APETALA2-like Floral Homeotic Protein Up-Regulating FaesAP1_2 Gene Involved in Floral Development in Long-Homostyle Common Buckwheat
Abstract
:1. Introduction
2. Results
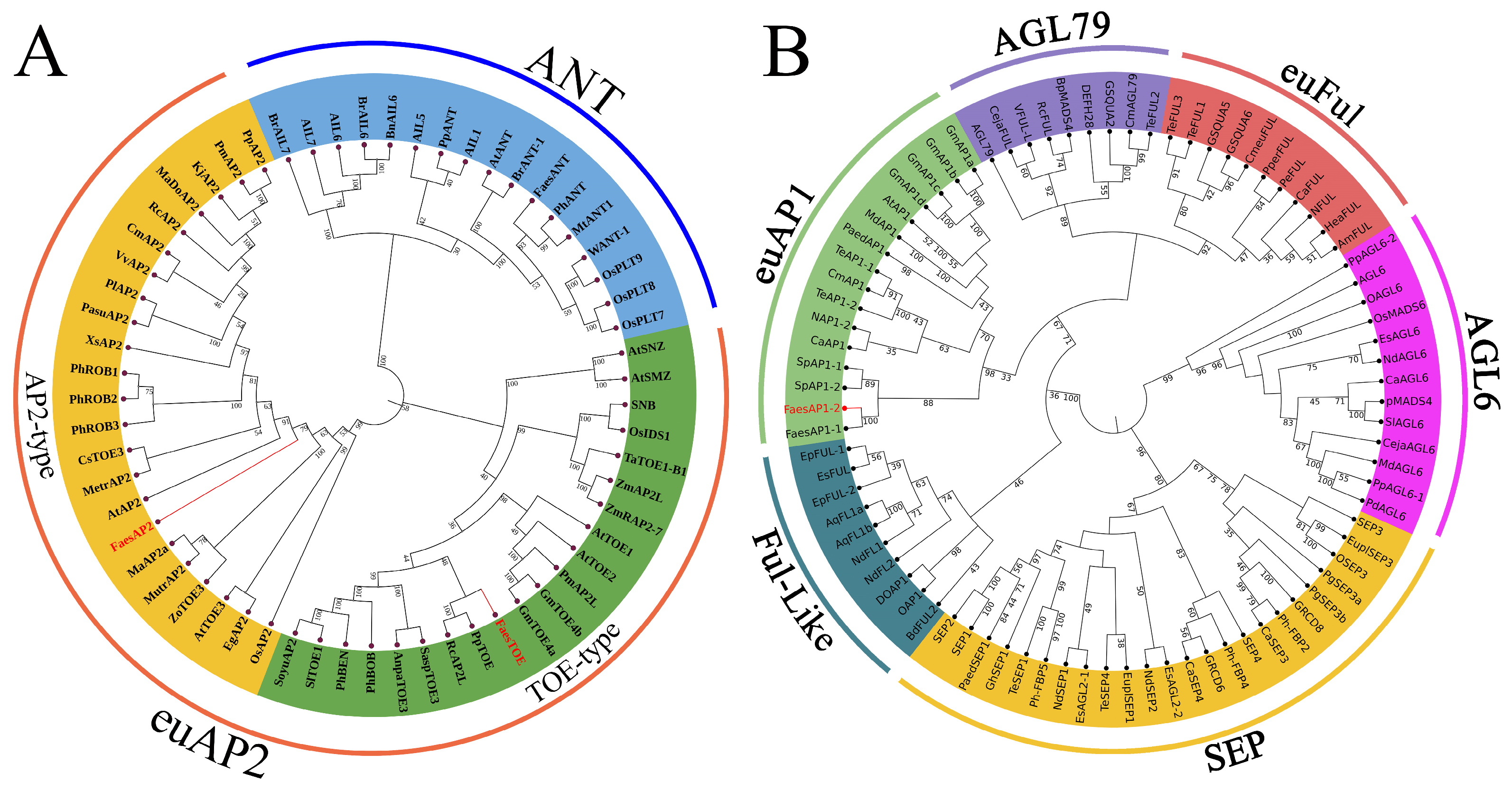
2.1. Isolation and Sequence Analysis of APETALA2 Homologous Genes, FaesAP1_2, and Its Promoter from Long-Homostyle Common Buckwheat
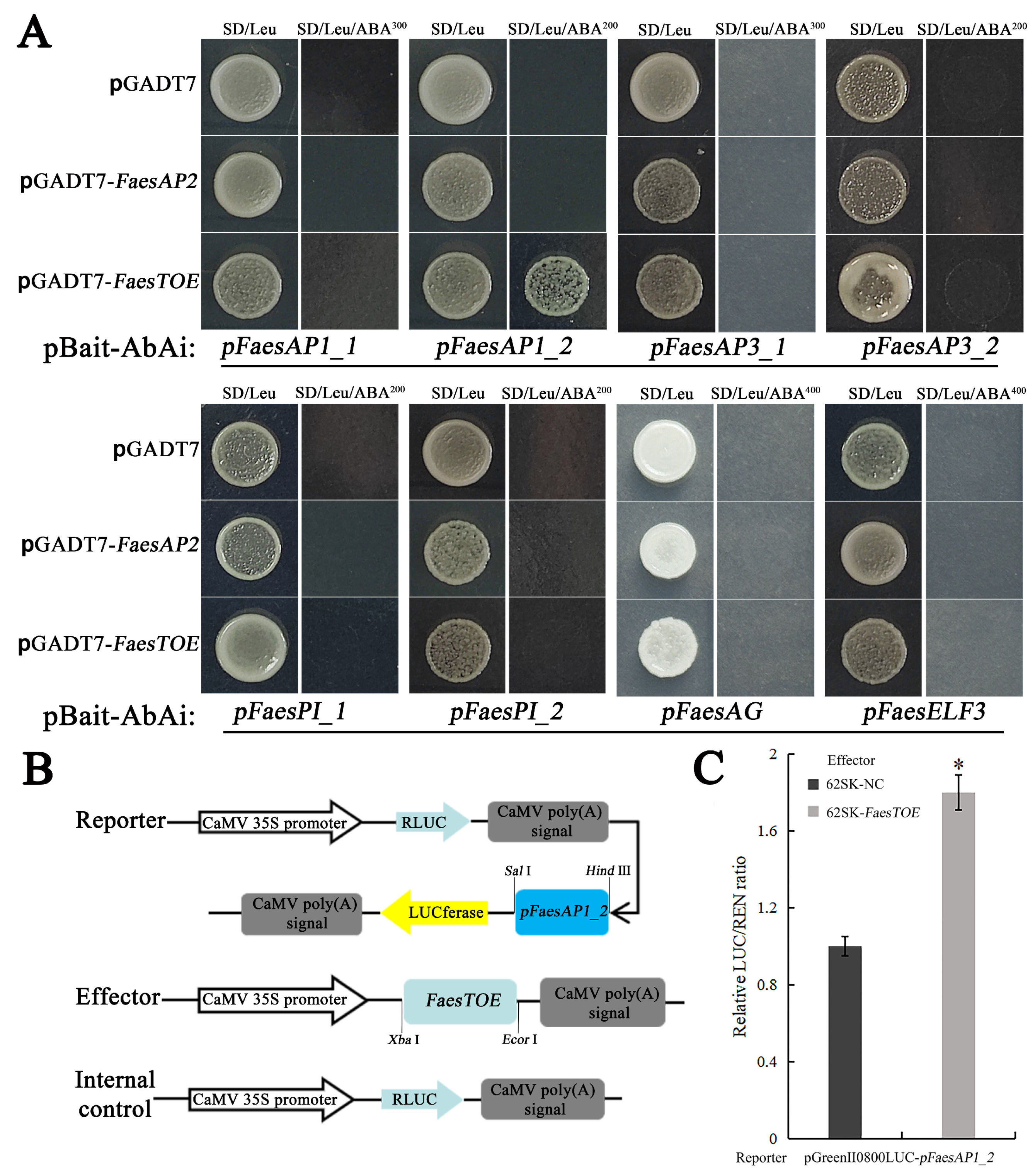
2.2. FaesTOE Up-Regulate FaesAP1_2 Involved in Floral Organ Development
2.3. Expression Analysis of FaesAP2 and FaesTOE in LH Flower F. esculentum
2.4. Characterization of FaesAP2, FaesTOE and FaesAP1_2-Silenced Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Isolation and Characterization of APETALA2 Floral Homeotic Genes Long-Homostyle Common Buckwheat
4.3. Isolation and Sequence Analysis of FaesAP1_2 and FaesAP1_2 Promoter (pFaesAP1_2) from Long-Homostyle Common Buckwheat
4.4. Yeast One-Hybrid Assay
4.5. Dual-Luciferase Reporter Assay
4.6. Expression Analysis of FaesAP2 and FaesTOE
4.7. VIGS Assay in Long-Homostyle Buckwheat
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowman, J.L.; Smyth, D.R.; Meyerowitz, E.M. Genetic interactions among floral homeotic genes of Arabidopsis. Development 1991, 112, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.T.; Girke, T.; Liu, X.; Yant, L.; Schmid, M.; Chen, X. The floral homeotic protein APETALA2 recognizes and acts through an AT-rich sequence element. Development 2012, 139, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shi, T.; Zheng, B.; Yumul, R.E.; Liu, X.; You, C.; Gao, Z.; Xiao, L.; Chen, X. APETALA2 antagonizes the transcriptional activity of AGAMOUS in regulating floral stem cells in Arabidopsis thaliana. New Phytol. 2017, 215, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, I.; Menezes de Moura, S.; Alves-Ferreira, M.; Ferrándiz, C.; Balanzà, V. Identification of Players Controlling Meristem Arrest Downstream of the FRUITFULL-APETALA2 Pathway. Plant Physiol. 2020, 184, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Yant, L.; Mathieu, J.; Dinh, T.T.; Ott, F.; Lanz, C.; Wollmann, H.; Chen, X.; Schmid, M. Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 2010, 22, 2156–2170. [Google Scholar] [CrossRef]
- Balanzà, V.; Martínez-Fernández, I.; Sato, S.; Yanofsky, M.F.; Kaufmann, K.; Angenent, G.C.; Bemer, M.; Ferrándiz, C. Genetic control of meristem arrest and life span in Arabidopsis by a FRUITFULL-APETALA2 pathway. Nat. Commun. 2018, 9, 565. [Google Scholar] [CrossRef]
- Ó’Maoiléidigh, D.S.; Van Driel, A.D.; Singh, A.; Sang, Q.; Le Bec, N.; Vincent, C.; De Olalla, E.B.G.; Vayssières, A.; Romera Branchat, M.; Severing, E. Systematic analyses of the MIR172 family members of Arabidopsis define their distinct roles in regulation of APETALA2 during floral transition. PLoS Biol. 2021, 19, e3001043. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, S.; Wang, X.; Liu, J.; Guo, X.; Mu, J.; Tian, J.; Wang, X. Defective APETALA2 Genes Lead to Sepal Modification in Brassica Crops. Front. Plant Sci. 2018, 9, 367. [Google Scholar] [CrossRef]
- Kim, S.; Soltis, P.S.; Wall, K.; Soltis, D.E. Phylogeny and domain evolution in the APETALA2-like gene family. Mol. Biol. Evol. 2006, 23, 107–120. [Google Scholar] [CrossRef]
- Morel, P.; Heijmans, K.; Rozier, F.; Zethof, J.; Chamot, S.; Bento, S.R.; Vialette-Guiraud, A.; Chambrier, P.; Trehin, C.; Vandenbussche, M. Divergence of the floral A-function between an asterid and a rosid species. Plant Cell 2017, 29, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, L.; Zeng, L.; Zhang, C.; Ma, H. Arabidopsis TOE proteins convey a photoperiodic signal to antagonize CONSTANS and regulate flowering time. Genes Dev. 2015, 29, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Kerstens, M.H.; Schranz, M.E.; Bouwmeester, K. Phylogenomic analysis of the APETALA2 transcription factor subfamily across angiosperms reveals both deep conservation and lineage-specific patterns. Plant J. 2020, 103, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Morel, P.; Heijmans, K.; Ament, K.; Chopy, M.; Trehin, C.; Chambrier, P.; Rodrigues Bento, S.; Bimbo, A.; Vandenbussche, M. The Floral C-Lineage Genes Trigger Nectary Development in Petunia and Arabidopsis. Plant Cell 2018, 30, 2020–2037. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, J.; Zhu, M.; Miao, X.; Shi, Z. OsMADS1 Represses microRNA172 in Elongation of Palea/Lemma Development in Rice. Front. Plant Sci. 2016, 7, 1891. [Google Scholar] [CrossRef]
- Yang, F.X.; Zhu, G.F.; Wang, Z.; Liu, H.L.; Huang, D. A putative miR172-targeted CeAPETALA2-like gene is involved in floral patterning regulation of the orchid Cymbidium ensifolium. Genet. Mol. Res. 2015, 14, 12049–12061. [Google Scholar] [CrossRef]
- Zumajo-Cardona, C.; Pabón-Mora, N.; Ambrose, B.A. The Evolution of euAPETALA2 Genes in Vascular Plants: From Plesiomorphic Roles in Sporangia to Acquired Functions in Ovules and Fruits. Mol. Biol. Evol. 2021, 38, 2319–2336. [Google Scholar] [CrossRef] [PubMed]
- Brockington, S.F.; Rudall, P.J.; Frohlich, M.W.; Oppenheimer, D.G.; Soltis, P.S.; Soltis, D.E. ‘Living stones’ reveal alternative petal identity programs within the core eudicots. Plant J. 2012, 69, 193–203. [Google Scholar] [CrossRef]
- You, W.; Chen, X.; Zeng, L.; Ma, Z.; Liu, Z. Characterization of PISTILLATA-like Genes and Their Promoters from the Distyly Fagopyrum esculentum. Plants 2022, 11, 1047. [Google Scholar] [CrossRef]
- Matsui, K.; Yasui, Y. Buckwheat heteromorphic self-incompatibility: Genetics, genomics and application to breeding. Breed. Sci. 2020, 70, 32–38. [Google Scholar] [CrossRef]
- Fawcett, J.A.; Takeshima, R.; Kikuchi, S.; Yazaki, E.; Katsube-Tanaka, T.; Dong, Y.; Li, M.; Hunt, H.V.; Jones, M.K.; Lister, D.L. Genome sequencing reveals the genetic architecture of heterostyly and domestication history of common buckwheat. Nat. Plants 2023, 9, 1236–1251. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Q.; Zeng, L.; Li, J.; Jiao, X.; Liu, Z. FaesAP3_1 Regulates the FaesELF3 Gene Involved in Filament-Length Determination of Long-Homostyle Fagopyrum esculentum. Int. J. Mol. Sci. 2022, 23, 14403. [Google Scholar] [CrossRef]
- Li, L.Y.; Fang, Z.W.; Li, X.P.; Liu, Z.X. Isolation and Characterization of the C-class MADS-box Gene from the Distylous Pseudo-cereal Fagopyrum esculentum. J. Plant Biol. 2017, 60, 189–198. [Google Scholar] [CrossRef]
- Liu, Z.; Fei, Y.; Zhang, K.; Fang, Z. Ectopic Expression of a Fagopyrum esculentum APETALA1 Ortholog Only Rescues Sepal Development in Arabidopsis Ap1 Mutant. Int. J. Mol. Sci. 2019, 20, 2021. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, J.; Wang, X.; Liu, Z. Isolation and Characterization of APETALA3 Orthologs and Promoters from the Distylous Fagopyrum esculentum. Plants 2021, 10, 1644. [Google Scholar] [CrossRef]
- Wenkel, S.; Turck, F.; Singer, K.; Gissot, L.; Le Gourrierec, J.; Samach, A.; Coupland, G. CONSTANS and the CCAAT Box Binding Complex Share a Functionally Important Domain and Interact to Regulate Flowering of Arabidopsis. Plant Cell 2006, 18, 2971–2984. [Google Scholar] [CrossRef]
- Rogers, H.J.; Bate, N.; Combe, J.; Sullivan, J.; Sweetman, J.; Swan, C.; Lonsdale, D.M.; Twell, D. Functional Analysis of cis-Regulatory Elements within the Promoter of the Tobacco Late Pollen Gene g10. Plant Mol. Biol. 2001, 45, 577–585. [Google Scholar] [CrossRef]
- Filichkin, S.A.; Leonard, J.M.; Monteros, A.; Liu, P.P.; Nonogaki, H. A Novel Endo-β-Mannanase Gene in Tomato LeMAN5 Is Associated with Anther and Pollen Development. Plant Physiol. 2004, 134, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- De Folter, S.; Angenent, G.C. Trans meets cis in MADS science. Trends Plant Sci. 2006, 11, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Sablowski, R.W.; Moyano, E.; Culianez-Macia, F.A.; Schuch, W.; Martin, C.; Bevan, M. A flower-specific Myb protein activates transcription of phenylpropanoid biosynthetic genes. EMBO J. 1994, 13, 128–137. [Google Scholar] [CrossRef]
- Hartmann, U.; Sagasser, M.; Mehrtens, F.; Stracke, R.; Weisshaar, B. Differential combinatorial interactions of cis-acting elements recognized by R2R3-MYB, BZIP, and BHLH factors control light-responsive and tissue-specific activation of phenylpropanoid biosynthesis genes. Plant Mol. Biol. 2005, 57, 155–171. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L.; encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Ketterling, M.G.; McCarty, D.R. Quantitative statistical analysis of cis-regulatory sequences in ABA/VP1- and CBF/DREB1-regulated genes of Arabidopsis. Plant Physiol. 2005, 139, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zheng, T.; Qiu, L.; Guo, X.; Li, P.; Yong, X.; Li, L.; Ahmad, S.; Wang, J.; Cheng, T.; et al. A 49-bp deletion of PmAP2L results in a double flower phenotype in Prunus mume. Hortic. Res. 2023, 11, uhad278. [Google Scholar] [CrossRef] [PubMed]
- Gattolin, S.; Cirilli, M.; Pacheco, I.; Ciacciulli, A.; Da Silva Linge, C.; Mauroux, J.B.; Lambert, P.; Cammarata, E.; Bassi, D.; Pascal, T.; et al. Deletion of the miR172 target site in a TOE-type gene is a strong candidate variant for dominant double-flower trait in Rosaceae. Plant J. 2018, 96, 358–371. [Google Scholar] [CrossRef]
- Cirilli, M.; Rossini, L.; Chiozzotto, R.; Baccichet, I.; Florio, F.E.; Mazzaglia, A.; Turco, S.; Bassi, D.; Gattolin, S. Less is more: Natural variation disrupting a miR172 gene at the di locus underlies the recessive double-flower trait in peach (P. persica L. Batsch). BMC Plant Biol. 2022, 22, 318. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, X.; Liu, Z.; Bai, J.; Song, J.; Li, R.; Cui, X. Tomato APETALA2 family member SlTOE1 regulates inflorescence branching by repressing SISTER OF TM3. Plant Physiol. 2023, 192, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Gattolin, S.; Cirilli, M.; Chessa, S.; Stella, A.; Bassi, D.; Rossini, L. Mutations in orthologous PETALOSA TOE-type genes cause a dominant double-flower phenotype in phylogenetically distant eudicots. J. Exp. Bot. 2020, 71, 2585–2595. [Google Scholar] [CrossRef] [PubMed]
- Sundström, J.F.; Nakayama, N.; Glimelius, K.; Irish, V.F. Direct regulation of the floral homeotic APETALA1 gene by APETALA3 and PISTILLATA in Arabidopsis. Plant J. 2006, 46, 593–600. [Google Scholar] [CrossRef]
- Hu, T.; Li, X.; Du, L.; Manuela, D.; Xu, M. LEAFY and APETALA1 down-regulate ZINC FINGER PROTEIN 1 and 8 to release their repression on class B and C floral homeotic genes. Proc. Natl. Acad. Sci. USA 2023, 120, e2221181120. [Google Scholar] [CrossRef]
- Morel, P.; Chambrier, P.; Boltz, V.; Chamot, S.; Rozier, F.; Rodrigues Bento, S.; Trehin, C.; Monniaux, M.; Zethof, J.; Vandenbussche, M. Divergent Functional Diversification Patterns in the SEP/AGL6/AP1 MADS-Box Transcription Factor Superclade. Plant Cell 2019, 31, 3033–3056. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Huang, W.; Li, Z.; Song, C.; Liu, D.; Liu, Y.; Hayward, A.; Liu, Y.; Huang, H.; Wang, Y. Functional and evolutionary analysis of the AP1/SEP/AGL6 superclade of MADS-box genes in the basal eudicot Epimedium sagittatum. Ann. Bot. 2014, 113, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, V.V.; Shahmuradov, I.A.; Salamov, A.A. Identification of promoter regions and regulatory sites. Methods Mol. Biol. 2010, 674, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S.P. Virus-induced gene silencing in tomato. Plant J. 2002, 31, 777–786. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Luo, L.; Jiao, X.; Chen, X.; Liu, Y.; Liu, Z. APETALA2-like Floral Homeotic Protein Up-Regulating FaesAP1_2 Gene Involved in Floral Development in Long-Homostyle Common Buckwheat. Int. J. Mol. Sci. 2024, 25, 7193. https://doi.org/10.3390/ijms25137193
Yang Q, Luo L, Jiao X, Chen X, Liu Y, Liu Z. APETALA2-like Floral Homeotic Protein Up-Regulating FaesAP1_2 Gene Involved in Floral Development in Long-Homostyle Common Buckwheat. International Journal of Molecular Sciences. 2024; 25(13):7193. https://doi.org/10.3390/ijms25137193
Chicago/Turabian StyleYang, Qingyu, Lan Luo, Xinyu Jiao, Xiangjian Chen, Yuzhen Liu, and Zhixiong Liu. 2024. "APETALA2-like Floral Homeotic Protein Up-Regulating FaesAP1_2 Gene Involved in Floral Development in Long-Homostyle Common Buckwheat" International Journal of Molecular Sciences 25, no. 13: 7193. https://doi.org/10.3390/ijms25137193





