. 2024, 260, 129253. [Google Scholar] [CrossRef]
- Teixé-Roig, J.; Oms-Oliu, G.; Ballesté-Muñoz, S.; Odriozola-Serrano, I.; Martín-Belloso, O. Encapsulation and controlled release of phycocyanin during the in vitro digestion using polysaccharide-added double emulsions (W1/O/W2). Food Struct. 2022, 31, 100249. [Google Scholar] [CrossRef]
- Sahin, O.I.; Dundar, A.N.; Ozdemir, S.; Uzuner, K.; Parlak, M.E.; Dagdelen, A.F.; Saricaoglu, F.T. Nanophytosomes as a protection system to improve the gastrointestinal stability and bioavailability of phycocyanin. Food Biosci. 2022, 50, 102052. [Google Scholar] [CrossRef]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Kiani, F.; Hajjari, M.M.; Sharif, N.; Fazaeli, M.; Hosseini, S.M.H. Electrospun zein incorporating phycocyanin and Spirulina extract: Fabrication, characterization, and potential application. LWT 2023, 188, 115408. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, W.; Li, Y.; Zhang, S.; Liang, H.; Ji, C.; Lin, X. High-speed electrospinning of phycocyanin and probiotics complex nanofibrous with higher probiotic activity and antioxidation. Food Res. Int. 2023, 167, 112715. [Google Scholar] [CrossRef] [PubMed]
- Schmatz, D.A.; da Silveira Mastrantonio, D.J.; Vieira Costa, J.A.; de Morais, M.G. Encapsulation of phycocyanin by electrospraying: A promising approach for the protection of sensitive compounds. Food Bioprod. Process. 2020, 119, 206–215. [Google Scholar] [CrossRef]
- Ghosh, J.; Haraguchi, Y.; Asahi, T.; Nakao, Y.; Shimizu, T. Muscle cell proliferation using water-soluble extract from nitrogen-fixing cyanobacteria Anabaena sp. PCC 7120 for sustainable cultured meat production. Biochem. Biophys. Res. Commun. 2023, 682, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jung, S.; Heo, J.; Koh, W.-G.; Lee, S.; Hong, J. Chitosan/Cellulose-Based Porous Nanofilm Delivering C-Phycocyanin: A Novel Platform for the Production of Cost-Effective Cultured Meat. ACS Appl. Mater. Interfaces 2021, 13, 32193–32204. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jung, S.; Choi, M.; Lee, M.; Choi, B.; Koh, W.-G.; Lee, S.; Hong, J. Gelatin MAGIC powder as nutrient-delivering 3D spacer for growing cell sheets into cost-effective cultured meat. Biomaterials 2021, 278, 121155. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Nourmohammadi, N.; Soleimanian-Zad, S.; Shekarchizadeh, H. Effect of Spirulina (Arthrospira platensis) microencapsulated in alginate and whey protein concentrate addition on physicochemical and organoleptic properties of functional stirred yogurt. J. Sci. Food Agric. 2020, 100, 5260–5268. [Google Scholar] [CrossRef]
- Koli, D.; Rudra, S.; Bhowmik, A.; Pabbi, S. Nutritional, Functional, Textural and Sensory Evaluation of Spirulina Enriched Green Pasta: A Potential Dietary and Health Supplement. Foods 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Tolpeznikaite, E.; Klupsaite, D.; Starkute, V.; Bartkevics, V.; Skrastina, A.; Pavlenko, R.; Mockus, E.; Lele, V.; Batkeviciute, G.; et al. Bio-Converted Spirulina for Nutraceutical Chewing Candy Formulations Rich in L-Glutamic and Gamma-Aminobutyric Acids. Microorganisms 2023, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Beisler, N.; Sandmann, M. Integration of Arthrospira platensis (spirulina) into the brewing process to develop new beers with unique sensory properties. Front. Sustain. Food Syst. 2022, 6, 918772. [Google Scholar] [CrossRef]
- Campos Assumpção de Amarante, M.; Cavalcante Braga, A.R.; Sala, L.; Juliano Kalil, S. Colour stability and antioxidant activity of C-phycocyanin-added ice creams after in vitro digestion. Food Res. Int. 2020, 137, 109602. [Google Scholar] [CrossRef] [PubMed]
- Stanic-Vucinic, D.; Minic, S.; Nikolic, M.R.; Velickovic, T.C. Spirulina Phycobiliproteins as Food Components and Complements. In Microalgal Biotechnology; Jacob-Lopes, E., Zepka, L.Q., Queiroz, M.I., Eds.; InTech: Rijeka, Croatia, 2018. [Google Scholar]
- Colonia, B.S.O.; de Melo Pereira, G.V.; de Carvalho, J.C.; Karp, S.G.; Rodrigues, C.; Soccol, V.T.; Fanka, L.S.; Soccol, C.R. Deodorization of algae biomass to overcome off-flavors and odor issues for developing new food products: Innovations, trends, and applications. Food Chem. Adv. 2023, 2, 100270. [Google Scholar] [CrossRef]
- Almeida, L.M.R.; da Silva Cruz, L.F.; Machado, B.A.S.; Nunes, I.L.; Costa, J.A.V.; de Souza Ferreira, E.; Lemos, P.V.F.; Druzian, J.I.; de Souza, C.O. Effect of the addition of Spirulina sp. biomass on the development and characterization of functional food. Algal Res. 2021, 58, 102387. [Google Scholar] [CrossRef]
- Grahl, S.; Strack, M.; Weinrich, R.; Mörlein, D. Consumer-Oriented Product Development: The Conceptualization of Novel Food Products Based on Spirulina (Arthrospira platensis) and Resulting Consumer Expectations. J. Food Qual. 2018, 2018, 1919482. [Google Scholar] [CrossRef]
- Grahl, S.; Strack, M.; Mensching, A.; Mörlein, D. Alternative protein sources in Western diets: Food product development and consumer acceptance of spirulina-filled pasta. Food Qual. Prefer. 2020, 84, 103933. [Google Scholar] [CrossRef]
- Sengupta, S.; Bhowal, J. Optimization of ingredient and processing parameter for the production of Spirulina platensis incorporated soy yogurt using response surface methodology. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1081–1085. [Google Scholar] [CrossRef]
- Palanisamy, M.; Töpfl, S.; Berger, R.G.; Hertel, C. Physico-chemical and nutritional properties of meat analogues based on Spirulina/lupin protein mixtures. Eur. Food Res. Technol. 2019, 245, 1889–1898. [Google Scholar] [CrossRef]
- Tiepo, C.B.V.; Gottardo, F.M.; Mortari, L.M.; Bertol, C.D.; Reinehr, C.O.; Colla, L.M. Addition of Spirulina platensis in handmade ice cream: Phisicochemical and sensory effects/Adição de Spirulina platensis em sorvete caseiro: Efeitos físico-químicos e sensoriais. Brazilian J. Dev. 2021, 7, 88106–88123. [Google Scholar] [CrossRef]
- Zen, C.K.; Tiepo, C.B.V.; da Silva, R.V.; Reinehr, C.O.; Gutkoski, L.C.; Oro, T.; Colla, L.M. Development of functional pasta with microencapsulated Spirulina: Technological and sensorial effects. J. Sci. Food Agric. 2020, 100, 2018–2026. [Google Scholar] [CrossRef]
- Galetović, A.; Seura, F.; Gallardo, V.; Graves, R.; Cortés, J.; Valdivia, C.; Núñez, J.; Tapia, C.; Neira, I.; Sanzana, S.; et al. Use of Phycobiliproteins from Atacama Cyanobacteria as Food Colorants in a Dairy Beverage Prototype. Foods 2020, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Shanmugam, M. Isolation of phycoerythrin from Kappaphycus alvarezii: A potential natural colourant in ice cream. J. Appl. Phycol. 2020, 32, 4221–4233. [Google Scholar] [CrossRef]
- da Silva Faresin, L.; Devos, R.J.B.; Reinehr, C.O.; Colla, L.M. Development of ice cream with reduction of sugar and fat by the addition of inulin, Spirulina platensis or phycocyanin. Int. J. Gastron. Food Sci. 2022, 27, 100445. [Google Scholar] [CrossRef]
- Durmaz, Y.; Kilicli, M.; Toker, O.S.; Konar, N.; Palabiyik, I.; Tamtürk, F. Using spray-dried microalgae in ice cream formulation as a natural colorant: Effect on physicochemical and functional properties. Algal Res. 2020, 47, 101811. [Google Scholar] [CrossRef]
- Benchikh, Y.; Filali, A.; Rebai, S. Modeling and optimizing the phycocyanins extraction from Arthrospira platensis (Spirulina) algae and preliminary supplementation assays in soft beverage as natural colorants and antioxidants. J. Food Process. Preserv. 2021, 45, e15170. [Google Scholar] [CrossRef]
- Taiti, C.; Stefano, G.; Percaccio, E.; Di Giacomo, S.; Iannone, M.; Marianelli, A.; Di Sotto, A.; Garzoli, S. Addition of Spirulina to Craft Beer: Evaluation of the Effects on Volatile Flavor Profile and Cytoprotective Properties. Antioxidants 2023, 12, 1021. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- El Baky, H.H.A.; El Baroty, G.S.; Ibrahem, E.A. Functional characters evaluation of biscuits sublimated with pure phycocyanin isolated from Spirulina and Spirulina biomass. Nutr. Hosp. 2015, 32, 231–241. [Google Scholar] [CrossRef]
- Rodrigues, E.F.; Vendruscolo, L.P.; Bonfante, K.; Reinehr, C.O.; Colla, E.; Colla, L.M. Phycocyanin as substitute for texture ingredients in ice creams. Br. Food J. 2019, 122, 693–707. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kang, S.-H.; Kim, M.-R. Changes in the Quality Characteristics and Antioxidant Activities of Spirulina Added Bread during Storage. Korean J. Food Preserv. 2011, 18, 111–118. [Google Scholar] [CrossRef][Green Version]
- Golmakani, M.-T.; Soleimanian-Zad, S.; Alavi, N.; Nazari, E.; Eskandari, M.H. Effect of Spirulina (Arthrospira platensis) powder on probiotic bacteriologically acidified feta-type cheese. J. Appl. Phycol. 2019, 31, 1085–1094. [Google Scholar] [CrossRef]
- Nefasa, A.N.; Wulandari, E.C.; Christwardana, M.; Hadiyanto, H. Quality of Macronutrient of Cow’s Milk with Addition of Soybean Oil and Phycocyanin Extract as Functional Food. Food Sci. Technol. 2020, 3, 17. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Garbowska, M.; Berthold-Pluta, A.; Stasiak-Różańska, L.; Kalisz, S.; Pluta, A. The Impact of White Mulberry, Green Barley, Chia Seeds, and Spirulina on Physicochemical Characteristics, Texture, and Sensory Quality of Processed Cheeses. Foods 2023, 12, 2862. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Cho, H.; Kim, K.; Kweon, M. Quality Characteristics and Antioxidant Activity of Fresh Noodles Formulated with Flour-Bran Blends Varied by Particle Size and Blend Ratio of Purple-Colored Wheat Bran. Processes 2022, 10, 584. [Google Scholar] [CrossRef]
- Atallah, A.A.; Morsy, O.M.; Gemiel, D.G. Characterization of functional low-fat yogurt enriched with whey protein concentrate, Ca-caseinate and spirulina. Int. J. Food Prop. 2020, 23, 1678–1691. [Google Scholar] [CrossRef]
- Hassanzadeh, H.; Ghanbarzadeh, B.; Galali, Y.; Bagheri, H. The physicochemical properties of the spirulina-wheat germ-enriched high-protein functional beverage based on pear-cantaloupe juice. Food Sci. Nutr. 2022, 10, 3651–3661. [Google Scholar] [CrossRef]
- Nazir, F.; Saeed, M.; Abbas, A.; Majeed, M.R.; Israr, M.; Zahid, H.; Ilyas, M.; Nasir, M. Development, quality assessment and nutritive valorization of Spirulina platensis in yogurt spread. Food Sci. Appl. Biotechnol. 2022, 5, 106. [Google Scholar] [CrossRef]
- Sanjari, S.; Sarhadi, H.; Shahdadi, F. Investigating the Effect of Spirulina platensis Microalgae on Textural and Sensory Properties of Baguette Bread. J. Nutr. Food Secur. 2018, 3, 218–225. [Google Scholar] [CrossRef]
- Fradinho, P.; Niccolai, A.; Soares, R.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Effect of Arthrospira platensis (spirulina) incorporation on the rheological and bioactive properties of gluten-free fresh pasta. Algal Res. 2020, 45, 101743. [Google Scholar] [CrossRef]
- Şahin, O.I. Functional and sensorial properties of cookies enriched with SPIRULINA and DUNALIELLA biomass. J. Food Sci. Technol. 2020, 57, 3639–3646. [Google Scholar] [CrossRef]
- Luo, A.; Feng, J.; Hu, B.; Lv, J.; Liu, Q.; Nan, F.; Oliver Chen, C.-Y.; Xie, S. Arthrospira (Spirulina) platensis extract improves oxidative stability and product quality of Chinese-style pork sausage. J. Appl. Phycol. 2018, 30, 1667–1677. [Google Scholar] [CrossRef]
- Vieira, M.V.; Oliveira, S.M.; Amado, I.R.; Fasolin, L.H.; Vicente, A.A.; Pastrana, L.M.; Fuciños, P. 3D printed functional cookies fortified with Arthrospira platensis: Evaluation of its antioxidant potential and physical-chemical characterization. Food Hydrocoll. 2020, 107, 105893. [Google Scholar] [CrossRef]
- Hussein, A.; Ibrahim, G.; Kamil, M.; El-Shamarka, M.; Mostafa, S.; Mohamed, D. Spirulina-Enriched Pasta as Functional Food Rich in Protein and Antioxidant. Biointerface Res. Appl. Chem. 2021, 11, 14736–14750. [Google Scholar] [CrossRef]
- Haghdoost, A.; Golestan, L.; Hasani, M.; Noghabi, M.S.; Shahidi, S.A. Assessment of the potential of algae phycobiliprotein nanoliposome for extending the shelf life of common carp burgers during refrigerated storage. Fish. Aquat. Sci. 2022, 25, 276–286. [Google Scholar] [CrossRef]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; D’Ottavio, M.; Batista, A.P.; Raymundo, A.; Granchi, L.; et al. Development of new microalgae-based sourdough “crostini”: Functional effects of Arthrospira platensis (spirulina) addition. Sci. Rep. 2019, 9, 19433. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Gouraji, E.; Soleimanian-Zad, S.; Ghiaci, M. Phycocyanin-enriched yogurt and its antibacterial and physicochemical properties during 21 days of storage. LWT 2019, 102, 230–236. [Google Scholar] [CrossRef]
- Ersyah, D.; Jaziri, A.A.; Setijawati, D. Effect of Spirulina (Arthrospira platensis) Powder on The Physico-chemical and Sensory Characterization of Dry Noodle. J. Aquac. Fish Health 2022, 11, 277–288. [Google Scholar] [CrossRef]
- De Oliveira, T.T.B.; dos Reis, I.M.; de Souza, M.B.; da Silva Bispo, E.; Fonseca Maciel, L.; Druzian, J.I.; Lordelo Guimarães Tavares, P.P.; de Oliveira Cerqueira, A.; dos Santos Boa Morte, E.; Abreu Glória, M.B.; et al. Microencapsulation of Spirulina sp. LEB-18 and its incorporation in chocolate milk: Properties and functional potential. LWT 2021, 148, 111674. [Google Scholar] [CrossRef]
- van der Spiegel, M.; Noordam, M.Y.; van der Fels-Klerx, H.J. Safety of Novel Protein Sources (Insects, Microalgae, Seaweed, Duckweed, and Rapeseed) and Legislative Aspects for Their Application in Food and Feed Production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef] [PubMed]
- Kleinübing, S.J.; Vieira, R.S.; Beppu, M.M.; Guibal, E.; Silva, M.G.C.D. Characterization and evaluation of copper and nickel biosorption on acidic algae Sargassum Filipendula. Mater. Res. 2010, 13, 541–550. [Google Scholar] [CrossRef]
- Desideri, D.; Cantaluppi, C.; Ceccotto, F.; Meli, M.A.; Roselli, C.; Feduzi, L. Essential and toxic elements in seaweeds for human consumption. J. Toxicol. Environ. Health Part A 2016, 79, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Niedzielski, P.; Kaczmarek, N.; Jurczak, T.; Klimaszyk, P. The multidisciplinary approach to safety and toxicity assessment of microalgae-based food supplements following clinical cases of poisoning. Harmful Algae 2015, 46, 34–42. [Google Scholar] [CrossRef]
- Gromek, W.; Kołdej, N.; Kurowski, M.; Majsiak, E. Spirulina (Arthrospira platensis): Antiallergic Agent or Hidden Allergen? A Literature Review. Foods 2024, 13, 1052. [Google Scholar] [CrossRef]
- Athané, A.; Demol, J.; Brosset-Vincent, S.; Aguenou, C.; Krisa, S.; Courtois, A.; Griffiths, H.; Cagnac, O. The safety evaluation of phycocyanin-enriched Galdieria sulphuraria extract using 90-day toxicity study in rats and in vitro genotoxicity studies. Toxicol. Res. Appl. 2020, 4, 239784732092999. [Google Scholar] [CrossRef]
- Jensen, G.S.; Drapeau, C.; Lenninger, M.; Benson, K.F. Clinical Safety of a High Dose of Phycocyanin-Enriched Aqueous Extract from Arthrospira (Spirulina) platensis: Results from a Randomized, Double-Blind, Placebo-Controlled Study with a Focus on Anticoagulant Activity and Platelet Activation. J. Med. Food 2016, 19, 645–653. [Google Scholar] [CrossRef]
- Fernand, F.; Israel, A.; Skjermo, J.; Wichard, T.; Timmermans, K.R.; Golberg, A. Offshore macroalgae biomass for bioenergy production: Environmental aspects, technological achievements and challenges. Renew. Sustain. Energy Rev. 2017, 75, 35–45. [Google Scholar] [CrossRef]
- Liu, D.; Keesing, J.K.; Xing, Q.; Shi, P. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar. Pollut. Bull. 2009, 58, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Radulovich, R.; Umanzor, S.; Cabrera, R.; Mata, R. Tropical seaweeds for human food, their cultivation and its effect on biodiversity enrichment. Aquaculture 2015, 436, 40–46. [Google Scholar] [CrossRef]
- Lotze, H.K.; Milewski, I.; Fast, J.; Kay, L.; Worm, B. Ecosystem-based management of seaweed harvesting. Bot. Mar. 2019, 62, 395–409. [Google Scholar] [CrossRef]
- Kumari, P.; Shukla, S.P.; Rathi Bhuvaneswari, G.; Kumar, S.; Xavier, M.; Kumar, M. High value pigment production and carbon sequestration through wastewater grown Spirulina (Arthrospira) platensis: A green technology for wastewater utilization. Waste Manag. Bull. 2023, 1, 1–10. [Google Scholar] [CrossRef]
- Yu, P.; Wu, Y.; Wang, G.; Jia, T.; Zhang, Y. Purification and bioactivities of phycocyanin. Crit. Rev. Food Sci. Nutr. 2017, 57, 3840–3849. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.R.; Minis, S.; Macvanin, M.; Stanic-Vucinic, D.; Cirkovic Velickovic, T. Analytical Protocols in Phycobiliproteins Analysis. In Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M., Zepka, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 179–201. [Google Scholar]
- Ruiz-Ruiz, F.; Benavides, J.; Rito-Palomares, M. Scaling-up of a B-phycoerythrin production and purification bioprocess involving aqueous two-phase systems: Practical experiences. Process Biochem. 2013, 48, 738–745. [Google Scholar] [CrossRef]
- Torres-Acosta, M.A.; Ruiz-Ruiz, F.; Aguilar-Yáñez, J.M.; Benavides, J.; Rito-Palomares, M. Economic analysis of pilot-scale production of B-phycoerythrin. Biotechnol. Prog. 2016, 32, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Lauceri, R.; Chini Zittelli, G.; Torzillo, G. A simple method for rapid purification of phycobiliproteins from Arthrospira platensis and Porphyridium cruentum biomass. Algal Res. 2019, 44, 101685. [Google Scholar] [CrossRef]
- Jaouen, P.; Lépine, B.; Rossignol, N.; Royer, R.; Quéméneur, F. Clarification and concentration with membrane technology of a phycocyanin solution extracted from Spirulina platensis. Biotechnol. Tech. 1999, 13, 877–881. [Google Scholar] [CrossRef]
- Ramos, A.; Acién, F.G.; Fernández-Sevilla, J.M.; González, C.V.; Bermejo, R. Large-scale isolation and purification of C-phycocyanin from the cyanobacteria Anabaena marina using expanded bed adsorption chromatography. J. Chem. Technol. Biotechnol. 2010, 85, 783–792. [Google Scholar] [CrossRef]
- Denis, C.; Massé, A.; Fleurence, J.; Jaouen, P. Concentration and pre-purification with ultrafiltration of a R-phycoerythrin solution extracted from macro-algae Grateloupia turuturu: Process definition and up-scaling. Sep. Purif. Technol. 2009, 69, 37–42. [Google Scholar] [CrossRef]
- Martins, M.; Soares, B.P.; Santos, J.H.P.M.; Bharmoria, P.; Torres Acosta, M.A.; Dias, A.C.R.V.; Coutinho, J.A.P.; Ventura, S.P.M. Sustainable Strategy Based on Induced Precipitation for the Purification of Phycobiliproteins. ACS Sustain. Chem. Eng. 2021, 9, 3942–3954. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Ragaza, J.A.; Hossain, M.S.; Meiler, K.A.; Velasquez, S.F.; Kumar, V. A review on Spirulina: Alternative media for cultivation and nutritive value as an aquafeed. Rev. Aquac. 2020, 12, 2371–2395. [Google Scholar] [CrossRef]
- Lucakova, S.; Branyikova, I.; Branyik, T.; Matoulkova, D.; Krausova, G. Wastewater from the demineralization of cheese whey for cost-efficient cultivation of spirulina. J. Appl. Phycol. 2022, 34, 89–99. [Google Scholar] [CrossRef]
- Lim, H.R.; Khoo, K.S.; Chew, K.W.; Chang, C.-K.; Munawaroh, H.S.H.; Kumar, P.S.; Huy, N.D.; Show, P.L. Perspective of Spirulina culture with wastewater into a sustainable circular bioeconomy. Environ. Pollut. 2021, 284, 117492. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yang, Z.; Lu, Z.; Wang, Q.; Liu, J.; Song, L. Combination of utilization of CO2 from flue gas of biomass power plant and medium recycling to enhance cost-effective Spirulina production. J. Appl. Phycol. 2019, 31, 2175–2185. [Google Scholar] [CrossRef]
- Thevarajah, B.; Nishshanka, G.K.S.H.; Premaratne, M.; Nimarshana, P.H.V.; Nagarajan, D.; Chang, J.-S.; Ariyadasa, T.U. Large-scale production of Spirulina-based proteins and c-phycocyanin: A biorefinery approach. Biochem. Eng. J. 2022, 185, 108541. [Google Scholar] [CrossRef]

| Protein (Source) | The Experimental Approach | The Results | Reference |
|---|---|---|---|
| C-phycocyanin (Arthrospira platensis) | Color stabilization of protein via λ-carrageenan (λC) in liquid formulations | Complexation with λC increased the protein color stability at pH < pI, especially at a pH of 3.0, even when heated to 90 °C. | [55] |
| C-Phycocyanin (Arthrospira platensis) | Stabilization effects of protein complexation with λ-carrageenan on its intrinsic blue color | The electrostatic complexation stabilized protein color in the acidic pH range (2.5–6.0) and against a heat treatment at 70 °C. | [56] |
| C-Phycocyanin (Spirulina platensis) | Stabilization of protein in aqueous solutions via sucrose and trehalose (20 and 40%, w/w) | The stabilizing effect of saccharides on the thermal discoloration of protein with sucrose performed better than trehalose. | [43] |
| C-Phycocyanin (Hawaiian Spirulina) | Investigation of the potential of twelve food-derived antioxidants to bind and stabilize the protein | Complexation of protein with quercetin and coenzyme Q10 improved its thermal stability (higher melting point). | [57] |
| C-phycocyanin (Arthrospira platensis) | Enhancement of protein productivity and stability using organic acids (citric, acetic, succinic, fumaric, and oxalic acid) | Organic acids, primarily citric acid (7.5%), act as preservatives to stabilize protein (promoting the half-live) at high temperatures. | [58] |
| C-phycocyanin (Arthrospira platensis) | Improvement of protein stability by adding saccharides (glucose, mannose, galactose, and maltose) and sugar alcohols (mannitol and maltitol) | Sugars effectively improved the protein’s thermal stability in correlation with the additive concentration and inhibited its oxidative degradation. | [59] |
| C-phycocyanin (Spirulina platensis) | Increasing protein stability via 0.5% cysteine addition during enzyme-assisted extraction | Cysteine increased the thermal stability of protein extracted with (endopeptidase) Collupulin. | [53] |
| C-phycocyanin (Spirulina) | Improvement of protein stability by forming soluble complexes with poly-saccharides (κ-/ι-/λ-carrageenans, xanthan gum, high-methoxyl pectin, and guar gum) | Improved protein’s colloidal and color stabilities against acidic pH (standard beverage processing) and heating conditions. | [60] |
| R-phycoerythrin (Porphyra haitanensis) | Stabilization of protein by self-assembly with oligochitosan (at a 1:20 reaction ratio) | The thermal (40–80 °C), natural light, and ultraviolet light irradiation (254 nm) protein stabilities were all improved. | [61] |
| Phycobiliproteins (Oscillatoria sp. BTA-170) | Stabilization of C-PC, A-PC, and PE in the presence of different monosaccharides (glucose, fructose, glucose, and lactose) | Glucose was the most critical monosaccharide that stabilizes the degradation of proteins at 65 °C and higher temperatures. | [62] |
| Phycobiliproteins (Spirulina platensis) | More efficient extraction of protein using NaCl as an extraction enhancer | Protein stability was improved by adding NaCl, which had unaffected antioxidant activity and a secondary structure. | [63] |
| C-Phycocyanin | Improving the protein color stability with epigallocatechin gallate (EGCG) | EGCG binding protected protein against color fading under light conditions. | [64] |
| R-phycocyanin (Cyanidioschyzon merolae) | Preservation of thermotolerant protein during storage with salts | The stabilizing effect of CaCl2 and MgCl2 (0.1 M) towards protein during seven days. | [65] |
| C-Phycocyanin | Improving the protein color stability in acidified conditions with whey protein isolate (WPI) | A low WPI concentration (0.05–0.1%) at pH 3.0 improved the protein’s color stability under light exposure. | [66] |
| C-phycocyanin (Spirulina) | Improvement of protein stability in acidified conditions using whey proteins (α-lactalbumin, β-lactoglobulin, BSA, immunoglobulins, and glycomacropeptides) | Native whey protein (10%) efficiently improves protein colloidal stability and prevents aggregation at pH 3.0. | [51] |
| Protein (Source) | Method and Conditions Used | Result | Reference |
|---|---|---|---|
| R-Phycoerythrin (Gracilaria gracilis) | Protein incorporation into the gelatin-based films | Improved the protein photochemical stability in the solid state for eight months. | [67] |
| C-Phycocyanin (Arthrospira platensis) | Preparation of pectin–phycocyanin complexes with different mixing ratios | Improved the colloidal stability of the protein wholly entrapped by the polysaccharide molecules at acidic pH after heating at 85 °C. | [68] |
| C-phycocyanin (Spirulina platensis) | Double encapsulation of protein using aqueous two-phase systems (PEG 4000/Potassium phosphate and PEG 6000/Dextran) by spray drying | Prolonged shelf life with the additional benefit of enhancing the purity of protein compared with conventional (maltodextrin) encapsulation. | [69] |
| Phycobiliproteins (Spirulina platensis) | Proteins were treated with high hydrostatic pressure (HPP) (600 MPa; 300 s) in the presence of sucrose, trehalose, and glucose (20 and 40%, w/w) | Sugars exerted baroprotective, concentration-dependent action on proteins’ (color) stability with preserved antioxidant activity. | [70] |
| C-Phycocyanin (Arthrospira platensis EGEMACC 38) | Spray-dried microencapsulation of protein using various combinations and ratios of wall materials (maltodextrin, gum arabic, whey protein isolate, and sodium caseinate) | The highest blueness index was observed in protein powder encapsulated with maltodextrin and whey protein isolate. | [71] |
| C-phycocyanin (Spirulina) | Modification of protein with 20 kDa methoxy polyethylene glycol polymers | The conjugates exhibited higher blue color intensity, improved thermodynamic stability, and a gain in pH stability and antioxidant activities. | [72] |
| Phycocyanin and B-Phycoerythrin | Intercalation of proteins into montmorillonite and laponite laminar nanoclays | Proteins’ optical and thermal properties were significantly improved. | [73] |
| C-Phycocyanin (Spirulina platensis) | The protein was modified using formaldehyde crosslinking | Increases photostability of modified protein only upon yellow light irradiation. | [74] |
| C-phycocyanin (Arthrospira platensis IFRPD 1182) | Freeze-dried maltodextrin and gum Arabic (fractions from 0 to 100%) were used as protein microencapsulation wall materials | Increased thermal stability of encapsulated protein, with high antioxidant properties. | [75] |
| Phycobiliprotein (Palmaria palmata) | Phycobiliprotein within liposome (soy lecithin) stabilized using polyethylene glycol adsorbed cellulose nanocrystals. | The encapsulated protein was stable below 60 ℃, above pH of 5.0, and against illumination. | [76] |
| C-Phycocyanin (Spirulina) | High-pressure processing treatment of the protein–whey protein and protein–carrageenan complexes at acidic pH | Protein’s complexations improved the color and, therefore, its storage stability under light exposure. | [77] |
| R-Phycoerythrin (Porphyra haitanensis) | Preparation of the various oligochitosan-modified protein complexes (OMPC) via the transglutaminase-catalyzed glycosylation reaction | Emulsifying stability, thermal stability, photostability, and pH stability of the OMPC were all significantly improved. | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minić, S.; Gligorijević, N.; Veličković, L.; Nikolić, M. Narrative Review of the Current and Future Perspectives of Phycobiliproteins’ Applications in the Food Industry: From Natural Colors to Alternative Proteins. Int. J. Mol. Sci. 2024, 25, 7187. https://doi.org/10.3390/ijms25137187
Minić S, Gligorijević N, Veličković L, Nikolić M. Narrative Review of the Current and Future Perspectives of Phycobiliproteins’ Applications in the Food Industry: From Natural Colors to Alternative Proteins. International Journal of Molecular Sciences. 2024; 25(13):7187. https://doi.org/10.3390/ijms25137187
Chicago/Turabian StyleMinić, Simeon, Nikola Gligorijević, Luka Veličković, and Milan Nikolić. 2024. "Narrative Review of the Current and Future Perspectives of Phycobiliproteins’ Applications in the Food Industry: From Natural Colors to Alternative Proteins" International Journal of Molecular Sciences 25, no. 13: 7187. https://doi.org/10.3390/ijms25137187
APA StyleMinić, S., Gligorijević, N., Veličković, L., & Nikolić, M. (2024). Narrative Review of the Current and Future Perspectives of Phycobiliproteins’ Applications in the Food Industry: From Natural Colors to Alternative Proteins. International Journal of Molecular Sciences, 25(13), 7187. https://doi.org/10.3390/ijms25137187








