Uncovering PheCLE1 and PheCLE10 Promoting Root Development Based on Genome-Wide Analysis
Abstract
1. Introduction
2. Results
2.1. Identification of Members of the CLE Family and Analysis of Protein Characteristics in Moso Bamboo
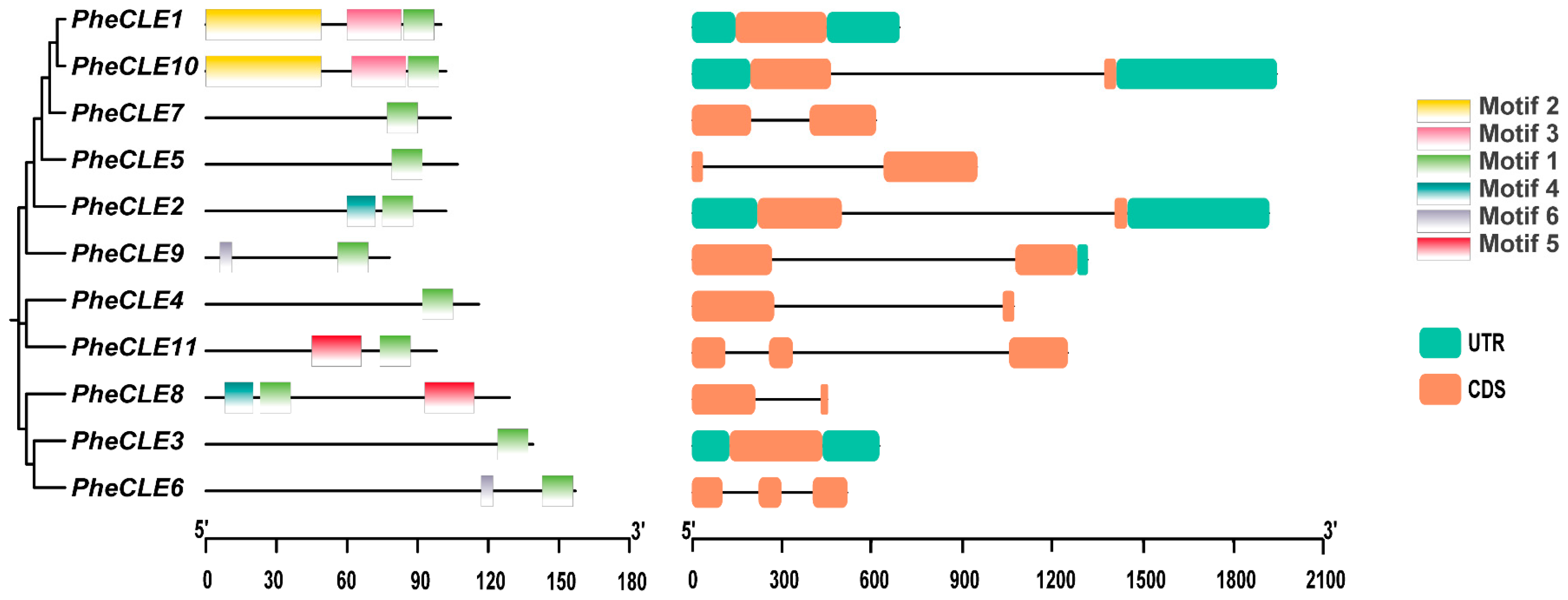
2.2. Analysis of PheCLE Gene Structure and Conserved Motifs
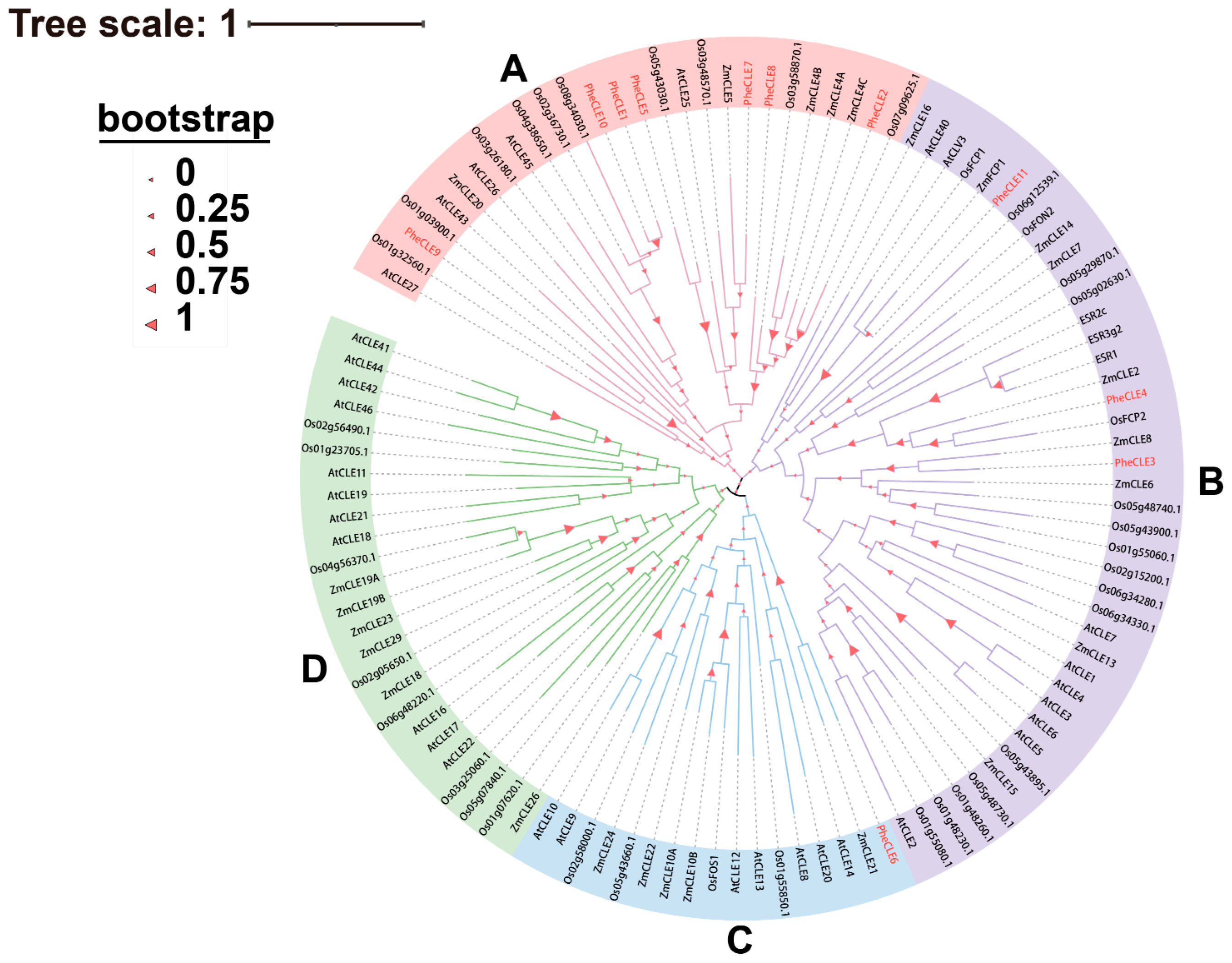
2.3. Evolutionary Analysis of CLE Genes
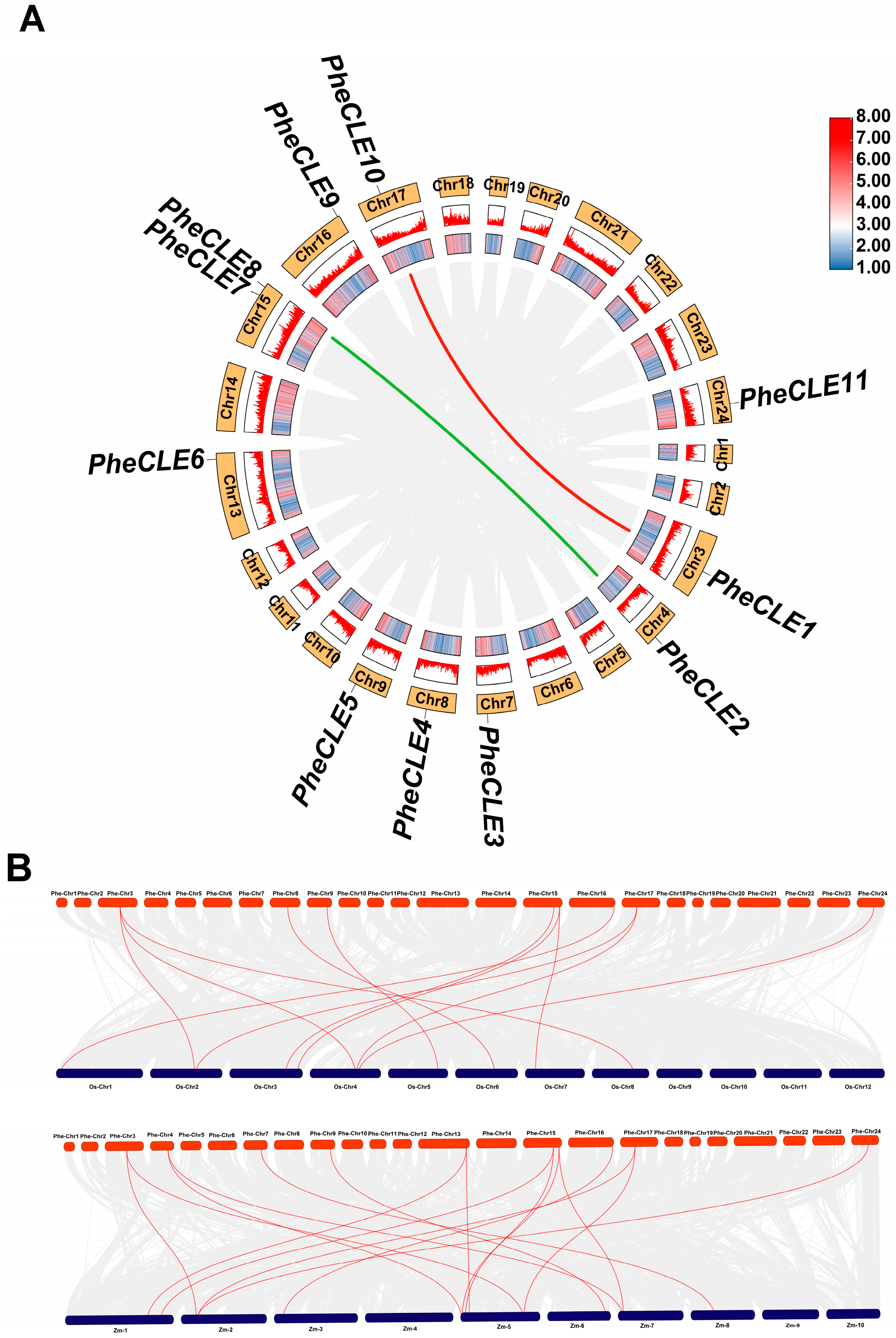
2.4. Collinearity Analysis of CLE Genes
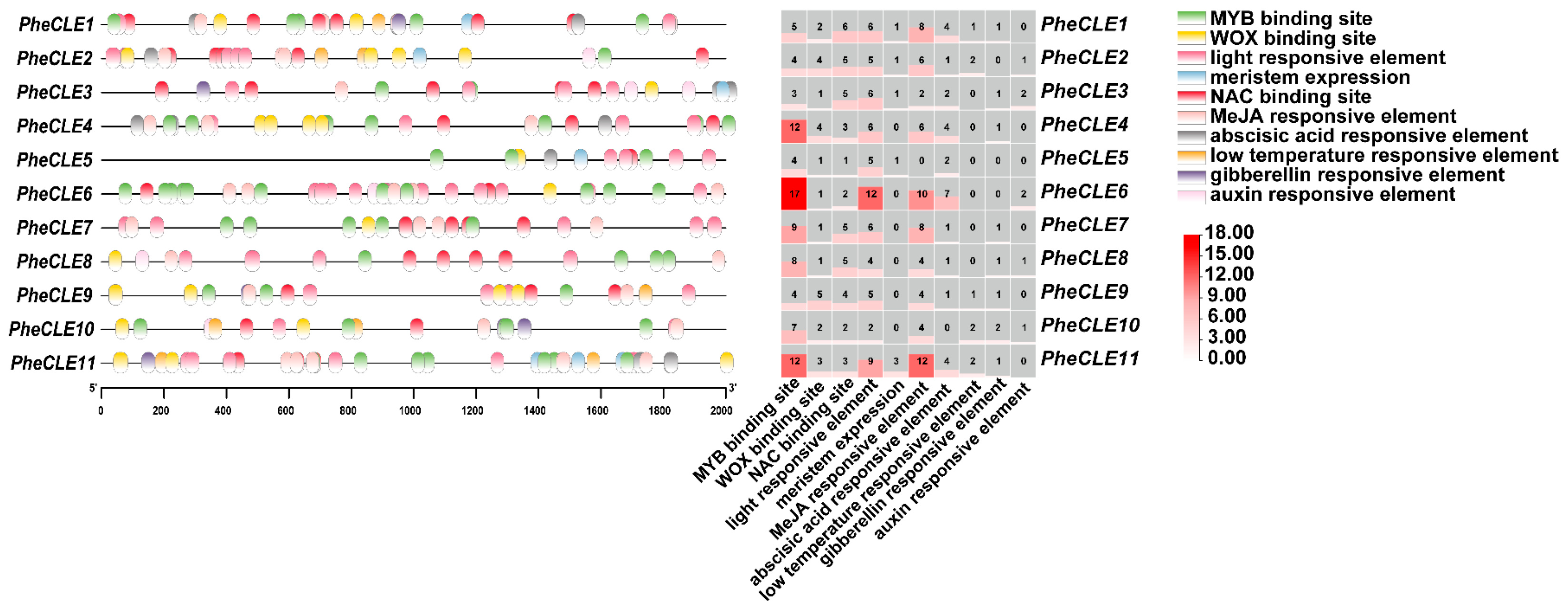
2.5. Analysis of Cis-Acting Elements in the PheCLE Gene Promoters
2.6. Expression Patterns of CLE Family Genes in Moso Bamboo
2.7. In Situ Hybridization Analysis of PheCLE1 and PheCLE10
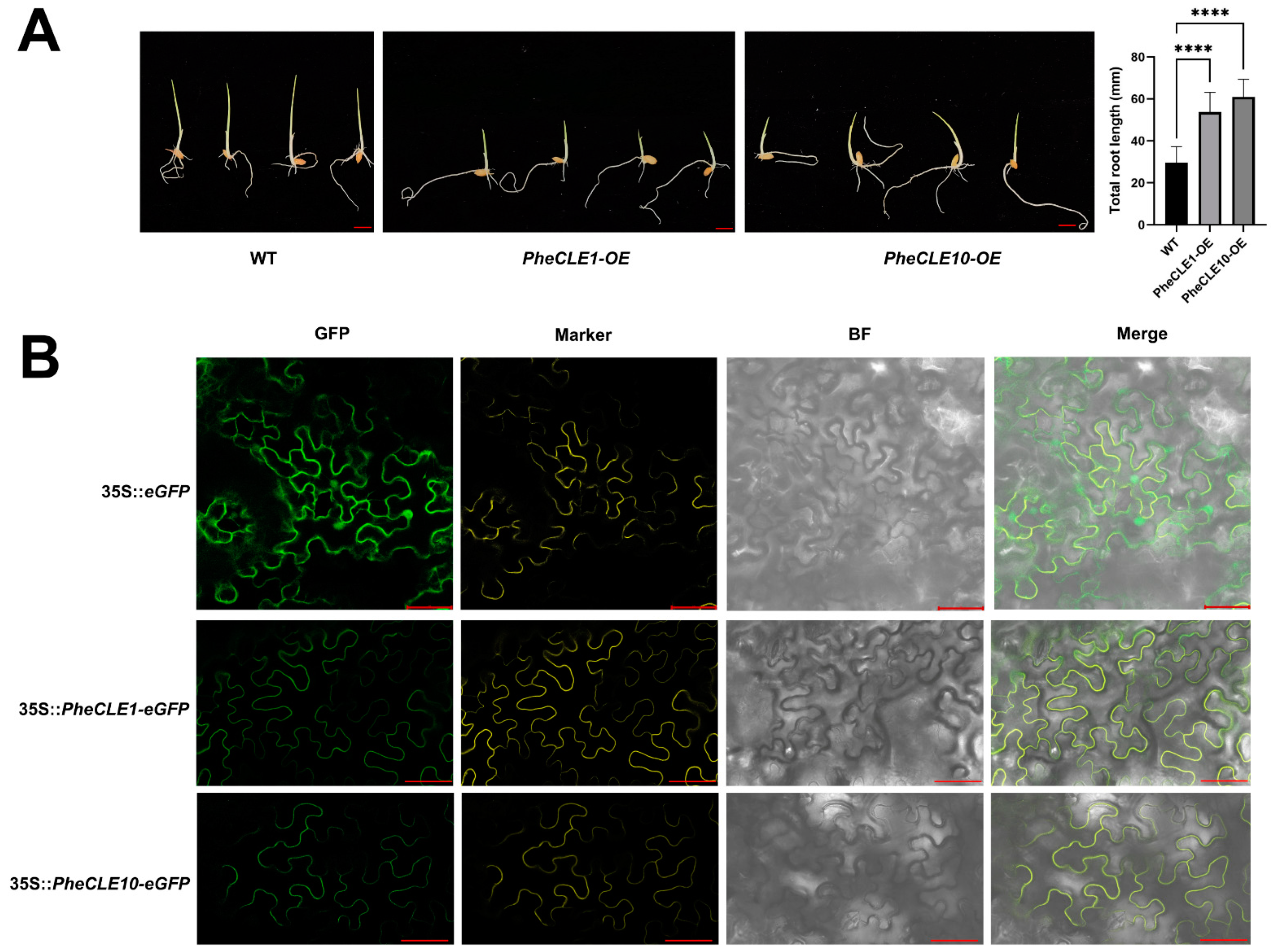
2.8. Overexpression of PheCLE1 and PheCLE10 Leads to Rice Root Elongation
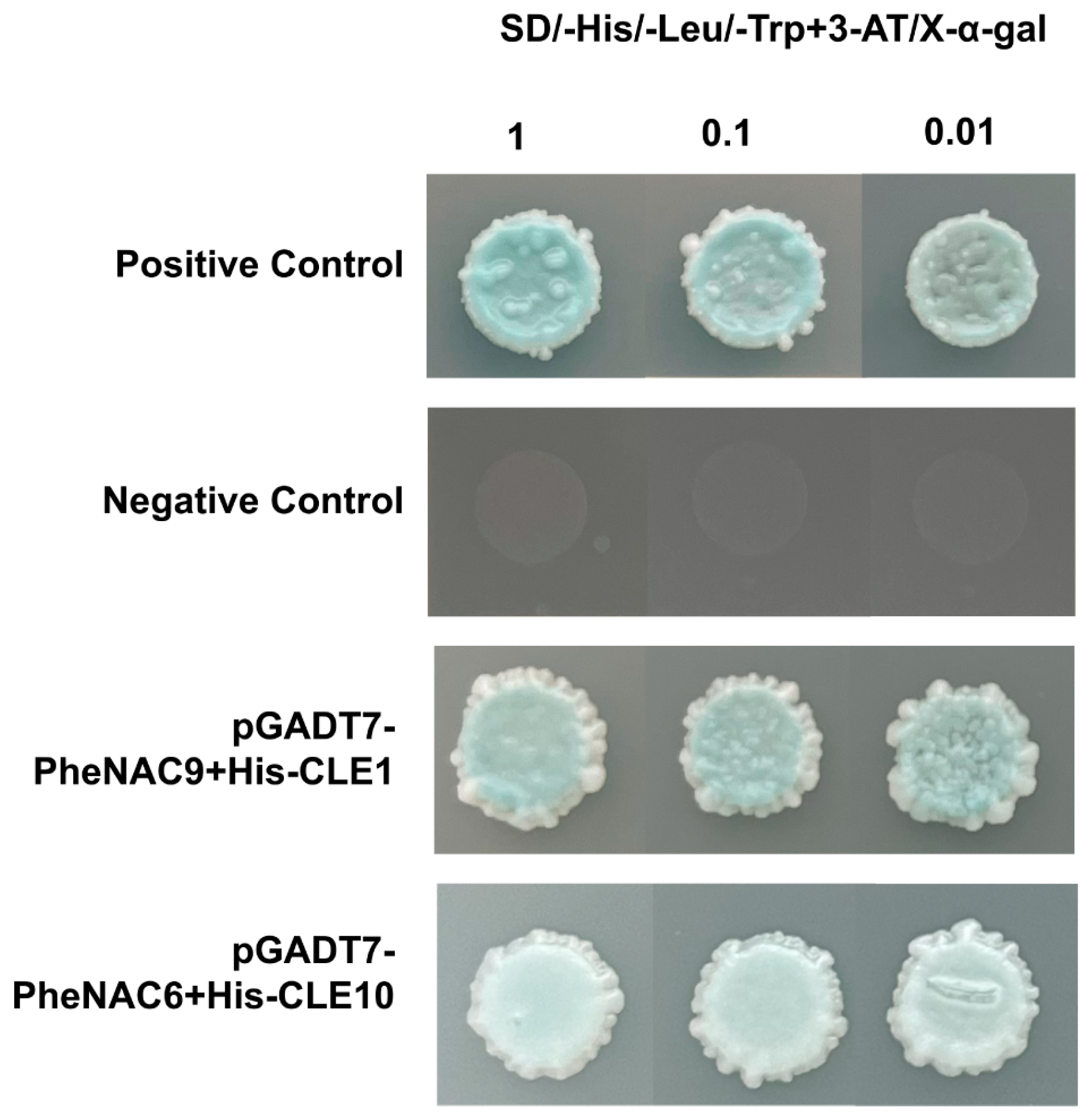
2.9. Screening for Upstream Regulatory Factors of PheCLE1 and PheCLE10 Genes
3. Discussion
4. Materials and Methods
4.1. Experimental Samples and Cultivation Conditions
4.2. Identification and Protein Characterization of CLE Family Members of Moso Bamboo
4.3. Structural and Motif Analysis of PheCLE Genes
4.4. Evolutionary Analysis of CLE Genes
4.5. Collinearity Analysis of CLE Genes
4.6. Promoter Analysis of PheCLE Genes
4.7. Gene Expression Pattern Analysis of PheCLE Genes
4.8. Subcellular Localization of PheCLE Proteins
4.9. In Situ Hybridization
4.10. Yeast One-Hybrid (Y1H) Assay
4.11. Dual Luciferase Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- INBAR. 2022 Global Bamboo and Rattan Commodities International Trade Report; International Bamboo and Rattan Organization: Beijing, China, 2023; Available online: https://www.inbar.int/resources/inbar_publications/2022zgztspgjmybg/ (accessed on 25 April 2024).
- Yang, J.B.; Dong, Y.R.; Wong, K.M.; Gu, Z.J.; Yang, H.Q.; Li, D.Z. Genetic structure and differentiation in Dendrocalamus sinicus (Poaceae: Bambusoideae) populations provide insight into evolutionary history and speciation of woody bamboos. Sci. Rep. 2018, 8, 16933. [Google Scholar] [CrossRef] [PubMed]
- Iroegbu, A.O.C.; Ray, S.S. Bamboos: From bioresource to sustainable materials and chemicals. Sustainability 2021, 13, 12200. [Google Scholar] [CrossRef]
- Zhou, G.; Gu, L.; Chai, Q.; Zhang, J.; Zhu, W.; Zhang, Z. Carbon footprints labeling of bamboo products: A powerful tool to promote innovative development of bamboo industry. World Bamboo Ratt. 2022, 20, 29. [Google Scholar]
- Chen, M.; Guo, L.; Ramakrishnan, M.; Fei, Z.; Vinod, K.K.; Ding, Y.; Jiao, C.; Gao, Z.; Zha, R.; Wang, C.; et al. Rapid growth of Moso bamboo (Phyllostachys edulis): Cellular roadmaps, transcriptome dynamics, and environmental factors. Plant Cell 2022, 34, 3577–3610. [Google Scholar] [CrossRef]
- Li, L.; Cheng, Z.; Ma, Y.; Bai, Q.; Li, X.; Cao, Z.; Wu, Z.; Gao, J. The association of hormone signaling genes, transcription and changes in shoot anatomy during moso bamboo growth. Plant Biotechnol. J. 2018, 16, 72–85. [Google Scholar] [CrossRef]
- Fletcher, J.C.; Brand, U.; Running, M.P.; Simon, R.; Meyerowitz, E.M. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 1999, 283, 1911–1914. [Google Scholar] [CrossRef]
- Opsahl-Ferstad, H.G.; Le Deunff, E.; Dumas, C.; Rogowsky, P.M. ZmEsr, a novel endosperm-specific gene expressed in a restricted region around the maize embryo. Plant J. Cell Mol. Biol. 1997, 12, 235–246. [Google Scholar] [CrossRef] [PubMed]
- April, H.H.; Peter, M.G.; Brett, J.F. The structure and activity of modulation suppressing CLE peptide hormones of legumes. Funct. Plant Biol. 2015, 42, 229–238. [Google Scholar]
- Imin, N.; Patel, N.; Corcilius, L.; Payne, R.J.; Djordjevic, M.A. CLE peptide tri-arabinosylation and peptide domain sequence composition are essential for SUNN-dependent autoregulation of nodulation in Medicago truncatula. New Phytol. 2018, 218, 73–80. [Google Scholar] [CrossRef]
- Shinohara, H.; Matsubayashi, Y. Chemical Synthesis of Arabidopsis CLV3 Glycopeptide Reveals the Impact of Hydroxyproline Arabinosylation on Peptide Conformation and Activity. Plant Cell Physiol. 2013, 54, 369–374. [Google Scholar] [CrossRef]
- Goad, D.M.; Zhu, C.; Kellogg, E.A. Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function. New Phytol. 2017, 216, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Oelkers, K.; Goffard, N.; Weiller, G.F.; Gresshoff, P.M.; Mathesius, U.; Frickey, T. Bioinformatic analysis of the CLE signaling peptide family. BMC Plant Biol. 2009, 9, 1. [Google Scholar] [CrossRef]
- Sawa, S.; Kinoshita, A.; Betsuyaku, S.; Fukuda, H. A large family of genes that share homology with CLE domain in Arabidopsis and rice. Plant Signal. Behav. 2008, 3, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Il Je, B.; Gruel, J.; Lee, Y.K.; Bommert, P.; Arevalo, E.D.; Eveland, A.L.; Wu, Q.; Goldshmidt, A.; Meeley, R.; Bartlett, M.; et al. Signaling from maize organ primordia via FASCIATED EAR3 regulates stem cell proliferation and yield traits. Nat. Genet. 2016, 48, 785–795. [Google Scholar]
- Han, H.; Zhang, G.; Wu, M.; Wang, G. Identification and characterization of the Populus trichocarpa CLE family. BMC Genom. 2016, 17, 174–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Song, Y.; Wang, J. Genome-wide characterization, expression and functional analysis of CLV3/ESR gene family in tomato. BMC Genom. 2014, 15, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Hastwell, A.H.; Gresshoff, P.M.; Ferguson, B.J. Genome-wide annotation and characterization of CLAVATA/ESR (CLE) peptide hormones of soybean (Glycine max) and common bean (Phaseolus vulgaris), and their orthologues of Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 5271–5287. [Google Scholar] [CrossRef]
- Hong, L.; Fletcher, J.C. Stem cells: Engines of plant growth and development. Int. J. Mol. Sci. 2023, 24, 14889. [Google Scholar] [CrossRef]
- Mueller, R.; Bleckmann, A.; Simon, R. The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell 2008, 20, 934–946. [Google Scholar] [CrossRef]
- Kinoshita, A.; Betsuyaku, S.; Osakabe, Y.; Mizuno, S.; Nagawa, S.; Stahl, Y.; Simon, R.; Yamaguchi-Shinozaki, K.; Fukuda, H.; Sawa, S. RPK2 is an essential receptor-like kinase that transmits the CLV3 signal in Arabidopsis. Development 2010, 137, 4327. [Google Scholar] [CrossRef][Green Version]
- Kayes, J.M.; Clark, S.E. CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Dev. (Camb. Engl.) 1998, 125, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Zhou, C.; Li, Y.J.; Yu, Y.; Tang, L.P.; Zhang, W.J.; Yao, W.J.; Huang, R.; Laux, T.; Zhang, X.S. Integration of pluripotency pathways regulates stem cell maintenance in the Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA 2020, 117, 22561–22571. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, M.; Jürgens, G.; Laux, T. The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development 2002, 129, 3195–3206. [Google Scholar] [CrossRef] [PubMed]
- Stahl, Y.; Wink, R.H.; Ingram, G.C.; Simon, R. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr. Biol. 2009, 19, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Hobe, M.; Müller, R.; Grünewald, M.; Brand, U.; Simon, R. Loss of CLE40, a protein functionally equivalent to the stem cell restricting signal CLV3, enhances root waving in Arabidopsis. Dev. Genes Evol. 2003, 213, 371–381. [Google Scholar] [CrossRef]
- Yamaguchi, Y.L.; Ishida, T.; Yoshimura, M.; Imamura, Y.; Shimaoka, C.; Sawa, S. A collection of mutants for CLE-Peptide-Encoding genes in Arabidopsis generated by CRISPR/Cas9-mediated gene targeting. Plant Cell Physiol. 2017, 58, 1848–1856. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Shinohara, H.; Kondo, Y.; Inoue, A.; Nakanomyo, I.; Ogawa, M.; Sawa, S.; Ohashi-Ito, K.; Matsubayashi, Y.; Fukuda, H. Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc. Natl. Acad. Sci. USA 2008, 105, 15208–15213. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Ryu, H.; Rho, S.; Hill, K. A secreted peptide acts on BIN2-mediated phosphorylation of ARFs to potentiate auxin response during lateral root development. Nat. Cell Biol. 2014, 16, 66–78. [Google Scholar] [CrossRef]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 2018, 556, 235–242. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, W.; Tian, H.; Zheng, K.; Dai, X.; Liu, S.; Hu, Q.; Wang, X.; Liu, B.; Wang, S. An auxin responsive CLE gene regulates shoot apical meristem development in Arabidopsis. Front. Plant Sci. 2015, 6, 295. [Google Scholar] [CrossRef]
- Whitford, R.; Fernandez, A.; De Groodt, R.; Ortega, E.; Hilson, P. Plant CLE peptides from two distinct functional classes synergistically induce division of vascular cells. Proc. Natl. Acad. Sci. USA 2008, 105, 18625–18630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gao, Z.; Wang, L.; Wang, J.; Wang, S.; Fei, B.; Chen, C.; Shi, C.; Liu, X.; Zhang, H.; et al. Chromosome-level reference genome and alternative splicing atlas of moso bamboo (Phyllostachys edulis). Gigascience 2018, 7, giy115. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.; Frank, M.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Kondo, Y.; Fukuda, H. Establishment and maintenance of vascular cell communities through local signaling. Curr. Opin. Plant Biol. 2011, 14, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Hou, D.; Ge, W.; Li, X.; Xie, L.; Zheng, H.; Cai, M.; Liu, J.; Gao, J. Integrated mRNA, MicroRNA transcriptome and degradome analyses provide insights into stamen development in Moso Bamboo. Plant Cell Physiol. 2020, 61, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Mu, C.; Li, X.; Cheng, W.; Cai, M.; Wu, C.; Jiang, J.; Fang, H.; Bai, Y.; Zheng, H.; et al. Single-cell transcriptome atlas reveals spatiotemporal developmental trajectories in the basal roots of moso bamboo (Phyllostachys edulis). Hortic. Res. 2023, 10, uhad122. [Google Scholar] [CrossRef]
- Betsuyaku, S.; Sawa, S.; Yamada, M. The function of the CLE peptides in plant development and plant-microbe interactions. Arab. Book 2011, 9, e0149. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Kondo, Y.; Fukuda, H. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 2010, 22, 2618–2629. [Google Scholar] [CrossRef]
- Willoughby, A.C.; Nimchuk, Z.L. WOX going on: CLE peptides in plant development. Curr. Opin. Plant Biol. 2021, 63, 102056. [Google Scholar] [CrossRef]
- Breiden, M.; Olsson, V.; Bluemke, P.; Schlegel, J.; Gustavo-Pinto, K.; Dietrich, P.; Butenko, M.A.; Simon, R. The cell fate controlling CLE40 peptide requires CNGCs to trigger highly localized Ca2+ transients in Arabidopsis thaliana root meristems. Plant Cell Physiol. 2021, 62, 1290–1301. [Google Scholar] [CrossRef]
- Richards, S.; Wink, R.H.; Simon, R. Mathematical modelling of WOX5- and CLE40-mediated columella stem cell homeostasis in Arabidopsis. J. Exp. Bot. 2015, 66, 5375–5384. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Vassileva, V.; De, R. Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 2008, 322, 594–597. [Google Scholar]
- Drisch, R.; Stahl, Y. Function and regulation of transcription factors involved in root apical meristem and stem cell maintenance. Front. Plant Sci. 2015, 6, 505. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, S.; Rodriguez-Villalon, A.; Santuari, L.; Wyser-Rmili, C.; Ragni, L.; Hardtke, C. Suppression of Arabidopsis protophloem differentiation and root meristem growth by CLE45 requires the receptor-like kinase BAM3. Proc. Natl. Acad. Sci. USA 2013, 110, 7074–7079. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, G.; Wu, M. CLE Peptide Signaling and crosstalk with phytohormones and environmental stimuli. Front. Plant Sci. 2015, 6, 1211. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Khan, M.H.U.; Ahmar, S.; Fan, C. Comprehensive study and multipurpose role of the CLV3/ESR-related (CLE) genes family in plant growth and development. J. Cell. Physiol. 2021, 236, 2298–2317. [Google Scholar] [CrossRef]
- Yang, J.H.; Lee, K.-H.; Du, Q.; Yang, S.; Yuan, B.; Qi, L.; Wang, H. A membrane-associated NAC domain transcription factor XVP interacts with TDIF co-receptor and regulates vascular meristem activity. New Phytol. 2020, 226, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, G.; Kiyosue, T.; Hirakawa, Y. Control of stem cell behavior by CLE-JINGASA signaling in the shoot apical meristem. Curr. Biol. 2023, 33, 5121–5131.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Evolutionary and Functional Analysis of WUSCHEL-Related Homeobox Family of Moso Bamboo. Doctoral Dissertation, Chinese Academy of Forestry, Beijing, China, 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Bai, Y.; Cai, M.; Mu, C.; Zheng, H.; Cheng, Z.; Xie, Y.; Gao, J. Integrative analysis of exogenous auxin mediated plant height regulation in Moso bamboo (Phyllostachys edulis). Ind. Crops Prod. 2023, 200, 116852. [Google Scholar] [CrossRef]
- Bai, Y.; Cai, M.; Mu, C.; Cheng, W.; Zheng, H.; Cheng, Z.; Li, J.; Mu, S.; Gao, J. New insights into the local Auxin biosynthesis and its effects on the rapid growth of Moso Bamboo (Phyllostachys edulis). Front. Plant Sci. 2022, 13, 858686. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, C.; Cheng, W.; Fang, H.; Geng, R.; Jiang, J.; Cheng, Z.; Gao, J. Uncovering PheCLE1 and PheCLE10 Promoting Root Development Based on Genome-Wide Analysis. Int. J. Mol. Sci. 2024, 25, 7190. https://doi.org/10.3390/ijms25137190
Mu C, Cheng W, Fang H, Geng R, Jiang J, Cheng Z, Gao J. Uncovering PheCLE1 and PheCLE10 Promoting Root Development Based on Genome-Wide Analysis. International Journal of Molecular Sciences. 2024; 25(13):7190. https://doi.org/10.3390/ijms25137190
Chicago/Turabian StyleMu, Changhong, Wenlong Cheng, Hui Fang, Ruiman Geng, Jutang Jiang, Zhanchao Cheng, and Jian Gao. 2024. "Uncovering PheCLE1 and PheCLE10 Promoting Root Development Based on Genome-Wide Analysis" International Journal of Molecular Sciences 25, no. 13: 7190. https://doi.org/10.3390/ijms25137190
APA StyleMu, C., Cheng, W., Fang, H., Geng, R., Jiang, J., Cheng, Z., & Gao, J. (2024). Uncovering PheCLE1 and PheCLE10 Promoting Root Development Based on Genome-Wide Analysis. International Journal of Molecular Sciences, 25(13), 7190. https://doi.org/10.3390/ijms25137190




