Aprotinin (II): Inhalational Administration for the Treatment of COVID-19 and Other Viral Conditions
Abstract
1. Introduction
2. Development of Aprotinin for Respiratory Viral Infections
3. Pharmacodynamic Actions of Aprotinin
3.1. Antiviral Activity
3.2. Anti-Inflammatory Activity
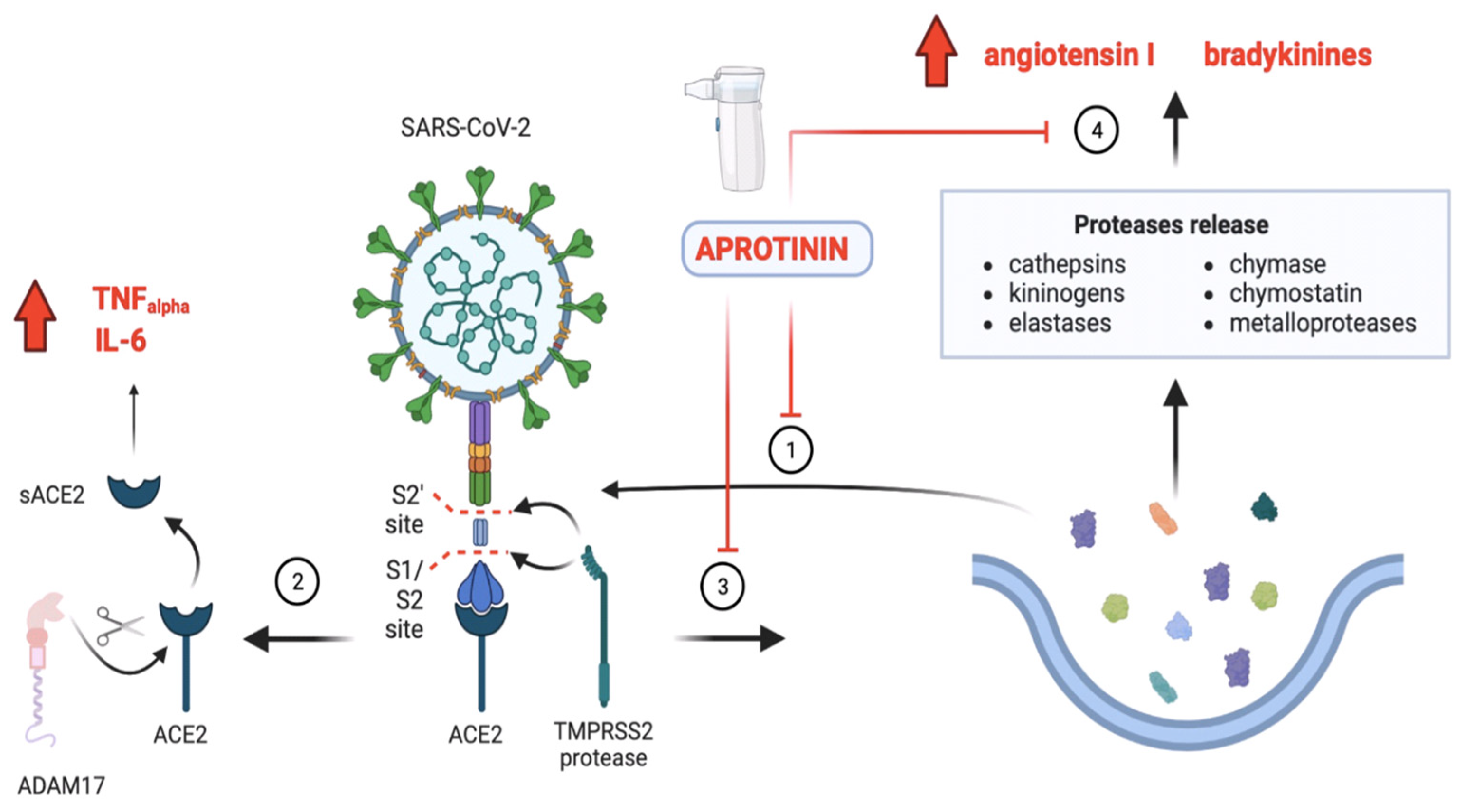
3.2.1. Aprotinin and Its Ability to Re-Establish the Imbalance between KKS and RAAS in COVID-19
3.2.2. Aprotinin Restores Protease and Antiprotease Balance in the Lung
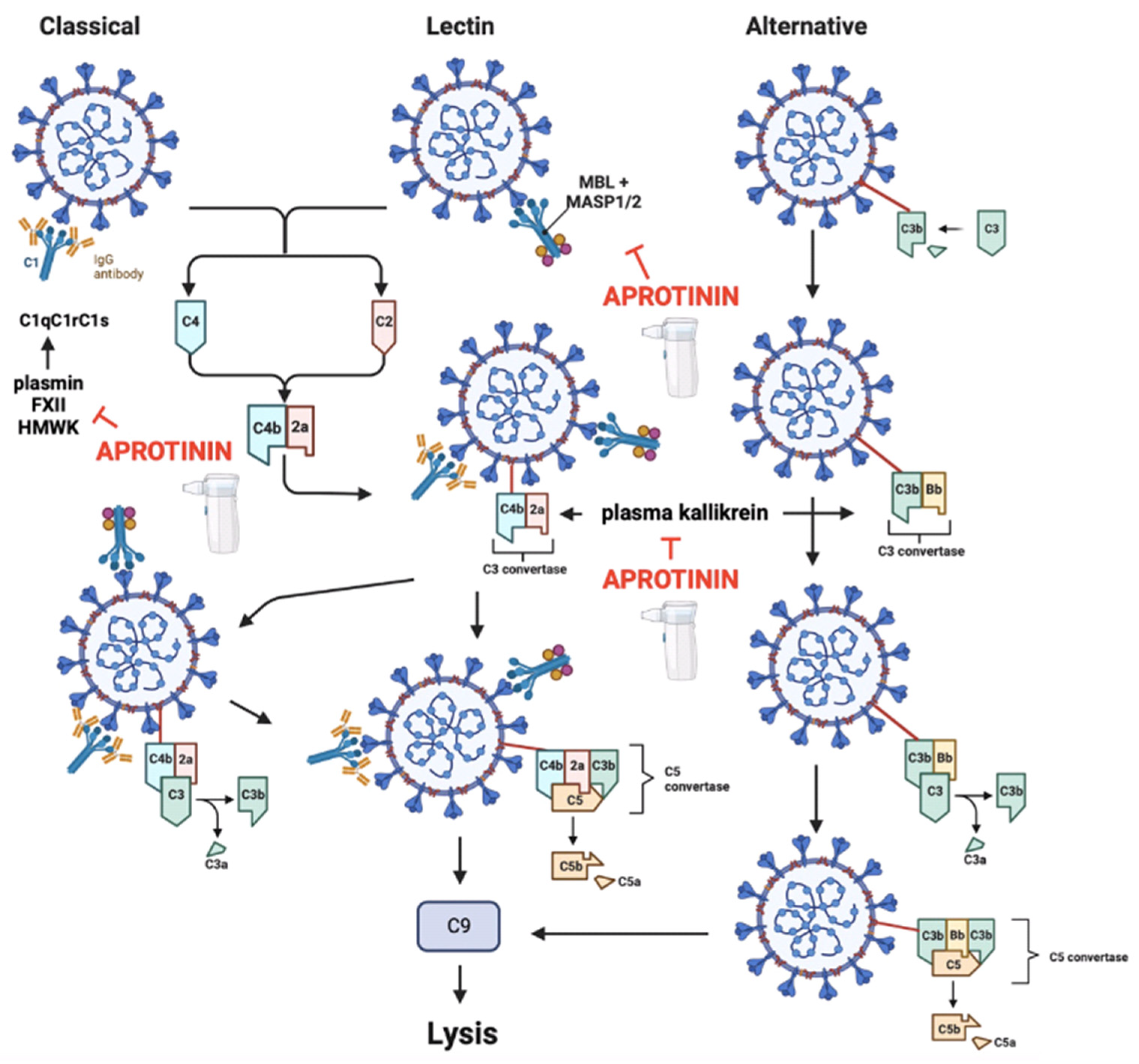
3.2.3. Aprotinin Re-Establishes the Contact System and Complement Activity of the Innate Immune System
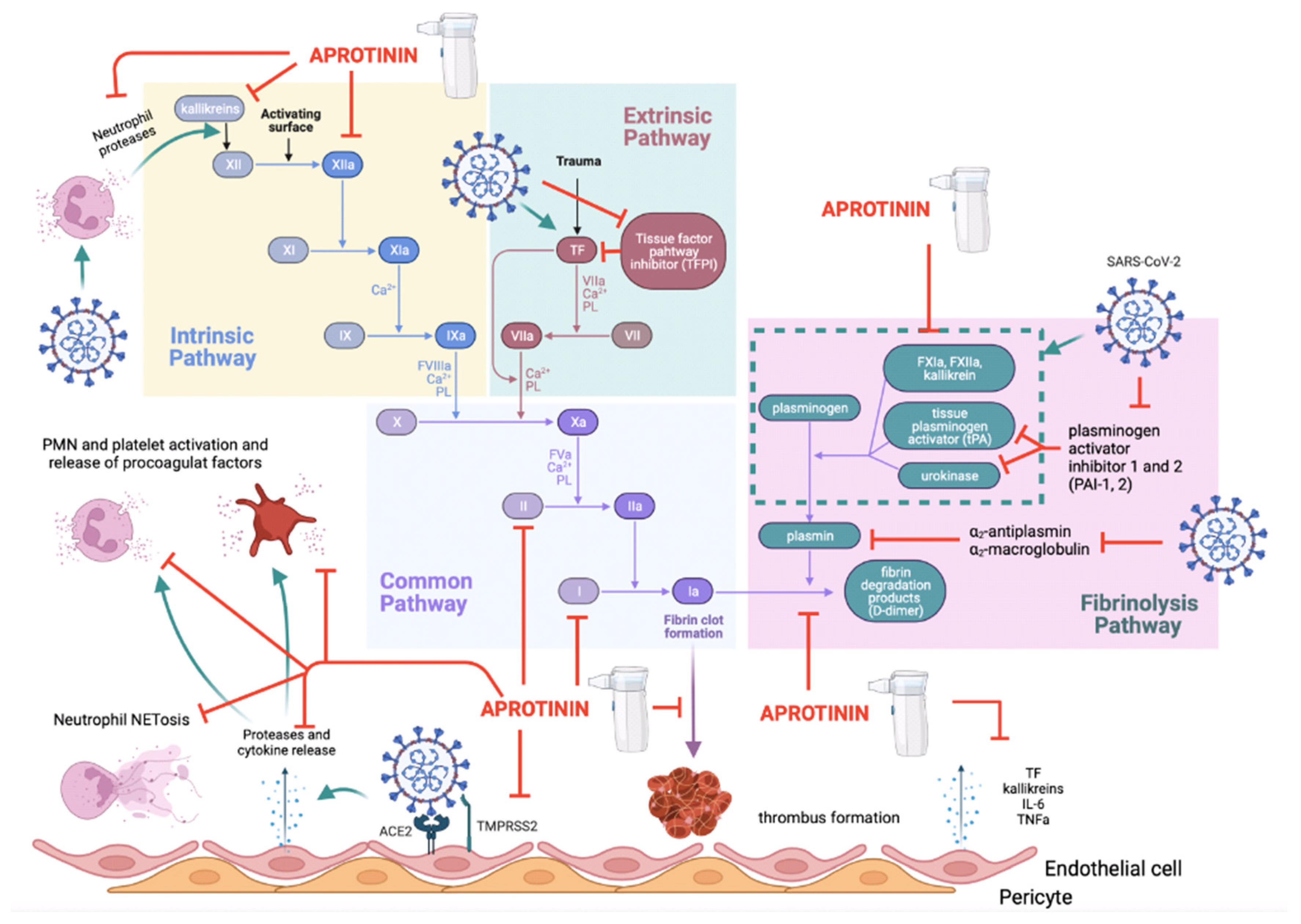
3.3. The Thromboinflammatory Activity of Aprotinin
3.4. Activity against the Symptomatic Processes of COVID-19
4. The Clinical Efficacy of Aprotinin for Treating SARS-CoV-2 in Experimental Animals and in Humans
5. Aprotinin Pharmacokinetics
5.1. Pharmacokinetics in Experimental Animals
5.2. Pharmacokinetics in Humans
5.3. Pulmonary Administration of Aprotinin
5.3.1. Administration Devices
5.3.2. Dose by the Pulmonary Route
5.3.3. Pharmacokinetics of Aprotinin via the Pulmonary Route
6. Toxicity of Aprotinin by the Pulmonary Route of Administration
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forgie, S.; Marrie, T.J. Healthcare-Associated Atypical Pneumonia. Semin. Respir. Crit. Care Med. 2009, 30, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y. Protease-Dependent Virus Tropism and Pathogenicity. Trends Microbiol. 1993, 1, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Izaguirre, G. The Proteolytic Regulation of Virus Cell Entry by Furin and Other Proprotein Convertases. Viruses 2019, 11, 837. [Google Scholar] [CrossRef] [PubMed]
- Zhirnov, O.P.; Klenk, H.D.; Wright, P.F. Aprotinin and Similar Protease Inhibitors as Drugs against Influenza. Antivir. Res. 2011, 92, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.; Wunderer, G. Biochemistry and Applications of Aprotinin, the Kallikrein Inhibitor from Bovine Organs. Arzneimittelforschung 1983, 33, 479–494. [Google Scholar] [PubMed]
- Deanda, A.; Spiess, B.D. Aprotinin Revisited. J. Thorac. Cardiovasc. Surg. 2012, 144, 998–1002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhirnov, O.P.; Ovcharenko, A.V.; Bukrinskaya, A.G. Suppression of Influenza Virus Replication in Infected Mice by Protease Inhibitors. J. Gen. Virol. 1984, 65 Pt 1, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Zhirnov, O.P.; Ovcharenko, A.V.; Bukrinskaia, A.G.; Ursaki, L.P.; Ivanova, L.A. Antiviral and therapeutic action of protease inhibitors in viral infections: Experimental and clinical observations. Vopr. Virusol. 1984, 29, 491–497. [Google Scholar] [PubMed]
- Ovcharenko, A.V.; Zhirnov, O.P. Aprotinin Aerosol Treatment of Influenza and Paramyxovirus Bronchopneumonia of Mice. Antivir. Res. 1994, 23, 107–118. [Google Scholar] [CrossRef]
- Zhirnov, O.P.; Kirzhner, L.S.; Ovcharenko, A.V.; Malyshev, N.A. Clinical effectiveness of aprotinin aerosol in influenza and parainfluenza. Vestn. Ross. Akad. Med. Nauk. 1996, 5, 26–31. [Google Scholar] [PubMed]
- Hayashi, T.; Hotta, H.; Itoh, M.; Homma, M. Protection of Mice by a Protease Inhibitor, Aprotinin, against Lethal Sendai Virus Pneumonia. J. Gen. Virol. 1991, 72 Pt 4, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Ebina, T.; Tsukada, K. Protease Inhibitors Prevent the Development of Human Rotavirus-Induced Diarrhea in Suckling Mice. Microbiol. Immunol. 1991, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, M.; Matsuyama, S.; Baba, M.; Suzuki, H.; Shigeta, S. Effects of Protease Inhibitors on Replication of Various Myxoviruses. Antimicrob. Agents Chemother. 1992, 36, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Krasota, A.; Belousova, R.; Duchêne, D.; Larionova, N. In Vitro Inhibition of Bovine Herpes Virus 1 Reproduction with Native and Microencapsulated Proteinase Inhibitor Aprotinin. J. Control Release 2002, 85, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Larionova, N.V.; Malykh, E.V.; Villemson, A.L.; Krasota, A.J.; Duchene, D.; Ollivon, M.; Gernet, M.V.; Belousova, R.V.; Shen, W.-C.; Larionova, N.I. Effect of Membranotropic and Mucoadhesive Formulations of Protein Proteinase Inhibitors on Bovine Herpes Virus-1 Reproduction. Int. J. Pharm. 2003, 256, 191–198. [Google Scholar] [CrossRef]
- Mueller, N.H.; Yon, C.; Ganesh, V.K.; Padmanabhan, R. Characterization of the West Nile Virus Protease Substrate Specificity and Inhibitors. Int. J. Biochem. Cell Biol. 2007, 39, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Steuer, C.; Klein, C.D. Arylcyanoacrylamides as Inhibitors of the Dengue and West Nile Virus Proteases. Bioorg. Med. Chem. 2011, 19, 7318–7337. [Google Scholar] [CrossRef]
- Bojkova, D.; Bechtel, M.; McLaughlin, K.-M.; McGreig, J.E.; Klann, K.; Bellinghausen, C.; Rohde, G.; Jonigk, D.; Braubach, P.; Ciesek, S.; et al. Aprotinin Inhibits SARS-CoV-2 Replication. Cells 2020, 9, 2377. [Google Scholar] [CrossRef]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Van Lam van, T.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; et al. TMPRSS2 and Furin Are Both Essential for Proteolytic Activation of SARS-CoV-2 in Human Airway Cells. Life Sci. Alliance 2020, 3, e202000786. [Google Scholar] [CrossRef]
- Redondo-Calvo, F.J.; Padín, J.F.; Muñoz-Rodríguez, J.R.; Serrano-Oviedo, L.; López-Juárez, P.; Porras Leal, M.L.; González Gasca, F.J.; Rodríguez Martínez, M.; Pérez Serrano, R.; Sánchez Cadena, A.; et al. Aprotinin Treatment against SARS-CoV-2: A Randomized Phase III Study to Evaluate the Safety and Efficacy of a Pan-Protease Inhibitor for Moderate COVID-19. Eur. J. Clin. Investig. 2022, 52, e13776. [Google Scholar] [CrossRef]
- Redondo-Calvo, F.J.; Padín, J.F.; Martínez-Alarcón, J.; Muñoz-Rodríguez, J.R.; Serrano-Oviedo, L.; López-Juárez, P.; Porras Leal, M.L.; González Gasca, F.J.; Rodríguez Martínez, M.; Pérez Serrano, R.; et al. Inhaled Aprotinin Reduces Viral Load in Mild-to-Moderate Inpatients with SARS-CoV-2 Infection. Eur. J. Clin. Investig. 2022, 52, e13850. [Google Scholar] [CrossRef]
- Turk, B. Targeting Proteases: Successes, Failures and Future Prospects. Nat. Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef]
- Greene, C.M.; McElvaney, N.G. Proteases and Antiproteases in Chronic Neutrophilic Lung Disease—Relevance to Drug Discovery. Br. J. Pharmacol. 2009, 158, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Szabo, R.; Wu, Q.; Dickson, R.B.; Netzel-Arnett, S.; Antalis, T.M.; Bugge, T.H. Type II Transmembrane Serine Proteases. Thromb. Haemost. 2003, 90, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Wachtfogel, Y.T.; Kucich, U.; Hack, C.E.; Gluszko, P.; Niewiarowski, S.; Colman, R.W.; Edmunds, L.H. Aprotinin Inhibits the Contact, Neutrophil, and Platelet Activation Systems during Simulated Extracorporeal Perfusion. J. Thorac. Cardiovasc. Surg. 1993, 106, 1–9; discussion 9–10. [Google Scholar] [CrossRef] [PubMed]
- Ivachtchenko, A.V.; Ivashchenko, A.A.; Shkil, D.O.; Ivashchenko, I.A. Aprotinin-Drug against Respiratory Diseases. Int. J. Mol. Sci. 2023, 24, 11173. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Siebeck, M.; Thetter, O.; Jochum, M.; Fritz, H. Aprotinin Concentrations Effective for the Inhibition of Tissue Kallikrein and Plasma Kallikrein in Vitro and in Vivo. Adv. Exp. Med. Biol. 1989, 247, 35–42. [Google Scholar] [CrossRef]
- Moreau, M.E.; Garbacki, N.; Molinaro, G.; Brown, N.J.; Marceau, F.; Adam, A. The Kallikrein-Kinin System: Current and Future Pharmacological Targets. J. Pharmacol. Sci. 2005, 99, 6–38. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, T.; Schnierer, S.; Tschesche, H. Recombinant Aprotinin Homologue with New Inhibitory Specificity for Cathepsin G. Eur. J. Biochem. 1991, 202, 95–99. [Google Scholar] [CrossRef]
- Pintigny, D.; Dachary-Prigent, J. Aprotinin can inhibit the proteolytic activity of thrombin. A fluorescence and an enzymatic study. Eur. J. Biochem. 1992, 207, 89–95. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, W.; Jiang, A.; Chen, J.; Tang, F.; Liu, J.-N. Expression, Purification and Characterization of Aprotinin and a Human Analogue of Aprotinin. Protein Expr. Purif. 2009, 65, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V.; Schuster, A.; Ueki, I.; Boushey, H.A.; Nadel, J.A. Mucus Hypersecretion in Bronchiectasis. The Role of Neutrophil Proteases. Am. Rev. Respir. Dis. 1992, 146, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, B.F.; Gyllstedt, E.; Andersson, K.E. Conversion of angiotensin I to angiotensinII by chymase activity in human pulmonary membranes. Peptides 1997, 18, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Kido, H.; Fukutomi, A.; Katunuma, N. A Novel Membrane-Bound Serine Esterase in Human T4+ Lymphocytes Immunologically Reactive with Antibody Inhibiting Syncytia Induced by HIV-1. Purification and Characterization. J. Biol. Chem. 1990, 265, 21979–21985. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, T.; Schäfers, J.; Gürtler, L.; Kido, H.; Niwa, Y.; Katunuma, N.; Tschesche, H. Inhibition of Tryptase TL2 from Human T4+ Lymphocytes and Inhibition of HIV-1 Replication in H9 Cells by Recombinant Aprotinin and Bikunin Homologues. J. Protein Chem. 1997, 16, 651–660. [Google Scholar] [CrossRef]
- Engel, M.; Hoffmann, T.; Manhart, S.; Heiser, U.; Chambre, S.; Huber, R.; Demuth, H.-U.; Bode, W. Rigidity and Flexibility of Dipeptidyl Peptidase IV: Crystal Structures of and Docking Experiments with DPIV. J. Mol. Biol. 2006, 355, 768–783. [Google Scholar] [CrossRef] [PubMed]
- Abramić, M.; Agić, D. Survey of Dipeptidyl Peptidase III Inhibitors: From Small Molecules of Microbial or Synthetic Origin to Aprotinin. Molecules 2022, 27, 3006. [Google Scholar] [CrossRef] [PubMed]
- Kuyvenhoven, J.P.; Molenaar, I.Q.; Verspaget, H.W.; Veldman, M.G.; Palareti, G.; Legnani, C.; Moolenburgh, S.E.; Terpstra, O.T.; Lamers, C.B.H.W.; van Hoek, B.; et al. Plasma MMP-2 and MMP-9 and Their Inhibitors TIMP-1 and TIMP-2 during Human Orthotopic Liver Transplantation. The Effect of Aprotinin and the Relation to Ischemia/Reperfusion Injury. Thromb. Haemost. 2004, 91, 506–513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chu, S.C.; Hu, D.N.; Yang, S.F.; Yang, P.Y.; Hsieh, Y.S.; Huang, S.M.; Yu, G.; McCormick, S.A. Uveal melanocytes produce matrix metalloproteinases-2 and -9 in vitro. Pigment. Cell Res. 2004, 17, 636–642. [Google Scholar] [CrossRef]
- Ripa, R.; Gilli, P. Protease inhibitors (trasylol and epsilon-aminocaproic acid) on human erythrocytic angiotensinase. Boll. Soc. Ital. Biol. Sper. 1968, 44, 1297–1300. [Google Scholar]
- Dahlheim, H. Analysis and reaction behavior of localized angiotensinase (N) in the juxtaglomerular apparatus of the rat kidney. Pflug. Arch. 1972, 332, R27. [Google Scholar]
- Day, J.R.S.; Landis, R.C.; Taylor, K.M. Aprotinin and the Protease-Activated Receptor 1 Thrombin Receptor: Antithrombosis, Inflammation, and Stroke Reduction. Semin. Cardiothorac. Vasc. Anesth. 2006, 10, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Landis, R.C. Protease Activated Receptors: Clinical Relevance to Hemostasis and Inflammation. Hematol. Oncol. Clin. N. Am. 2007, 21, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Bianchi, C.; Voisine, P.; Sandmeyer, J.; Feng, J.; Sellke, F.W. Aprotinin Inhibits Protease-Dependent Platelet Aggregation and Thrombosis. Ann. Thorac. Surg. 2005, 79, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Gomides, L.F.; Duarte, I.D.; Ferreira, R.G.; Perez, A.C.; Francischi, J.N.; Klein, A. Proteinase-Activated Receptor-4 Plays a Major Role in the Recruitment of Neutrophils Induced by Trypsin or Carrageenan during Pleurisy in Mice. Pharmacology 2012, 89, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Yamaya, M.; Shimotai, Y.; Hatachi, Y.; Lusamba Kalonji, N.; Tando, Y.; Kitajima, Y.; Matsuo, K.; Kubo, H.; Nagatomi, R.; Hongo, S.; et al. The Serine Protease Inhibitor Camostat Inhibits Influenza Virus Replication and Cytokine Production in Primary Cultures of Human Tracheal Epithelial Cells. Pulm. Pharmacol. Ther. 2015, 33, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.V.; Thiel, S.; Jensen, L.; Vorup-Jensen, T.; Koch, C.; Jensenius, J.C. Control of the Classical and the MBL Pathway of Complement Activation. Mol. Immunol. 2000, 37, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Cortesio, C.L.; Jiang, W. Mannan-Binding Lectin-Associated Serine Protease 3 Cleaves Synthetic Peptides and Insulin-like Growth Factor-Binding Protein 5. Arch. Biochem. Biophys. 2006, 449, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Keizer, M.P.; Pouw, R.B.; Kamp, A.M.; Patiwael, S.; Marsman, G.; Hart, M.H.; Zeerleder, S.; Kuijpers, T.W.; Wouters, D. TFPI Inhibits Lectin Pathway of Complement Activation by Direct Interaction with MASP-2. Eur. J. Immunol. 2015, 45, 544–550. [Google Scholar] [CrossRef]
- Vanneste, Y.; Pauwels, S.; Lambotte, L.; Michel, A.; Dimaline, R.; Deschodt-Lanckman, M. Respective Roles of Kallikrein and Endopeptidase 24.11 in the Metabolic Pathway of Atrial Natriuretic Peptide in the Rat. Biochem. J. 1990, 269, 801–806. [Google Scholar] [CrossRef]
- Aoyagi, T.; Wada, T.; Nagai, M.; Kojima, F.; Harada, S.; Takeuchi, T.; Takahashi, H.; Hirokawa, K.; Tsumita, T. Deficiency of Kallikrein-like Enzyme Activities in Cerebral Tissue of Patients with Alzheimer’s Disease. Experientia 1990, 46, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.E.; Springall, D.R.; Robbins, R.A. Aprotinin Is Associated with a Decrease in Nitric Oxide Production during Cardiopulmonary Bypass. Surgery 1997, 121, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.E.; Robbins, R.A. Aprotinin but Not Tranexamic Acid Inhibits Cytokine-Induced Inducible Nitric Oxide Synthase Expression. Anesth. Analg. 1997, 84, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Bruda, N.L.; Hurlbert, B.J.; Hill, G.E. Aprotinin Reduces Nitric Oxide Production in Vitro and in Vivo in a Dose-Dependent Manner. Clin. Sci. 1998, 94, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Adebamiro, A.; Cheng, Y.; Johnson, J.P.; Bridges, R.J. Endogenous Protease Activation of ENaC: Effect of Serine Protease Inhibition on ENaC Single Channel Properties. J. General. Physiol. 2005, 126, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, S.K.; Cui, S.; Vorum, H.; Bregengård, C.; Bjørn, S.E.; Norris, K.; Gliemann, J.; Christensen, E.I. Evidence That Epithelial Glycoprotein 330/Megalin Mediates Uptake of Polybasic Drugs. J. Clin. Investig. 1995, 96, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Rahbar Saadat, Y.; Hosseiniyan Khatibi, S.M.; Zununi Vahed, S.; Ardalan, M. Host Serine Proteases: A Potential Targeted Therapy for COVID-19 and Influenza. Front. Mol. Biosci. 2021, 8, 725528. [Google Scholar] [CrossRef] [PubMed]
- Azouz, N.P.; Klingler, A.M.; Callahan, V.; Akhrymuk, I.V.; Elez, K.; Raich, L.; Henry, B.M.; Benoit, J.L.; Benoit, S.W.; Noé, F.; et al. Alpha 1 Antitrypsin Is an Inhibitor of the SARS-CoV-2-Priming Protease TMPRSS2. Pathog. Immun. 2021, 6, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Bollavaram, K.; Leeman, T.H.; Lee, M.W.; Kulkarni, A.; Upshaw, S.G.; Yang, J.; Song, H.; Platt, M.O. Multiple Sites on SARS-CoV-2 Spike Protein Are Susceptible to Proteolysis by Cathepsins B, K, L, S, and V. Protein Sci. 2021, 30, 1131–1143. [Google Scholar] [CrossRef]
- Zabiegala, A.; Kim, Y.; Chang, K.-O. Roles of Host Proteases in the Entry of SARS-CoV-2. Anim. Dis. 2023, 3, 12. [Google Scholar] [CrossRef]
- Minakshi, R.; Padhan, K. The YXXΦ Motif within the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) 3a Protein Is Crucial for Its Intracellular Transport. Virol. J. 2014, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.M.; Jabs, S.; Qiao, W.; Varanese, L.D.; Schweizer, M.; Mosen, P.R.; Riley, N.M.; Klüssendorf, M.; Zengel, J.R.; Flynn, R.A.; et al. The Human Disease Gene LYSET Is Essential for Lysosomal Enzyme Transport and Viral Infection. Science 2022, 378, eabn5648. [Google Scholar] [CrossRef] [PubMed]
- Scarcella, M.; d’Angelo, D.; Ciampa, M.; Tafuri, S.; Avallone, L.; Pavone, L.M.; De Pasquale, V. The Key Role of Lysosomal Protease Cathepsins in Viral Infections. Int. J. Mol. Sci. 2022, 23, 9089. [Google Scholar] [CrossRef] [PubMed]
- Buijsers, B.; Yanginlar, C.; de Nooijer, A.; Grondman, I.; Maciej-Hulme, M.L.; Jonkman, I.; Janssen, N.A.F.; Rother, N.; de Graaf, M.; Pickkers, P.; et al. Increased Plasma Heparanase Activity in COVID-19 Patients. Front. Immunol. 2020, 11, 575047. [Google Scholar] [CrossRef] [PubMed]
- Kinaneh, S.; Khamaysi, I.; Karram, T.; Hamoud, S. Heparanase as a Potential Player in SARS-CoV-2 Infection and Induced Coagulopathy. Biosci. Rep. 2021, 41, BSR20210290. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Smits, S.L.; Provacia, L.B.; van den Brand, J.M.A.; Wiersma, L.; Ouwendijk, W.J.D.; Bestebroer, T.M.; Spronken, M.I.; van Amerongen, G.; Rottier, P.J.M.; et al. Adenosine Deaminase Acts as a Natural Antagonist for Dipeptidyl Peptidase 4-Mediated Entry of the Middle East Respiratory Syndrome Coronavirus. J. Virol. 2014, 88, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Cao, L.; Ma, C.; Tortorici, M.A.; Liu, C.; Si, J.; Liu, P.; Gu, M.; Walls, A.C.; Wang, C.; et al. Close Relatives of MERS-CoV in Bats Use ACE2 as Their Functional Receptors. Nature 2022, 612, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Vankadari, N.; Wilce, J.A. Emerging WuHan (COVID-19) Coronavirus: Glycan Shield and Structure Prediction of Spike Glycoprotein and Its Interaction with Human CD26. Emerg. Microbes Infect. 2020, 9, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Sebastián-Martín, A.; Sánchez, B.G.; Mora-Rodríguez, J.M.; Bort, A.; Díaz-Laviada, I. Role of Dipeptidyl Peptidase-4 (DPP4) on COVID-19 Physiopathology. Biomedicines 2022, 10, 2026. [Google Scholar] [CrossRef]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single Cell RNA Sequencing of 13 Human Tissues Identify Cell Types and Receptors of Human Coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Arruda-Junior, D.F.; Salles, T.A.; Martins, F.L.; Antonio, E.L.; Tucci, P.J.F.; Gowdak, L.H.W.; Tavares, C.A.M.; Girardi, A.C. Unraveling the Interplay between Dipeptidyl Peptidase 4 and the Renin-Angiotensin System in Heart Failure. Life Sci. 2022, 305, 120757. [Google Scholar] [CrossRef] [PubMed]
- Bassendine, M.F.; Bridge, S.H.; McCaughan, G.W.; Gorrell, M.D. COVID-19 and Comorbidities: A Role for Dipeptidyl Peptidase 4 (DPP4) in Disease Severity? J. Diabetes 2020, 12, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Solerte, S.B.; Di Sabatino, A.; Galli, M.; Fiorina, P. Dipeptidyl Peptidase-4 (DPP4) Inhibition in COVID-19. Acta Diabetol. 2020, 57, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Schmaier, A.H. The Plasma Kallikrein-Kinin System Counterbalances the Renin-Angiotensin System. J. Clin. Investig. 2002, 109, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Bekassy, Z.; Lopatko Fagerström, I.; Bader, M.; Karpman, D. Crosstalk between the Renin-Angiotensin, Complement and Kallikrein-Kinin Systems in Inflammation. Nat. Rev. Immunol. 2022, 22, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The Pivotal Link between ACE2 Deficiency and SARS-CoV-2 Infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Nery, L.; Martins, L.; Jabour, L.; Dias, R.; Simões E Silva, A.C. Downregulation of Membrane-Bound Angiotensin Converting Enzyme 2 (ACE2) Receptor Has a Pivotal Role in COVID-19 Immunopathology. Curr. Drug Targets 2021, 22, 254–281. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, S.; Sisti, D.; Vandini, D.; Barocci, S.; Sudano, M.; Carlotti, E.; Teng, J.L.L.; Zamai, L. Circulating ACE2 Level and Zinc/Albumin Ratio as Potential Biomarkers for a Precision Medicine Approach to COVID-19. Adv. Biol. Regul. 2023, 89, 100973. [Google Scholar] [CrossRef]
- Liu, P.P.; Blet, A.; Smyth, D.; Li, H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation 2020, 142, 68–78. [Google Scholar] [CrossRef]
- El-Arif, G.; Khazaal, S.; Farhat, A.; Harb, J.; Annweiler, C.; Wu, Y.; Cao, Z.; Kovacic, H.; Abi Khattar, Z.; Fajloun, Z.; et al. Angiotensin II Type I Receptor (AT1R): The Gate towards COVID-19-Associated Diseases. Molecules 2022, 27, 2048. [Google Scholar] [CrossRef]
- Tolouian, R.; Vahed, S.Z.; Ghiyasvand, S.; Tolouian, A.; Ardalan, M. COVID-19 Interactions with Angiotensin-Converting Enzyme 2 (ACE2) and the Kinin System; Looking at a Potential Treatment. J. Ren. Inj. Prev. 2020, 9, e19. [Google Scholar] [CrossRef]
- Garvin, M.R.; Alvarez, C.; Miller, J.I.; Prates, E.T.; Walker, A.M.; Amos, B.K.; Mast, A.E.; Justice, A.; Aronow, B.; Jacobson, D. A Mechanistic Model and Therapeutic Interventions for COVID-19 Involving a RAS-Mediated Bradykinin Storm. Elife 2020, 9, e59177. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.P.; Ghebrehiwet, B. Pathways for Bradykinin Formation and Interrelationship with Complement as a Cause of Edematous Lung in COVID-19 Patients. J. Allergy Clin. Immunol. 2021, 147, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, A.; Iqbal, M.S.; Sultan, S.; Alhuthali, R.A.; Alshubaili, D.I.; Sayyam, R.S.; Abyad, L.M.; Qasem, A.H.; Arbaeen, A.F. Dysregulated Bradykinin: Mystery in the Pathogenesis of COVID-19. Mediat. Inflamm. 2022, 2022, 7423537. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.A.; Roche, R. A Hypothesized Role for Dysregulated Bradykinin Signaling in COVID-19 Respiratory Complications. FASEB J. 2020, 34, 7265–7269. [Google Scholar] [CrossRef]
- Osman, I.O.; Melenotte, C.; Brouqui, P.; Million, M.; Lagier, J.-C.; Parola, P.; Stein, A.; La Scola, B.; Meddeb, L.; Mege, J.-L.; et al. Expression of ACE2, Soluble ACE2, Angiotensin I, Angiotensin II and Angiotensin-(1-7) Is Modulated in COVID-19 Patients. Front. Immunol. 2021, 12, 625732. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; An, Y. ACE2 Shedding and the Role in COVID-19. Front. Cell Infect. Microbiol. 2021, 11, 789180. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of Biological Peptides by Human Angiotensin-Converting Enzyme-Related Carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef]
- Björkqvist, J.; Jämsä, A.; Renné, T. Plasma Kallikrein: The Bradykinin-Producing Enzyme. Thromb. Haemost. 2013, 110, 399–407. [Google Scholar] [CrossRef]
- Dell’Italia, L.J.; Collawn, J.F.; Ferrario, C.M. Multifunctional Role of Chymase in Acute and Chronic Tissue Injury and Remodeling. Circ. Res. 2018, 122, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Klickstein, L.B.; Kaempfer, C.E.; Wintroub, B.U. The Granulocyte-Angiotensin System. Angiotensin I-Converting Activity of Cathepsin G. J. Biol. Chem. 1982, 257, 15042–15046. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, K. Serine Protease Angiotensin II Systems. J. Hypertens. Suppl. 1996, 14, S3–S7. [Google Scholar] [PubMed]
- Licker, M.; Morel, D.R. Inhibitors of the Renin Angiotensin System: Implications for the Anaesthesiologist. Curr. Opin. Anaesthesiol. 1998, 11, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, M.; Maeda, H.; Noda, K.; Tsuji, E.; Kinoshita, A.; Ideishi, M.; Ogata, S.; Arakawa, K. Purification and Characterization of a Kinin- and Angiotensin II-Forming Enzyme in the Dog Heart. J. Hypertens. 1997, 15, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.; Zuberek, M.; Duta, C.; Meuth, A.; Sowers, J.R.; Whaley-Connell, A.; Nistala, R. Angiotensin II Stimulation of DPP4 Activity Regulates Megalin in the Proximal Tubules. Int. J. Mol. Sci. 2016, 17, 780. [Google Scholar] [CrossRef] [PubMed]
- Nistala, R.; Meuth, A.I.; Smith, C.; An, J.; Habibi, J.; Hayden, M.R.; Johnson, M.; Aroor, A.; Whaley-Connell, A.; Sowers, J.R.; et al. DPP4 Inhibition Mitigates ANG II-Mediated Kidney Immune Activation and Injury in Male Mice. Am. J. Physiol. Ren. Physiol. 2021, 320, F505–F517. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, K.; Takahashi, N.; Yamochi, T.; Hosono, O.; Dang, N.H.; Morimoto, C. Role of CD26/Dipeptidyl Peptidase IV in Human T Cell Activation and Function. Front. Biosci. 2008, 13, 2299–2310. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Yamamoto, N.; Nakai-Murakami, C.; Osawa, Y.; Tokunaga, K.; Sata, T.; Yamamoto, N.; Sasazuki, T.; Ishizaka, Y. Modulation of TNF-Alpha-Converting Enzyme by the Spike Protein of SARS-CoV and ACE2 Induces TNF-Alpha Production and Facilitates Viral Entry. Proc. Natl. Acad. Sci. USA 2008, 105, 7809–7814. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Zipeto, D.; Palmeira, J.d.F.; Argañaraz, G.A.; Argañaraz, E.R. ACE2/ADAM17/TMPRSS2 Interplay May Be the Main Risk Factor for COVID-19. Front. Immunol. 2020, 11, 576745. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, Y.; Xia, H.; Wang, C.; Tan, C.Y.; Cai, X.; Liu, Y.; Ji, F.; Xiong, P.; Liu, R.; et al. Transcriptional and Proteomic Insights into the Host Response in Fatal COVID-19 Cases. Proc. Natl. Acad. Sci. USA 2020, 117, 28336–28343. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Y.; Anderson, D.E.; Rathore, A.P.; O’Neill, A.; Mantri, C.K.; Saron, W.A.; Lee, C.Q.; Cui, C.W.; Kang, A.E.; Foo, R.; et al. Mast Cell Activation in Lungs during SARS-CoV-2 Infection Associated with Lung Pathology and Severe COVID-19. J. Clin. Investig. 2023, 133, e149834. [Google Scholar] [CrossRef] [PubMed]
- Lascano, J.; Oshins, R.; Eagan, C.; Wadood, Z.; Qiang, X.; Flagg, T.; Scindia, Y.; Mehrad, B.; Brantly, M.; Khodayari, N. Correlation of Alpha-1 Antitrypsin Levels and Exosome Associated Neutrophil Elastase Endothelial Injury in Subjects with SARS-CoV2 Infection. PLoS ONE 2022, 17, e0274427. [Google Scholar] [CrossRef] [PubMed]
- Siwy, J.; Wendt, R.; Albalat, A.; He, T.; Mischak, H.; Mullen, W.; Latosinska, A.; Lübbert, C.; Kalbitz, S.; Mebazaa, A.; et al. CD99 and Polymeric Immunoglobulin Receptor Peptides Deregulation in Critical COVID-19: A Potential Link to Molecular Pathophysiology? Proteomics 2021, 21, e2100133. [Google Scholar] [CrossRef] [PubMed]
- Zerimech, F.; Jourdain, M.; Onraed, B.; Bouchecareilh, M.; Sendid, B.; Duhamel, A.; Balduyck, M.; Pigny, P. LICORNE Study Groupa Protease-Antiprotease Imbalance in Patients with Severe COVID-19. Clin. Chem. Lab. Med. 2021, 59, e330–e334. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Ní Choileáin, O.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef]
- Reichert, R.; Hochstrasser, K.; Werle, E. Der Proteaseninhibitorspiegel in menschlichem Nasensekret unter physiologischen und pathophysiologischen Bedingungen. Klin. Wochenschr. 1971, 49, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Akaike, T.; Molla, A.; Ando, M.; Araki, S.; Maeda, H. Molecular Mechanism of Complex Infection by Bacteria and Virus Analyzed by a Model Using Serratial Protease and Influenza Virus in Mice. J. Virol. 1989, 63, 2252–2259. [Google Scholar] [CrossRef]
- Beppu, Y.; Imamura, Y.; Tashiro, M.; Towatari, T.; Ariga, H.; Kido, H. Human Mucus Protease Inhibitor in Airway Fluids Is a Potential Defensive Compound against Infection with Influenza A and Sendai Viruses. J. Biochem. 1997, 121, 309–316. [Google Scholar] [CrossRef]
- Hennet, T.; Peterhans, E.; Stocker, R. Alterations in Antioxidant Defences in Lung and Liver of Mice Infected with Influenza A Virus. J. Gen. Virol. 1992, 73 Pt 1, 39–46. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Rezaei, N. Immune-Epidemiological Parameters of the Novel Coronavirus—A Perspective. Expert Rev. Clin. Immunol. 2020, 16, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Lipcsey, M.; Persson, B.; Eriksson, O.; Blom, A.M.; Fromell, K.; Hultström, M.; Huber-Lang, M.; Ekdahl, K.N.; Frithiof, R.; Nilsson, B. The Outcome of Critically Ill COVID-19 Patients Is Linked to Thromboinflammation Dominated by the Kallikrein/Kinin System. Front. Immunol. 2021, 12, 627579. [Google Scholar] [CrossRef]
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The Immune Response to SARS-CoV-2 and COVID-19 Immunopathology—Current Perspectives. Pulmonology 2021, 27, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Donahue, M.A.; Price, P.M. Aprotinin: Antifibrinolytic and Anti-Inflammatory Mechanisms of Action in Cardiac Surgery with Cardiopulmonary Bypass. Dynamics 2002, 13, 16–23. [Google Scholar]
- Asimakopoulos, G.; Lidington, E.A.; Mason, J.; Haskard, D.O.; Taylor, K.M.; Landis, R.C. Effect of Aprotinin on Endothelial Cell Activation. J. Thorac. Cardiovasc. Surg. 2001, 122, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H.; Sypniewski, E. Aprotinin: A Pharmacologic Overview. Orthopedics 2004, 27, s653–s658. [Google Scholar] [CrossRef]
- Harig, F.; Feyrer, R.; Mahmoud, F.O.; Blum, U.; von der Emde, J. Reducing the Post-Pump Syndrome by Using Heparin-Coated Circuits, Steroids, or Aprotinin. Thorac. Cardiovasc. Surg. 1999, 47, 111–118. [Google Scholar] [CrossRef]
- Gilliland, H.E.; Armstrong, M.A.; Uprichard, S.; Clarke, G.; McMurray, T.J. The Effect of Aprotinin on Interleukin-8 Concentration and Leukocyte Adhesion Molecule Expression in an Isolated Cardiopulmonary Bypass System. Anaesthesia 1999, 54, 427–433. [Google Scholar] [CrossRef]
- van Oeveren, W.; Jansen, N.J.; Bidstrup, B.P.; Royston, D.; Westaby, S.; Neuhof, H.; Wildevuur, C.R. Effects of Aprotinin on Hemostatic Mechanisms during Cardiopulmonary Bypass. Ann. Thorac. Surg. 1987, 44, 640–645. [Google Scholar] [CrossRef]
- Gao, T.; Zhu, L.; Liu, H.; Zhang, X.; Wang, T.; Fu, Y.; Li, H.; Dong, Q.; Hu, Y.; Zhang, Z.; et al. Highly Pathogenic Coronavirus N Protein Aggravates Inflammation by MASP-2-Mediated Lectin Complement Pathway Overactivation. Signal Transduct. Target. Ther. 2022, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.W.; Amarilla, A.A.; Lee, J.D.; Albornoz, E.A.; Modhiran, N.; Clark, R.J.; Ferro, V.; Chhabra, M.; Khromykh, A.A.; Watterson, D.; et al. SARS-CoV-2 Triggers Complement Activation through Interactions with Heparan Sulfate. Clin. Transl. Immunol. 2022, 11, e1413. [Google Scholar] [CrossRef] [PubMed]
- Sayyadi, M.; Hassani, S.; Shams, M.; Dorgalaleh, A. Status of Major Hemostatic Components in the Setting of COVID-19: The Effect on Endothelium, Platelets, Coagulation Factors, Fibrinolytic System, and Complement. Ann. Hematol. 2023, 102, 1307–1322. [Google Scholar] [CrossRef] [PubMed]
- Cugno, M.; Meroni, P.L.; Gualtierotti, R.; Griffini, S.; Grovetti, E.; Torri, A.; Panigada, M.; Aliberti, S.; Blasi, F.; Tedesco, F.; et al. Complement Activation in Patients with COVID-19: A Novel Therapeutic Target. J. Allergy Clin. Immunol. 2020, 146, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, O.; Hultström, M.; Persson, B.; Lipcsey, M.; Ekdahl, K.N.; Nilsson, B.; Frithiof, R. Mannose-Binding Lectin Is Associated with Thrombosis and Coagulopathy in Critically Ill COVID-19 Patients. Thromb. Haemost. 2020, 120, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. The Plasma Contact System as a Modulator of Innate Immunity. Curr. Opin. Hematol. 2018, 25, 389–394. [Google Scholar] [CrossRef]
- Rhaleb, N.-E.; Yang, X.-P.; Carretero, O.A. The Kallikrein-Kinin System as a Regulator of Cardiovascular and Renal Function. Compr. Physiol. 2011, 1, 971–993. [Google Scholar] [CrossRef]
- Dietrich, W.; Spannagl, M.; Jochum, M.; Wendt, P.; Schramm, W.; Barankay, A.; Sebening, F.; Richter, J.A. Influence of High-Dose Aprotinin Treatment on Blood Loss and Coagulation Patterns in Patients Undergoing Myocardial Revascularization. Anesthesiology 1990, 73, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Ghebrehiwet, B.; Geisbrecht, B.V.; Xu, X.; Savitt, A.G.; Peerschke, E.I.B. The C1q Receptors: Focus on gC1qR/P33 (C1qBP, P32, HABP-1)1. Semin. Immunol. 2019, 45, 101338. [Google Scholar] [CrossRef]
- Savitt, A.G.; Manimala, S.; White, T.; Fandaros, M.; Yin, W.; Duan, H.; Xu, X.; Geisbrecht, B.V.; Rubenstein, D.A.; Kaplan, A.P.; et al. SARS-CoV-2 Exacerbates COVID-19 Pathology Through Activation of the Complement and Kinin Systems. Front. Immunol. 2021, 12, 767347. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Holbrook, D.; McMonagle, E. Effects of Aprotinin on Complement and Granulocyte Activation during Ex Vivo Hemodialysis. Am. J. Kidney Dis. 1994, 24, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Bernard, I.; Limonta, D.; Mahal, L.K.; Hobman, T.C. Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19. Viruses 2020, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 Expression in Human Heart Indicates New Potential Mechanism of Heart Injury among Patients Infected with SARS-CoV-2. Cardiovasc. Res. 2020, 116, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Rayner, S.G.; Hung, C.F.; Liles, W.C.; Altemeier, W.A. Lung Pericytes as Mediators of Inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L1–L8. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Anyfanti, P.; Gavriilaki, M.; Lazaridis, A.; Douma, S.; Gkaliagkousi, E. Endothelial Dysfunction in COVID-19: Lessons Learned from Coronaviruses. Curr. Hypertens. Rep. 2020, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Drost, C.C.; Rovas, A.; Osiaevi, I.; Rauen, M.; van der Vlag, J.; Buijsers, B.; Salmenov, R.; Lukasz, A.; Pavenstädt, H.; Linke, W.A.; et al. Heparanase Is a Putative Mediator of Endothelial Glycocalyx Damage in COVID-19—A Proof-of-Concept Study. Front. Immunol. 2022, 13, 916512. [Google Scholar] [CrossRef] [PubMed]
- Masola, V.; Bellin, G.; Gambaro, G.; Onisto, M. Heparanase: A Multitasking Protein Involved in Extracellular Matrix (ECM) Remodeling and Intracellular Events. Cells 2018, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ner, M.; Mayer, M.; Schirrmacher, V.; Vlodavsky, I. Involvement of Both Heparanase and Plasminogen Activator in Lymphoma Cell-Mediated Degradation of Heparan Sulfate in the Subendothelial Extracellular Matrix. J. Cell. Physiol. 1986, 128, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Choi, H.C.; Lee, K.Y.; Kang, Y.J. Aprotinin Inhibits Vascular Smooth Muscle Cell Inflammation and Proliferation via Induction of HO-1. Korean J. Physiol. Pharmacol. 2009, 13, 123–129. [Google Scholar] [CrossRef][Green Version]
- Royston, B.D.; Royston, D.; Pearson, J.D. Aprotinin Inhibits Platelet Adhesion to Endothelial Cells. Blood Coagul. Fibrinolysis 1992, 3, 737–742. [Google Scholar] [CrossRef]
- Iba, T.; Helms, J.; Levi, M.; Levy, J.H. Thromboinflammation in Acute Injury: Infections, Heatstroke, and Trauma. J. Thromb. Haemost. 2024, 22, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Zelek, W.M.; Harrison, R.A. Complement and COVID-19: Three Years on, What We Know, What We Don’t Know, and What We Ought to Know. Immunobiology 2023, 228, 152393. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.; Linden, D.; Guo-Parke, H.; Earley, O.; Peto, T.; McAuley, D.F.; Taggart, C.; Kidney, J. Vascular Risk Factors for COVID-19 ARDS: Endothelium, Contact-Kinin System. Front. Med. 2023, 10, 1208866. [Google Scholar] [CrossRef]
- Renné, T.; Stavrou, E.X. Roles of Factor XII in Innate Immunity. Front. Immunol. 2019, 10, 2011. [Google Scholar] [CrossRef] [PubMed]
- Colling, M.E.; Kanthi, Y. COVID-19-Associated Coagulopathy: An Exploration of Mechanisms. Vasc. Med. 2020, 25, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Becerril, B.; Campi-Caballero, R.; Sevilla-Fuentes, S.; Hernández-Regino, L.M.; Hanono, A.; Flores-Bustamante, A.; González-Flores, J.; García-Ávila, C.A.; Aquino-Gálvez, A.; Castillejos-López, M.; et al. Immunothrombosis in COVID-19: Implications of Neutrophil Extracellular Traps. Biomolecules 2021, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Dharra, R.; Kumar Sharma, A.; Datta, S. Emerging Aspects of Cytokine Storm in COVID-19: The Role of Proinflammatory Cytokines and Therapeutic Prospects. Cytokine 2023, 169, 156287. [Google Scholar] [CrossRef] [PubMed]
- Grignani, G.; Maiolo, A. Cytokines and Hemostasis. Haematologica 2000, 85, 967–972. [Google Scholar] [PubMed]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Kakuta, S.; Iwakura, Y.; Takayama, N.; Ooehara, J.; Otsu, M.; Kamiya, A.; Petrich, B.G.; et al. In Vivo Imaging Visualizes Discoid Platelet Aggregations without Endothelium Disruption and Implicates Contribution of Inflammatory Cytokine and Integrin Signaling. Blood 2012, 119, e45–e56. [Google Scholar] [CrossRef]
- Chang, J.C. COVID-19 Sepsis: Pathogenesis and Endothelial Molecular Mechanisms Based on “Two-Path Unifying Theory” of Hemostasis and Endotheliopathy-Associated Vascular Microthrombotic Disease, and Proposed Therapeutic Approach with Antimicrothrombotic Therapy. Vasc. Health Risk Manag. 2021, 17, 273–298. [Google Scholar] [CrossRef]
- van Wissen, M.; Keller, T.T.; van Gorp, E.C.M.; Gerdes, V.E.A.; Meijers, J.C.M.; van Doornum, G.J.J.; Büller, H.R.; Brandjes, D.P.M. Acute Respiratory Tract Infection Leads to Procoagulant Changes in Human Subjects. J. Thromb. Haemost. 2011, 9, 1432–1434. [Google Scholar] [CrossRef] [PubMed]
- Landis, R.C.; Asimakopoulos, G.; Poullis, M.; Haskard, D.O.; Taylor, K.M. The Antithrombotic and Antiinflammatory Mechanisms of Action of Aprotinin. Ann. Thorac. Surg. 2001, 72, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Moons, L.; Dewerchin, M.; Mackman, N.; Luther, T.; Breier, G.; Ploplis, V.; Müller, M.; Nagy, A.; Plow, E.; et al. Insights in Vessel Development and Vascular Disorders Using Targeted Inactivation and Transfer of Vascular Endothelial Growth Factor, the Tissue Factor Receptor, and the Plasminogen System. Ann. N. Y. Acad. Sci. 1997, 811, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R. Plasmin and Matrix Metalloproteinases in Vascular Remodeling. Thromb. Haemost. 2001, 86, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Buerke, M.; Pruefer, D.; Sankat, D.; Carter, J.M.; Buerke, U.; Russ, M.; Schlitt, A.; Friedrich, I.; Börgermann, J.; Vahl, C.F.; et al. Effects of Aprotinin on Gene Expression and Protein Synthesis After Ischemia and Reperfusion in Rats. Circulation 2007, 116, I121–I126. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.A.; Roath, O.S.; Thompson, J.F.; Chant, A.D.; Francis, J.L. Effect of Aprotinin on Neutrophil Function after Major Vascular Surgery. Br. J. Surg. 1992, 79, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Lu, Y.B.; Jiang, B.; Xu, S.W.; Chen, R.K.; Zhou, H.L. Effects of Methylprednisolone and Aprotinin on Phospholipase D Activity of Leukocytes in Systemic Inflammatory Response Induced by Cardiopulmonary Bypass. Acta Pharmacol. Sin. 2001, 22, 913–917. [Google Scholar]
- Carter, J.M.; Buerke, U.; Rössner, E.; Russ, M.; Schubert, S.; Schmidt, H.; Ebelt, H.; Pruefer, D.; Schlitt, A.; Werdan, K.; et al. Anti-Inflammatory Actions of Aprotinin Provide Dose-Dependent Cardioprotection from Reperfusion Injury. Br. J. Pharmacol. 2008, 155, 93–102. [Google Scholar] [CrossRef][Green Version]
- Nakanishi-Matsui, M.; Zheng, Y.W.; Sulciner, D.J.; Weiss, E.J.; Ludeman, M.J.; Coughlin, S.R. PAR3 Is a Cofactor for PAR4 Activation by Thrombin. Nature 2000, 404, 609–613. [Google Scholar] [CrossRef]
- Takeuchi, T.; Harris, J.L.; Huang, W.; Yan, K.W.; Coughlin, S.R.; Craik, C.S. Cellular Localization of Membrane-Type Serine Protease 1 and Identification of Protease-Activated Receptor-2 and Single-Chain Urokinase-Type Plasminogen Activator as Substrates. J. Biol. Chem. 2000, 275, 26333–26342. [Google Scholar] [CrossRef]
- Camerer, E.; Huang, W.; Coughlin, S.R. Tissue Factor- and Factor X-Dependent Activation of Protease-Activated Receptor 2 by Factor VIIa. Proc. Natl. Acad. Sci. USA 2000, 97, 5255–5260. [Google Scholar] [CrossRef] [PubMed]
- Poullis, M.; Manning, R.; Laffan, M.; Haskard, D.O.; Taylor, K.M.; Landis, R.C. The Antithrombotic Effect of Aprotinin: Actions Mediated via the Proteaseactivated Receptor 1. J. Thorac. Cardiovasc. Surg. 2000, 120, 370–378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thurner, L.R.; Höcherl, K. Role of Protease-Activated Receptor 2 in Regulation of Renin Synthesis and Secretion in Mice. Naunyn Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.E.; Lazos, S.A.; Tong, K. Angiotensin II Regulates the Expression of Plasminogen Activator Inhibitor-1 in Cultured Endothelial Cells. A Potential Link between the Renin-Angiotensin System and Thrombosis. J. Clin. Investig. 1995, 95, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Tsuji, H.; Masuda, H.; Nakagawa, K.; Nakahara, Y.; Kitamura, H.; Kasahara, T.; Sugano, T.; Yoshizumi, M.; Sawada, S.; et al. Angiotensin II Increases Plasminogen Activator Inhibitor-1 and Tissue Factor mRNA Expression without Changing That of Tissue Type Plasminogen Activator or Tissue Factor Pathway Inhibitor in Cultured Rat Aortic Endothelial Cells. Thromb. Haemost. 1997, 77, 1189–1195. [Google Scholar] [CrossRef]
- Wright, F.L.; Vogler, T.O.; Moore, E.E.; Moore, H.B.; Wohlauer, M.V.; Urban, S.; Nydam, T.L.; Moore, P.K.; McIntyre, R.C. Fibrinolysis Shutdown Correlation with Thromboembolic Events in Severe COVID-19 Infection. J. Am. Coll. Surg. 2020, 231, 193–203.e1. [Google Scholar] [CrossRef]
- Nougier, C.; Benoit, R.; Simon, M.; Desmurs-Clavel, H.; Marcotte, G.; Argaud, L.; David, J.S.; Bonnet, A.; Negrier, C.; Dargaud, Y. Hypofibrinolytic State and High Thrombin Generation May Play a Major Role in SARS-CoV2 Associated Thrombosis. J. Thromb. Haemost. 2020, 18, 2215–2219. [Google Scholar] [CrossRef]
- Maas, C. Plasminflammation—An Emerging Pathway to Bradykinin Production. Front. Immunol. 2019, 10, 2046. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.-J.; Rho, J.-R. Aprotinin Attenuates the Elevation of Pulmonary Vascular Resistance After Cardiopulmonary Bypass. J. Korean Med. Sci. 2006, 21, 25–29. [Google Scholar] [CrossRef][Green Version]
- Ragnoli, B.; Da Re, B.; Galantino, A.; Kette, S.; Salotti, A.; Malerba, M. Interrelationship between COVID-19 and Coagulopathy: Pathophysiological and Clinical Evidence. Int. J. Mol. Sci. 2023, 24, 8945. [Google Scholar] [CrossRef]
- Gupta, V.; Acharya, S.; Keerti, A. Common Coagulopathies Associated With COVID-19 Patients. Cureus 2023, 15, e38067. [Google Scholar] [CrossRef]
- Didiasova, M.; Wujak, L.; Schaefer, L.; Wygrecka, M. Factor XII in Coagulation, Inflammation and Beyond. Cell Signal 2018, 51, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Braat, E.A.M.; Dooijewaard, G.; Rijken, D.C. Fibrinolytic Properties of Activated FXII. Eur. J. Biochem. 1999, 263, 904–911. [Google Scholar] [CrossRef]
- Simão, F.; Feener, E.P. The Effects of the Contact Activation System on Hemorrhage. Front. Med. 2017, 4, 121. [Google Scholar] [CrossRef]
- Luo, S.; Vasbinder, A.; Du-Fay-de-Lavallaz, J.M.; Gomez, J.M.D.; Suboc, T.; Anderson, E.; Tekumulla, A.; Shadid, H.; Berlin, H.; Pan, M.; et al. Soluble Urokinase Plasminogen Activator Receptor and Venous Thromboembolism in COVID-19. J. Am. Heart Assoc. 2022, 11, e025198. [Google Scholar] [CrossRef]
- Abdellatif, H.A.A.; Sultan, B.O.; Nassar, H.M.; Gomaa, M.E.E.; Sakr, M.G.; Riad, E.; Al-Harbi, A.I.; Abdulhakim, J.A.; Fawzy, M.S.; Abd El-Fadeal, N.M. Circulating Soluble Urokinase Plasminogen Activator Receptor as a Predictive Indicator for COVID-19-Associated Acute Kidney Injury and Mortality: Clinical and Bioinformatics Analysis. Int. J. Mol. Sci. 2023, 24, 7177. [Google Scholar] [CrossRef]
- Shmakova, A.A.; Popov, V.S.; Romanov, I.P.; Khabibullin, N.R.; Sabitova, N.R.; Karpukhina, A.A.; Kozhevnikova, Y.A.; Kurilina, E.V.; Tsokolaeva, Z.I.; Klimovich, P.S.; et al. Urokinase System in Pathogenesis of Pulmonary Fibrosis: A Hidden Threat of COVID-19. Int. J. Mol. Sci. 2023, 24, 1382. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, A.; Rabin, J.; Menaker, J.; Madathil, R.; Galvagno, S.; Menne, A.; Chow, J.H.; Grazioli, A.; Herr, D.; Tanaka, K.; et al. Factor VIII and Functional Protein C Activity in Critically Ill Patients With Coronavirus Disease 2019: A Case Series. A A Pract. 2020, 14, e01236. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Lopez, M.-T.; Garcia-Leon, N.; Gomez-Arevalillo, S.; Martin-Serrano, P.; Matilla-Garcia, A. Coronavirus Disease 2019 and Coagulopathy: Other Prothrombotic Coagulation Factors. Blood Coagul. Fibrinolysis 2021, 32, 44–49. [Google Scholar] [CrossRef]
- Shama, N.; Mahmood, A.; Mehmood, S.; Zhang, W. Pathological Effects of SARS-CoV-2 Associated with Hematological Abnormalities. Curr. Issues Mol. Biol. 2023, 45, 7161–7182. [Google Scholar] [CrossRef]
- Ranucci, M.; Ballotta, A.; Di Dedda, U.; Baryshnikova, E.; Dei Poli, M.; Resta, M.; Falco, M.; Albano, G.; Menicanti, L. The Procoagulant Pattern of Patients with COVID-19 Acute Respiratory Distress Syndrome. J. Thromb. Haemost. 2020, 18, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. Hypercoagulability of COVID-19 Patients in Intensive Care Unit: A Report of Thromboelastography Findings and Other Parameters of Hemostasis. J. Thromb. Haemost. 2020, 18, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.; Häberle, H.; Schlensak, C.; Bitzer, M.; Malek, N.P.; Handgretinger, R.; Lang, P.; Hörber, S.; Peter, A.; Martus, P.; et al. Severe SARS-CoV-2 Infection Inhibits Fibrinolysis Leading to Changes in Viscoelastic Properties of Blood Clot: A Descriptive Study of Fibrinolysis in COVID-19. Thromb. Haemost. 2021, 121, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Akbasheva, O.E.; Mitrofanova, D.K.; Spirina, L.V.; Samoilova, Y.G.; Matveeva, M.V.; Podchinenova, D.V.; Oleynik, O.A. Alpha-2 Macroglobulin Activity in SARS-CoV-2 Induced Infection and in the Post-COVID-19 Period. Biomed. Khim. 2023, 69, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of Coronavirus Disease 2019. Crit. Care Med. 2020, 48, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Basmaci, R.; Bensaid, P.; Bru, C.B.; Coinde, E.; Dessioux, E.; Fournial, C.; Gashignard, J.; Haas, H.; Hentgen, V.; et al. Changes in Reverse Transcription Polymerase Chain Reaction–Positive Severe Acute Respiratory Syndrome Coronavirus 2 Rates in Adults and Children According to the Epidemic Stages. Pediatr. Infect. Dis. J. 2020, 39, e369. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Hisada, Y.; Denorme, F.; Grover, S.P.; Bouck, E.G.; Middleton, E.A.; Wolberg, A.S.; Rondina, M.T.; Mackman, N. Comparison of the Coagulopathies Associated with COVID-19 and Sepsis. Res. Pract. Thromb. Haemost. 2021, 5, e12525. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, M.-Q.; Shen, Q.; Wang, L.-Z.; Yan, R.-D.; Zhang, M.-Y.; Liu, J.-Y.; Qu, Y.-Q. Analysis of Inflammatory Parameters and Disease Severity for 88 Hospitalized COVID-19 Patients in Wuhan, China. Int. J. Med. Sci. 2020, 17, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Colarusso, C.; Terlizzi, M.; Pinto, A.; Sorrentino, R. A Lesson from a Saboteur: High-MW Kininogen Impact in Coronavirus-induced Disease 2019. Br. J. Pharmacol. 2020, 177, 4866–4872. [Google Scholar] [CrossRef]
- Lam, M.; Beqo, A.; Thumar, R. Overcoming Cough and Angioedema: Advocating for the Use of ARBs Over ACE Inhibitors. Ann. Pharmacother. 2022, 56, 358–362. [Google Scholar] [CrossRef]
- Davies, G.E.; Holman, G.; Johnston, T.P.; Lowe, J.S. Studies on Kallikrein: Failure of Some Anti-Inflammatory Drugs to Affect Release of Kinin. Br. J. Pharmacol. Chemother. 1966, 28, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Csaba, B.; Tóth, S. The Effect of Temperature and Some Mediator Antagonists on Anaphylactic Shock in Mice. Int. Arch. Allergy Appl. Immunol. 1971, 40, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, B.C. Protease Inhibitors in the Treatment of Hereditary Angioedema. Transfus. Apher. Sci. 2003, 29, 259–267. [Google Scholar] [CrossRef]
- Ghahestani, S.-M.; Mahmoudi, J.; Hajebrahimi, S.; Sioofy-Khojine, A.-B.; Salehi-Pourmehr, H.; Sadeghi-Ghyassi, F.; Mostafaei, H. Bradykinin as a Probable Aspect in SARS-Cov-2 Scenarios: Is Bradykinin Sneaking out of Our Sight? Iran. J. Allergy Asthma Immunol. 2020, 19, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Bankhead, A.; Jeng, S.; Menachery, V.D.; Proll, S.; Belisle, S.E.; Matzke, M.; Webb-Robertson, B.-J.M.; Luna, M.L.; Shukla, A.K.; et al. Mechanisms of Severe Acute Respiratory Syndrome Coronavirus-Induced Acute Lung Injury. mBio 2013, 4, e00271-13. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, S.-Y. Pathological Study of the 2019 Novel Coronavirus Disease (COVID-19) through Postmortem Core Biopsies. Mod. Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Kwaan, H.C. Coronavirus Disease 2019: The Role of the Fibrinolytic System from Transmission to Organ Injury and Sequelae. Semin. Thromb. Hemost. 2020, 46, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B. Pathophysiology of Acute Lung Injury and the Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2006, 27, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Bojkova, D.; Klann, K.; Koch, B.; Widera, M.; Krause, D.; Ciesek, S.; Cinatl, J.; Münch, C. Proteomics of SARS-CoV-2-Infected Host Cells Reveals Therapy Targets. Nature 2020, 583, 469–472. [Google Scholar] [CrossRef]
- Zecha, J.; Lee, C.-Y.; Bayer, F.P.; Meng, C.; Grass, V.; Zerweck, J.; Schnatbaum, K.; Michler, T.; Pichlmair, A.; Ludwig, C.; et al. Data, Reagents, Assays and Merits of Proteomics for SARS-CoV-2 Research and Testing. Mol. Cell Proteom. 2020, 19, 1503–1522. [Google Scholar] [CrossRef]
- Garcia-Verdugo, I.; Descamps, D.; Chignard, M.; Touqui, L.; Sallenave, J.-M. Lung Protease/Anti-Protease Network and Modulation of Mucus Production and Surfactant Activity. Biochimie 2010, 92, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Jaspers, I. Respiratory Protease/Antiprotease Balance Determines Susceptibility to Viral Infection and Can Be Modified by Nutritional Antioxidants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L1189–L1201. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, M.C.; Brown, R.; Ryan, S.; Mall, M.A.; Weldon, S.; Taggart, C.C. Proteases, Mucus, and Mucosal Immunity in Chronic Lung Disease. Int. J. Mol. Sci. 2021, 22, 5018. [Google Scholar] [CrossRef] [PubMed]
- Rasche, B.; Marcic, I.; Ulmer, W.T. Effect of the protease inhibitor aprotinin on pulmonary function and on the inhibitory activity of sputum in patients with chronic obstructive bronchitis. Arzneimittelforschung 1975, 25, 110–116. [Google Scholar] [PubMed]
- Planès, C.; Caughey, G.H. Regulation of the Epithelial Na+ Channel by Peptidases. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 78, pp. 23–46. ISBN 978-0-12-373748-9. [Google Scholar]
- Kleyman, T.R.; Eaton, D.C. Regulating ENaC’s Gate. Am. J. Physiol. Cell Physiol. 2020, 318, C150–C162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, X.; Yan, Z.; Zhang, S.; Zhang, J.; Guo, W. Role of Epithelial Sodium Channel-Related Inflammation in Human Diseases. Front. Immunol. 2023, 14, 1178410. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.F.; Mitaera, T.; Fronius, M. COVID-19 and Liquid Homeostasis in the Lung-A Perspective through the Epithelial Sodium Channel (ENaC) Lens. Cells 2022, 11, 1801. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yu, T.; Wang, T.; Ding, Y.; Cui, Y.; Nie, H. Competitive Cleavage of SARS-CoV-2 Spike Protein and Epithelial Sodium Channel by Plasmin as a Potential Mechanism for COVID-19 Infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 323, L569–L577. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Xue, H.; Zhou, W.; Ding, Y.; Nie, H. Regulation of Epithelial Sodium Transport by SARS-CoV-2 Is Closely Related with Fibrinolytic System-Associated Proteins. Biomolecules 2023, 13, 578. [Google Scholar] [CrossRef]
- Fu, Y.; Xue, H.; Wang, T.; Ding, Y.; Cui, Y.; Nie, H. Fibrinolytic System and COVID-19: From an Innovative View of Epithelial Ion Transport. Biomed. Pharmacother. 2023, 163, 114863. [Google Scholar] [CrossRef]
- Calkovska, A.; Kolomaznik, M.; Calkovsky, V. Alveolar Type II Cells and Pulmonary Surfactant in COVID-19 Era. Physiol. Res. 2021, 70, S195–S208. [Google Scholar] [CrossRef] [PubMed]
- Muhanna, D.; Arnipalli, S.R.; Kumar, S.B.; Ziouzenkova, O. Osmotic Adaptation by Na+-Dependent Transporters and ACE2: Correlation with Hemostatic Crisis in COVID-19. Biomedicines 2020, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Carson-Chahhoud, K.; Ardalan, M.; Kolahi, A.-A.; Safiri, S. How SARS-CoV-2 Might Affect Potassium Balance via Impairing Epithelial Sodium Channels? Mol. Biol. Rep. 2021, 48, 6655–6661. [Google Scholar] [CrossRef] [PubMed]
- Sandle, G.I.; Herod, M.R.; Fontana, J.; Lippiat, J.D.; Stockley, P.G. Is Intestinal Transport Dysfunctional in COVID-19-Related Diarrhea? Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G415–G418. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.J.; Yue, Q.; Singla, B.; Hamacher, J.; Sridhar, S.; Moseley, A.S.; Song, C.; Mraheil, M.A.; Fischer, B.; Zeitlinger, M.; et al. Direct Endothelial ENaC Activation Mitigates Vasculopathy Induced by SARS-CoV2 Spike Protein. Front. Immunol. 2023, 14, 1241448. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.S.; Narayanan, S.P.; Somanath, P.R. Is Amiloride a Promising Cardiovascular Medication to Persist in the COVID-19 Crisis? Drug Discov. Ther. 2020, 14, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Myerburg, M.M.; Harvey, P.R.; Heidrich, E.M.; Pilewski, J.M.; Butterworth, M.B. Acute Regulation of the Epithelial Sodium Channel in Airway Epithelia by Proteases and Trafficking. Am. J. Respir. Cell Mol. Biol. 2010, 43, 712–719. [Google Scholar] [CrossRef]
- Tan, C.D.; Selvanathar, I.A.; Baines, D.L. Cleavage of Endogenous γENaC and Elevated Abundance of αENaC Are Associated with Increased Na+ Transport in Response to Apical Fluid Volume Expansion in Human H441 Airway Epithelial Cells. Pflug. Arch. 2011, 462, 431–441. [Google Scholar] [CrossRef]
- Musante, I.; Scudieri, P.; Venturini, A.; Guidone, D.; Caci, E.; Castellani, S.; Conese, M.; Galietta, L.J.V. Peripheral Localization of the Epithelial Sodium Channel in the Apical Membrane of Bronchial Epithelial Cells. Exp. Physiol. 2019, 104, 866–875. [Google Scholar] [CrossRef]
- Coote, K.J.; Atherton, H.; Young, A.; Sugar, R.; Burrows, R.; Smith, N.J.; Schlaeppi, J.-M.; Groot-Kormelink, P.J.; Gosling, M.; Danahay, H. The Guinea-Pig Tracheal Potential Difference as an in Vivo Model for the Study of Epithelial Sodium Channel Function in the Airways. Br. J. Pharmacol. 2008, 155, 1025–1033. [Google Scholar] [CrossRef]
- Bohnert, B.N.; Menacher, M.; Janessa, A.; Wörn, M.; Schork, A.; Daiminger, S.; Kalbacher, H.; Häring, H.-U.; Daniel, C.; Amann, K.; et al. Aprotinin Prevents Proteolytic Epithelial Sodium Channel (ENaC) Activation and Volume Retention in Nephrotic Syndrome. Kidney Int. 2018, 93, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Zhirnov, O.P.; Ovcharenko, A.V.; Bukrinskaya, A.G. Protective Effect of Protease Inhibitors in Influenza Virus Infected Animals. Arch. Virol. 1982, 73, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Song, E.-J.; Españo, E.; Shim, S.-M.; Nam, J.-H.; Kim, J.; Lee, K.; Park, S.-K.; Lee, C.-K.; Kim, J.-K. Inhibitory Effects of Aprotinin on Influenza A and B Viruses in Vitro and in Vivo. Sci. Rep. 2021, 11, 9427. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, A.A.; Zagribelnyy, B.A.; Ivanenkov, Y.A.; Ivashchenko, I.A.; Karapetian, R.N.; Kravchenko, D.V.; Savchuk, N.P.; Yakubova, E.V.; Ivachtchenko, A.V. The Efficacy of Aprotinin Combinations with Selected Antiviral Drugs in Mouse Models of Influenza Pneumonia and Coronavirus Infection Caused by SARS-CoV-2. Molecules 2022, 27, 4975. [Google Scholar] [CrossRef]
- Ivashchenko, A.; Svistunov, A.; Khorobryh, T.; Loginov, V.; Karapetian, R.; Mishchenko, N.; Poyarkov, S.; Volgin, M.; Yakubova, E.; Topr, M.; et al. Aprotinin—A New Drug Candidate for The Prevention of SARS-CoV-2 (COVID-19). COVID-19 Prepr. 2020. [Google Scholar] [CrossRef]
- Ivashchenko, A.A.; Azarova, V.N.; Egorova, A.N.; Karapetian, R.N.; Kravchenko, D.V.; Krivonos, N.V.; Loginov, V.G.; Poyarkov, S.V.; Merkulova, E.A.; Rosinkova, O.S.; et al. Effect of Aprotinin and Avifavir® Combination Therapy for Moderate COVID-19 Patients. Viruses 2021, 13, 1253. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.M.; Van Thillo, Q.; Betrains, A.; Gyselinck, I.; Martens, C.P.; Spalart, V.; Ockerman, A.; Devooght, C.; Wauters, J.; Gunst, J.; et al. Modulation of Thromboinflammation in Hospitalized COVID-19 Patients with Aprotinin, Low Molecular Weight Heparin, and Anakinra: The DAWn-Antico Study. Res. Pract. Thromb. Haemost. 2022, 6, e12826. [Google Scholar] [CrossRef] [PubMed]
- Hori, R.; Saito, Y.; Yasuhara, M.; Okumura, K. In Vivo Conversion of Peptide Drugs into High Molecular Weight Forms. J. Pharmacobio-Dyn. 1984, 7, 910–916. [Google Scholar] [CrossRef]
- Bianchi, C.; Donadio, C.; Tramonti, G.; Auner, I.; Lorusso, P.; Deleide, G.; Lunghi, F.; Salvadori, P. Renal Handling of Cationic and Anionic Small Proteins: Experiments in Intact Rats; Karger Publishers: Basel, Switzerland, 1988; Volume 68, pp. 37–44. [Google Scholar] [CrossRef]
- Karasulu, H.Y.; Oruç, N.; Üstündağ-Okur, N.; İlem Özdemir, D.; Ay Şenyiğit, Z.; Barbet Yılmaz, F.; Aşıkoğlu, M.; Özkılıç, H.; Akçiçek, E.; Güneri, T.; et al. Aprotinin Revisited: Formulation, Characterization, Biodistribution and Therapeutic Potential of New Aprotinin Microemulsion in Acute Pancreatitis. J. Drug Target. 2015, 23, 525–537. [Google Scholar] [CrossRef]
- Tenstad, O.; Williamson, H.E.; Clausen, G.; Oien, A.H.; Aukland, K. Glomerular Filtration and Tubular Absorption of the Basic Polypeptide Aprotinin. Acta Physiol. Scand. 1994, 152, 33–50. [Google Scholar] [CrossRef]
- Baran, D.; Tenstad, O.; Aukland, K. Aprotinin Uptake in the Proximal Tubules in the Rat Kidney. II. Uptake Site Relative to Glomerulus. J. Struct. Biol. 2003, 142, 409–415. [Google Scholar] [CrossRef]
- Baran, D.; Tenstad, O.; Aukland, K. Aprotinin Uptake in the Proximal Tubules in the Rat Kidney I.: Length of Proximal Tubular Uptake Segment. J. Struct. Biol. 2003, 142, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vio, C.P.; Oestreicher, E.; Olavarria, V.; Velarde, V.; Mayfield, R.K.; Jaffa, A.A. Cellular Distribution of Exogenous Aprotinin in the Rat Kidney. Biol. Chem. 1998, 379, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Nausch, I.; Mentlein, R.; Heymann, E. The Degradation of Bioactive Peptides and Proteins by Dipeptidyl Peptidase IV from Human Placenta. Biol. Chem. Hoppe Seyler 1990, 371, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Agić, D.; Brkić, H.; Kazazić, S.; Tomić, A.; Abramić, M. Aprotinin Interacts with Substrate-Binding Site of Human Dipeptidyl Peptidase III. J. Biomol. Struct. Dyn. 2019, 37, 3596–3606. [Google Scholar] [CrossRef] [PubMed]
- Rustom, R.; Grime, S.; Maltby, P.; Stockdale, H.R.; Critchley, M.; Bone, J.M. A New Method to Measure Renal Tubular Degradation of Small Filtered Proteins in Man Using Radiolabelled Aprotinin (Trasylol). Clin. Sci. 1992, 83, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H.; Bailey, J.M.; Salmenperä, M. Pharmacokinetics of Aprotinin in Preoperative Cardiac Surgical Patients. Anesthesiology 1994, 80, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Aprile, C.; Saponaro, R.; Villa, G.; Lunghi, F. 99mTc-Aprotinin: Comparison with 99mTc-DMSA in Normal and Diseased Kidneys. Nuklearmedizin 1984, 23, 22–26. [Google Scholar] [PubMed]
- Sojan, S.M.; Smyth, D.R.; Tsopelas, C.; Mudge, D.; Collins, P.J.; Chatterton, B.E. Pharmacokinetics and Normal Scintigraphic Appearance of 99mTc Aprotinin. Nucl. Med. Commun. 2005, 26, 535. [Google Scholar] [CrossRef]
- Kramer, H.J.; Düsing, R.; Glänzer, K.; Kipnowski, J.; Klingmüller, D.; Meyer-Lehnert, H. Effects of Aprotinin on Renal Function. Contrib. Nephrol. 1984, 42, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Rustom, R.; Grime, J.S.; Maltby, P.; Stockdale, H.R.; Jackson, M.J.; Critchley, M.; Bone, J.M. Renal Tubular Protein Degradation of Radiolabelled Aprotinin (Trasylol) in Patients with Chronic Renal Failure. Clin. Sci. 1993, 85, 733–736. [Google Scholar] [CrossRef]
- O’Connor, C.J.; Brown, D.V.; Avramov, M.; Barnes, S.; O’Connor, H.N.; Tuman, K.J. The Impact of Renal Dysfunction on Aprotinin Pharmacokinetics during Cardiopulmonary Bypass. Anesth. Analg. 1999, 89, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.O.; Schall, R.; Hundt, H.K.L.; Groenewoud, G.; Ungerer, M.J.; Cronje, H.S.; Schumann, F. Pharmacokinetics of Aprotinin in Two Patients with Chronic Renal Impairment. Br. J. Clin. Pharmacol. 1996, 41, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros Sanz, M.Á.; Hernández-Tejedor, A.; Estella, Á.; Jiménez Rivera, J.J.; González de Molina Ortiz, F.J.; Sandiumenge Camps, A.; Vidal Cortés, P.; de Haro, C.; Aguilar Alonso, E.; Bordejé Laguna, L.; et al. Recommendations of the Working Groups from the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) for the Management of Adult Critically Ill Patients in the Coronavirus Disease (COVID-19). Med. Intensiv. 2020, 44, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Zhirnov, O.P.; Bokova, N.O.; Isaeva, E.I.; Vorobieva, I.V.; Malyshev, N.A. Pathogenetic Treatment of Influenza Patients with Aerosolized Form of Aprotinin, a Protease Inhibitor. Biol. Products. Prev. Diagn. Treat. 2015, 4, 59–64. [Google Scholar]
- Guyton, A.C. Measurement of the Respiratory Volumes of Laboratory Animals. Am. J. Physiol. 1947, 150, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, H.G.; Matthay, M.A.; Westrom, B.R.; Kim, K.J.; Karlsson, B.W.; Hastings, R.H. Alveolar Epithelial Clearance of Protein. J. Appl. Physiol. 1996, 80, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Hastings, R.H.; Folkesson, H.G.; Matthay, M.A. Mechanisms of Alveolar Protein Clearance in the Intact Lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L679–L689. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Umemori, S.; Muranishi, S. Absorption Enhancement of Intrapulmonary Administered Insulin by Various Absorption Enhancers and Protease Inhibitors in Rats. J. Pharm. Pharmacol. 1994, 46, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Kwon, J.-H.; Lim, S.-H.; Park, H.W.; Kim, C.-W. Characterization of Human Insulin Microcrystals and Their Absorption Enhancement by Protease Inhibitors in Rat Lungs. Int. J. Pharm. 2007, 339, 205–212. [Google Scholar] [CrossRef]
- Amancha, K.P.; Hussain, A. Effect of Protease Inhibitors on Pulmonary Bioavailability of Therapeutic Proteins and Peptides in the Rat. Eur. J. Pharm. Sci. 2015, 68, 1–10. [Google Scholar] [CrossRef]
- Morimoto, K.; Uehara, Y.; Iwanaga, K.; Kakemi, M. Effects of Sodium Glycocholate and Protease Inhibitors on Permeability of TRH and Insulin across Rabbit Trachea. Pharm. Acta Helv. 2000, 74, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Anders, J.; Johnson, M.; Dickson, R.B. Purification and Characterization of a Complex Containing Matriptase and a Kunitz-Type Serine Protease Inhibitor from Human Milk. J. Biol. Chem. 1999, 274, 18237–18242. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.S.; Irokawa, T.; Robbins, R.C.; Wine, J.J. Hyposecretion, Not Hyperabsorption, Is the Basic Defect of Cystic Fibrosis Airway Glands. J. Biol. Chem. 2006, 281, 7392–7398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nedredal, G.I.; Elvevold, K.; Chedid, M.F.; Ytrebø, L.M.; Rose, C.F.; Sen, S.; Smedsrød, B.; Jalan, R.; Revhaug, A. Pulmonary Vascular Clearance of Harmful Endogenous Macromolecules in a Porcine Model of Acute Liver Failure. Ann. Hepatol. 2016, 15, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Boucher, R.C. Mucus Clearance as a Primary Innate Defense Mechanism for Mammalian Airways. J. Clin. Investig. 2002, 109, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Wine, J.J.; Joo, N.S. Submucosal Glands and Airway Defense. Proc. Am. Thorac. Soc. 2004, 1, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Bachinsky, D.R.; Stamenkovic, I.; Strickland, D.K.; Brown, D.; Andres, G.; McCluskey, R.T. Organ Distribution in Rats of Two Members of the Low-Density Lipoprotein Receptor Gene Family, Gp330 and LRP/Alpha 2MR, and the Receptor-Associated Protein (RAP). J. Histochem. Cytochem. 1994, 42, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Ashton Acton. Phagocytosis: New Insights for the Healthcare Professional: 2013 Edition: ScholarlyBrief; ScholarlyEditions: Atlanta, GA, USA, 2013; ISBN 978-1-4816-6135-5. [Google Scholar]
- Brain, J.D.; Molina, R.M.; DeCamp, M.M.; Warner, A.E. Pulmonary Intravascular Macrophages: Their Contribution to the Mononuclear Phagocyte System in 13 Species. Am. J. Physiol. 1999, 276, L146–L154. [Google Scholar] [CrossRef]
- Kounnas, M.Z.; Moir, R.D.; Rebeck, G.W.; Bush, A.I.; Argraves, W.S.; Tanzi, R.E.; Hyman, B.T.; Strickland, D.K. LDL Receptor-Related Protein, a Multifunctional ApoE Receptor, Binds Secreted Beta-Amyloid Precursor Protein and Mediates Its Degradation. Cell 1995, 82, 331–340. [Google Scholar] [CrossRef]
- Cardoso, I.; Pereira, P.J.; Damas, A.M.; Saraiva, M.J. Aprotinin Binding to Amyloid Fibrils. Eur. J. Biochem. 2000, 267, 2307–2311. [Google Scholar] [CrossRef]
- Awaya, T.; Minamimoto, R.; Iwama, K.; Kubota, S.; Hotta, M.; Hirai, R.; Yamamoto, M.; Okazaki, O.; Hara, H.; Hiroi, Y.; et al. Performance of 99mTc-Aprotinin Scintigraphy for Diagnosing Light Chain (AL) Cardiac Amyloidosis Confirmed by Endomyocardial Biopsy. J. Nucl. Cardiol. 2020, 27, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Aprile, C.; Lodola, L. A Narrative Review of 99mTc-Aprotinin in the Diagnosis of Cardiac Amyloidosis and a New Life for an Unfairly Abandoned Drug. Biomedicines 2022, 10, 1377. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, S.; Kounnas, M.Z.; Henkin, J.; Mallampalli, R.K.; Chappell, D.A.; Strickland, D.K.; Argraves, W.S. Gp330 on Type II Pneumocytes Mediates Endocytosis Leading to Degradation of Pro-Urokinase, Plasminogen Activator Inhibitor-1 and Urokinase-Plasminogen Activator Inhibitor-1 Complex. J. Cell Sci. 1995, 108 Pt 6, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.E.; Howie, S.E.M. The Role of Megalin (LRP-2/Gp330) during Development. Dev. Biol. 2006, 296, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Buchäckert, Y.; Rummel, S.; Vohwinkel, C.U.; Gabrielli, N.M.; Grzesik, B.A.; Mayer, K.; Herold, S.; Morty, R.E.; Seeger, W.; Vadász, I. Megalin Mediates Transepithelial Albumin Clearance from the Alveolar Space of Intact Rabbit Lungs. J. Physiol. 2012, 590, 5167–5181. [Google Scholar] [CrossRef] [PubMed]
- Vohwinkel, C.U.; Buchäckert, Y.; Al-Tamari, H.M.; Mazzocchi, L.C.; Eltzschig, H.K.; Mayer, K.; Morty, R.E.; Herold, S.; Seeger, W.; Pullamsetti, S.S.; et al. Restoration of Megalin-Mediated Clearance of Alveolar Protein as a Novel Therapeutic Approach for Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2017, 57, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, J.K.; Malanga, C.J.; Rojanasakul, Y. Characterization of Proteolytic Activities of Pulmonary Alveolar Epithelium. Int. J. Pharm. 2000, 195, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Juillerat-Jeanneret, L.; Aubert, J.D.; Leuenberger, P. Peptidases in Human Bronchoalveolar Lining Fluid, Macrophages, and Epithelial Cells: Dipeptidyl (Amino)Peptidase IV, Aminopeptidase N, and Dipeptidyl (Carboxy)Peptidase (Angiotensin-Converting Enzyme). J. Lab. Clin. Med. 1997, 130, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Jeske, N.A.; Berg, K.A.; Cousins, J.C.; Ferro, E.S.; Clarke, W.P.; Glucksman, M.J.; Roberts, J.L. Modulation of Bradykinin Signaling by EP24.15 and EP24.16 in Cultured Trigeminal Ganglia. J. Neurochem. 2006, 97, 13–21. [Google Scholar] [CrossRef]
- Baginski, L.; Tachon, G.; Falson, F.; Patton, J.S.; Bakowsky, U.; Ehrhardt, C. Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis of Proteolytic Enzymes in Cultures of Human Respiratory Epithelial Cells. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 89–101. [Google Scholar] [CrossRef]
- Batah, S.S.; Fabro, A.T. Pulmonary Pathology of ARDS in COVID-19: A Pathological Review for Clinicians. Respir. Med. 2021, 176, 106239. [Google Scholar] [CrossRef] [PubMed]
- Koçak Tufan, Z.; Kayaaslan, B.; Mer, M. COVID-19 and Sepsis. Turk. J. Med. Sci. 2021, 51, 3301–3311. [Google Scholar] [CrossRef] [PubMed]
- Busuttil, R.W. A Comparison of Antifibrinolytic Agents Used in Hemostatic Fibrin Sealants. J. Am. Coll. Surg. 2003, 197, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Beierlein, W.; Scheule, A.M.; Antoniadis, G.; Braun, C.; Schosser, R. An Immediate, Allergic Skin Reaction to Aprotinin after Reexposure to Fibrin Sealant. Transfusion 2000, 40, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Meta, A.; Nakatake, H.; Imamura, T.; Nozaki, C.; Sugimura, K. High-Yield Production and Characterization of Biologically Active Recombinant Aprotinin Expressed in Saccharomyces Cerevisiae. Protein Expr. Purif. 2009, 66, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lage, A.; Díaz, J.W.; González, I. Effect of Proteinase Inhibitor in Experimental Tumors. Neoplasma 1978, 25, 257–259. [Google Scholar] [PubMed]
- Csiszar, A.; Kutay, B.; Wirth, S.; Schmidt, U.; Macho-Maschler, S.; Schreiber, M.; Alacakaptan, M.; Vogel, G.F.; Aumayr, K.; Huber, L.A.; et al. Interleukin-like Epithelial-to-Mesenchymal Transition Inducer Activity Is Controlled by Proteolytic Processing and Plasminogen–Urokinase Plasminogen Activator Receptor System–Regulated Secretion during Breast Cancer Progression. Breast Cancer Res. 2014, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Zhirnov, O.P.; Ovcharenko, A.V.; Goliando, P.B.; Svinogeeva, T.P.; Dolgova, G.V.; Nikitin, A.V. Aprotinin antiviral aerosol: Study of the local irritating and allergenic action after inhalation. Antibiot. Khimioter. 1994, 39, 54–58. [Google Scholar]
- Zhirnov, O.P.; Ovcharenko, A.V.; Goliando, P.B.; Svinogeeva, T.P.; Dolgova, G.V. The antiviral aerosol aprotinin. General effect on the body after inhalation administration. Antibiot. Khimioter. 1994, 39, 25–32. [Google Scholar]

| Targets | Provenance and Affinity | Participation in COVID-19 | Bibliography | |
|---|---|---|---|---|
| Proteases | Kallikreins (FXII—Hageman factor; HMWK—Fitzgerald factor) | They are of serum (Fletcher’s factor) and tissue origin, they are produced as a precursor (prekallikreins)
|
| Hoffmann et al., 1989 [27] Moreau et al., 2005 [28] Brinkmann et al., 1991 [29] Fritz and Wunderer, 1983 [5] Ivachtchenko et al., 2023 [26] |
| Thrombin (FII) | It is synthesised in hepatocytes as a precursor (prothrombin)
|
| Fritz and Wunderer, 1983 [5] Zhirnov et al., 2011 [4] Pintigny and Dachary-Prigent, 1992 [30] | |
| Plasmin | It is synthesised in hepatocytes as a precursor (plasminogen)
|
| Fritz and Wunderer, 1983 [5] Ivachtchenko et al., 2023 [26] Sun et al., 2009 [31] | |
| Fibrinogen-fibrin (FI-FIa) | It is synthesised in the liver |
| Fritz and Wunderer, 1983 [5] | |
| Tissue plasminogen activator (TPa) | Aprotinin has a Kunitz-like domain similar to tissue factor inhibitor peptide
|
| Fritz and Wunderer, 1983 [5] Ivachtchenko et al., 2023 [26] | |
| Cathepsins | They are synthesised by epithelial, inflammatory cells.
|
| Brinkmann et al., 1991 [29] Fahy et al., 1992 [32] | |
| Chymostatin | It is an anti-serine and cysteine-protease released from epithelial and inflammatory cells that potently inhibits cathepsin G or chymotrypsin
|
| Fritz and Wunderer, 1983 [5] Ivachtchenko et al., 2023 [26] | |
| Chymotrypsin | Glandular tissues
|
| Fritz and Wunderer, 1983 [5] Ivachtchenko et al., 2023 [26] | |
| Trypsin | It is a serine protease released from epithelial and inflammatory cells
|
| Fritz and Wunderer, 1983 [5] Ivachtchenko et al., 2023 [26] Brinkmann et al., 1991 [29] | |
| Chimases | Epithelial cells and myeloid cells
|
| Lindberg et al., 1997 [33] | |
| Neutrophil elastase | Released by neutrophils, contained in their azurophilic granules
|
| Fritz and Wunderer, 1983 [5] Ivachtchenko et al., 2023 [26] Brinkmann et al., 1991 [29] | |
| Tryptase TL2 |
|
| Kido et al., 1990, [34] Brinkmann et al., 1997 [35] Fritz and Wunderer, 1983 [5] Ivachtchenko et al., 2023 [26] | |
| Dipeptidyl peptidase 3 and 4 (DPP-3 and 4) | Ubiquitous
|
| Engel et al., 2006 [36] Abramić and Agić, 2022 [37] | |
| Matrix metalloproteases (MMP2 and 9) | Zinc metalloproteases. Ubiquitous
|
| Kuyvanhoven et al., 2004 [38] Shu-Chen Chu et al., 2004 [39] | |
| Angiotensinase C | It is a polycarboxypeptidase of lysosomal origin. Structural homology with DPP-2 |
| Ripa and Gilli, 1968 [40] Dahlheim, 1972 [41] | |
| PAR-1 and 2 | GPCRs activated by thrombin and trypsin. Intracellular signalling and intrinsic protease activity
|
| Day et al., 2006 [42] Landis, 2007 [43] Khan et al., 2005 [44] Gomides et al., 2012 [45] | |
| Transmembrane serine protease 2 (TMPRSS2) | Epithelial cells |
| Yamaya et al., 2015 [46] | |
| Mannose-associated serine protease (MASP) 2 and 3 |
|
| Petersen et al., 2000 [47] Cortesio and Jiang 2006 [48] Keizer et al., 2015 [49] | |
| Oligopeptidases (EP24.15) | Cytosolic, associated with membranes and secreted to the outside of the cellProtease that degrades peptides such as bradykinin and angiotensin |
| Vanneste et al., 1990 [50] Aoyagi et al., 1990 [51] | |
| Not too much (EP24.11) | Metallopeptides. Killers |
| Vanneste et al., 1990 [50] Aoyagi et al., 1990 [51] | |
| Other targets | Nitric oxide synthase (iNOS) | Ubiquitous
|
| Hill et al., 1997 [52] Hill and Robbins, 1997 [53] Bruda et al., 1998 [54] |
| ENaC | Epithelial cells
|
| Adebamiro et al., 2005 [55] | |
| α2-Macroblobulin receptor | Bronchial and alveolar epithelial fibroblasts, dendritic cells, and alveolar and vascular macrophages |
| Moestrup et al., 1995 [56] | |
| GP-330/Megaline receiver | Type I and II pneumocytes and alveolar epithelial cells
|
| Moestrup et al., 1995 [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padín, J.-F.; Pérez-Ortiz, J.M.; Redondo-Calvo, F.J. Aprotinin (II): Inhalational Administration for the Treatment of COVID-19 and Other Viral Conditions. Int. J. Mol. Sci. 2024, 25, 7209. https://doi.org/10.3390/ijms25137209
Padín J-F, Pérez-Ortiz JM, Redondo-Calvo FJ. Aprotinin (II): Inhalational Administration for the Treatment of COVID-19 and Other Viral Conditions. International Journal of Molecular Sciences. 2024; 25(13):7209. https://doi.org/10.3390/ijms25137209
Chicago/Turabian StylePadín, Juan-Fernando, José Manuel Pérez-Ortiz, and Francisco Javier Redondo-Calvo. 2024. "Aprotinin (II): Inhalational Administration for the Treatment of COVID-19 and Other Viral Conditions" International Journal of Molecular Sciences 25, no. 13: 7209. https://doi.org/10.3390/ijms25137209
APA StylePadín, J.-F., Pérez-Ortiz, J. M., & Redondo-Calvo, F. J. (2024). Aprotinin (II): Inhalational Administration for the Treatment of COVID-19 and Other Viral Conditions. International Journal of Molecular Sciences, 25(13), 7209. https://doi.org/10.3390/ijms25137209





