Exploring the Effect of Arsenic-Containing Hydrocarbon on the Bidirectional Synaptic Plasticity of the Dorsal Hippocampus
Abstract
:1. Introduction
2. Results
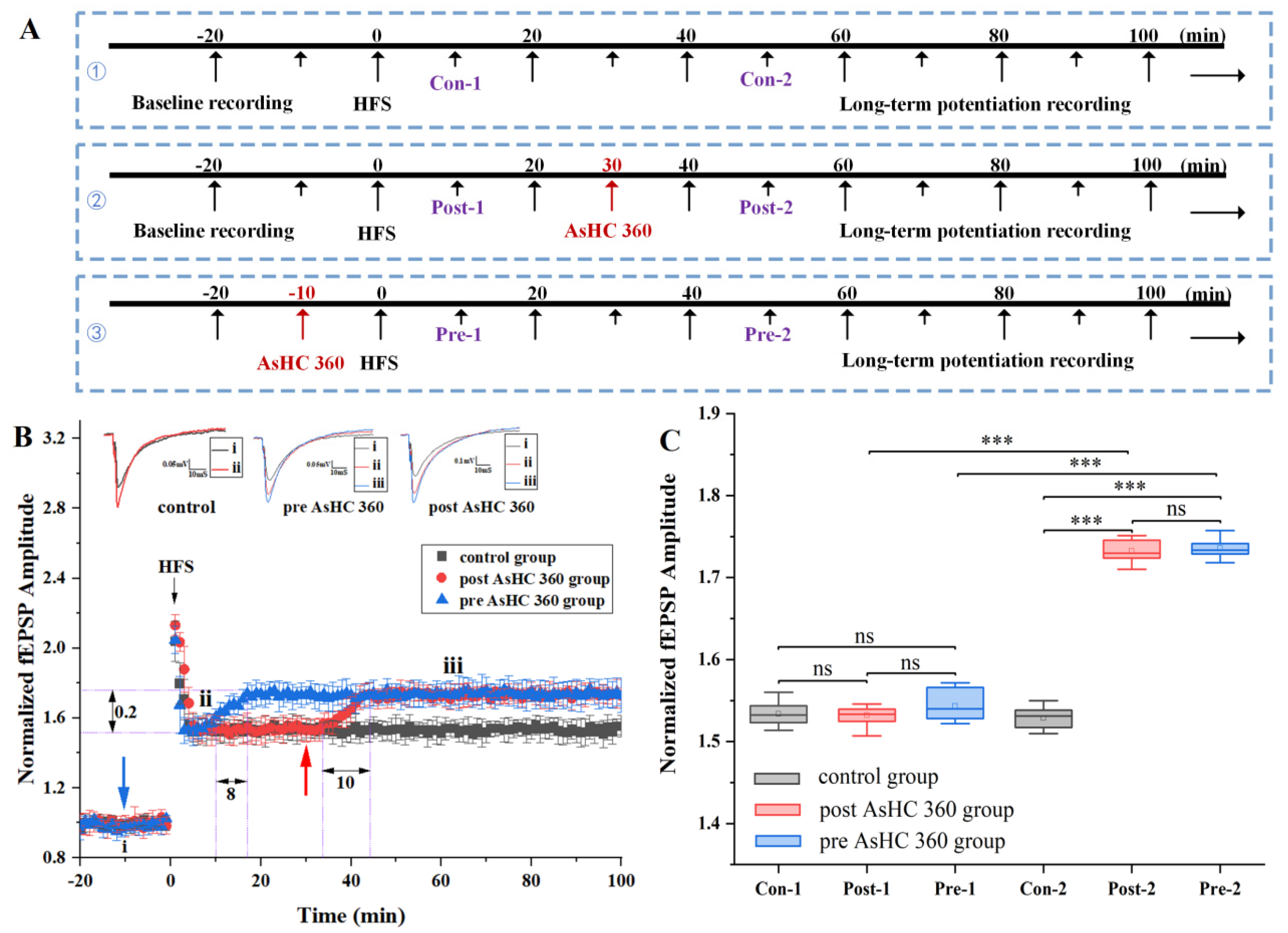
2.1. AsHC 360 Enhances HFS-LTP
2.2. AsHC 360 Enhances TBS-LTP
2.3. AsHC 360 Reduces LFS-LTD
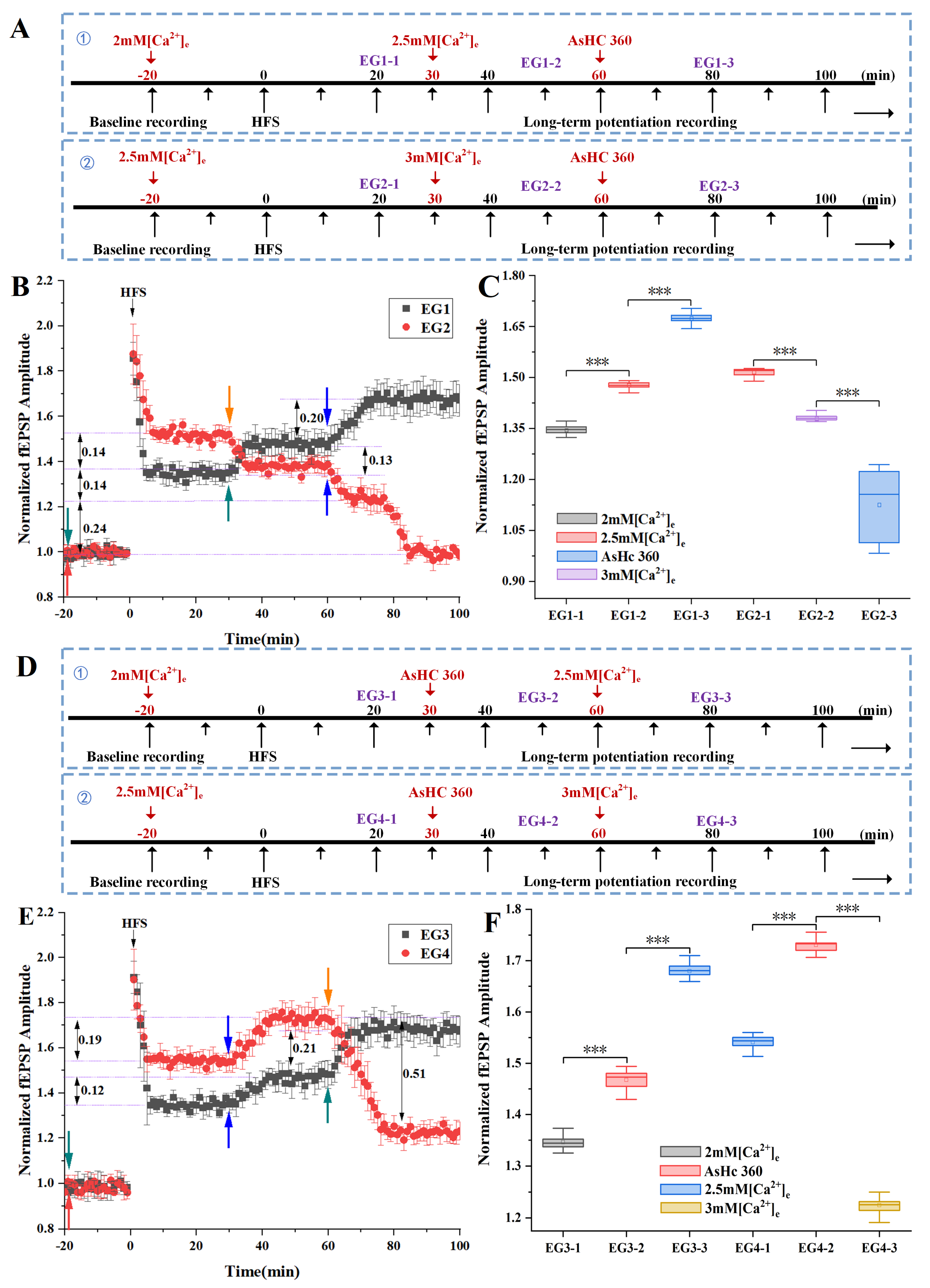
2.4. Ca2+ Activity Was Involved in the Process of LTP Enhancement Caused by Post AsHC 360
2.5. Ca2+ Activity Was Involved in the Process of LTD Enhancement Caused by Post AsHC 360
3. Discussion
3.1. AsHC 360 Facilitates Bidirectional Synaptic Plasticity during the Maintenance Phase Rather Than the Baseline Stage
3.2. Different [Ca2+]e Has Different Effects on Bidirectional Synaptic Plasticity
3.3. AsHC 360 Promotes Bidirectional Synaptic Plasticity by Influencing Ca2+ Levels
4. Materials and Methods
4.1. Animals
4.2. Experimental Pharmaceuticals and Reagents
4.3. The Preparation of Hippocampal Brain Slices
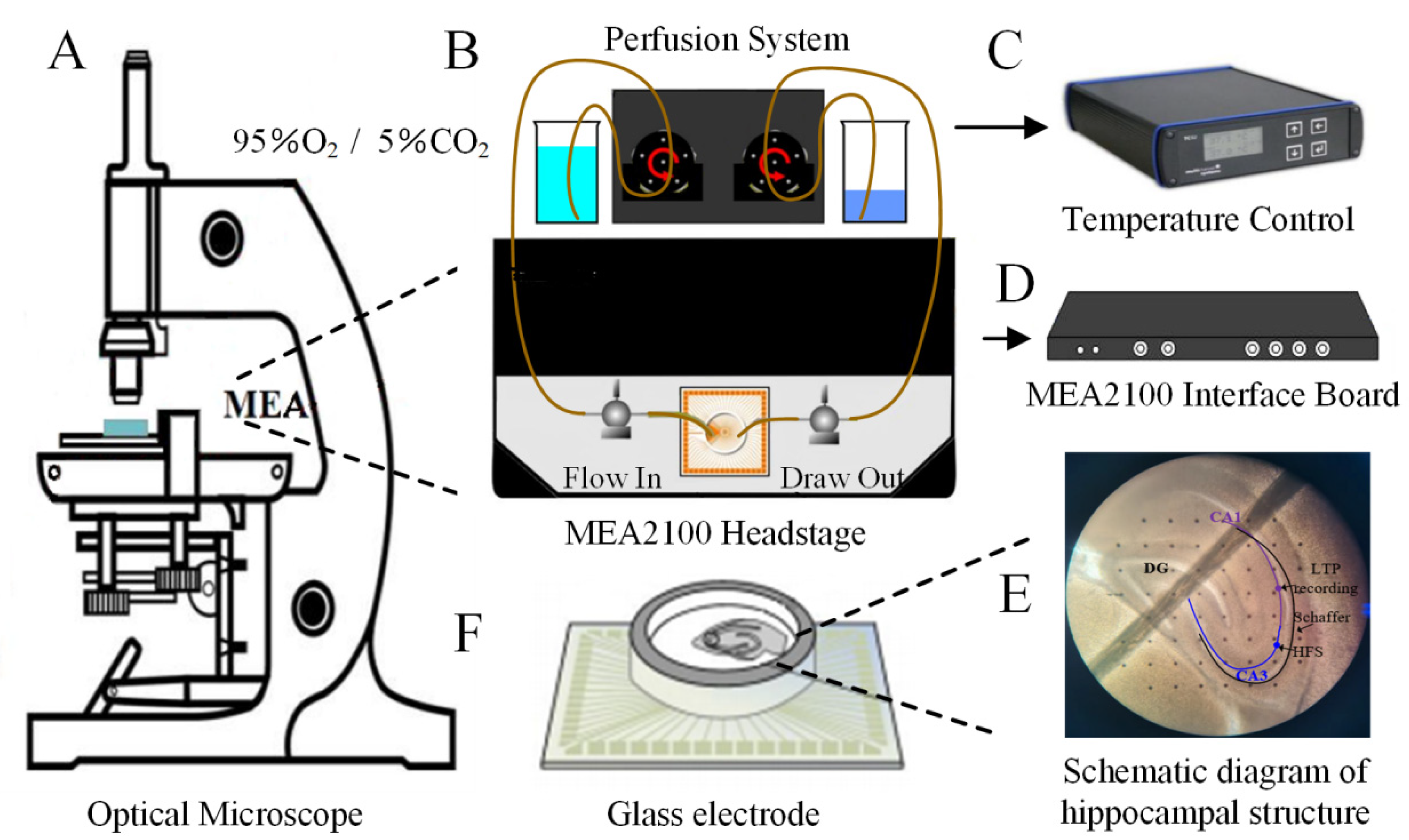
4.4. Electrophysiological Experimental Setup and fEPSPs Recording Method
4.5. Experimental Design
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunwittaya, S.; Ruksee, N.; Khamnong, T.; Jiawiwatkul, A.; Kleebpung, N.; Chumchua, V.; Plitponkarnpim, A.; Nopparat, C.; Permpoonputtana, K. Inorganic arsenic contamination and the health of children living near an inactive mining site: Northern Thailand. EXCLI J. 2022, 21, 1007. [Google Scholar] [PubMed]
- Zhang, J.; Hu, T.; Wang, Y.; Zhang, X.; Zhang, H.; Lin, J.; Tang, X.; Liu, X.; Chen, M.; Khan, N.U.; et al. Investigating the neurotoxic impacts of arsenic and the neuroprotective effects of dictyophora polysaccharide using SWATH-MS-based proteomics. Molecules 2022, 27, 1495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, S.; Sun, Y.; Dong, W.; Piao, F.; Piao, Y.; Guan, H.; Yu, S. Arsenic downregulates gene expression at the postsynaptic density in mouse cerebellum, including genes responsible for long-term potentiation and depression. Toxicol. Lett. 2014, 228, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Borak, J.; Hosgood, H.D. Seafood arsenic: Implications for human risk assessment. Regul. Toxicol. Pharmacol. 2007, 47, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Taleshi, M.S.; Seidler-Egdal, R.K.; Jensen, K.B.; Schwerdtle, T.; Francesconi, K.A. Synthesis and characterization of arsenolipids: Naturally occurring arsenic compounds in fish and algae. Organometallics 2014, 33, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Matissek, M.; Müller, S.M.; Taleshi, M.S.; Ebert, F.; Francesconi, K.A.; Schwerdtle, T. In vitro toxicological characterisation of three arsenic-containing hydrocarbons. Metallomics 2014, 6, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhao, L.; Tian, L.; Zhao, W.; Xiong, C.; Zheng, Y. AsHC 360 Exposure Influence on Epileptiform Discharges in Hippocampus of Infantile Male Rats In Vitro. Int. J. Mol. Sci. 2023, 24, 16806. [Google Scholar] [CrossRef]
- Meyer, S.; Schulz, J.; Jeibmann, A.; Taleshi, M.S.; Ebert, F.; Francesconi, K.A.; Schwerdtle, T. Arsenic-containing hydrocarbons are toxic in the in vivo model Drosophila melanogaster. Metallomics 2014, 6, 2010–2014. [Google Scholar] [CrossRef]
- Niehoff, A.-C.; Schulz, J.; Soltwisch, J.; Meyer, S.; Kettling, H.; Sperling, M.; Jeibmann, A.; Dreisewerd, K.; Francesconi, K.A.; Schwerdtle, T.; et al. Imaging by elemental and molecular mass spectrometry reveals the uptake of an arsenolipid in the brain of Drosophila melanogaster. Anal. Chem. 2016, 88, 5258–5263. [Google Scholar] [CrossRef]
- Müller, S.M.; Ebert, F.; Raber, G.; Meyer, S.; Bornhorst, J.; Hüwel, S.; Galla, H.J.; Francesconi, K.A.; Schwerdtle, T. Effects of arsenolipids on in vitro blood-brain barrier model. Arch. Toxicol. 2018, 92, 823–832. [Google Scholar] [CrossRef]
- Stiboller, M.; Raber, G.; Lenters, V.; Gjengedal, E.L.F.; Eggesbø, M.; Francesconi, K.A. Arsenolipids detected in the milk of nursing mothers. Environ. Sci. Technol. Lett. 2017, 4, 273–279. [Google Scholar] [CrossRef]
- Xiong, C.; Stiboller, M.; Glabonjat, R.A.; Rieger, J.; Paton, L.; Francesconi, K.A. Transport of arsenolipids to the milk of a nursing mother after consuming salmon fish. J. Trace Elem. Med. Biol. 2020, 61, 126502. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Petursdottir, A.H.; Rikhardsson, G.; Stergiadis, S.; Raab, A.; Feldmann, J. Speciation of arsenic in milk from cows fed seaweed. J. Sci. Food Agric. 2024. [Google Scholar] [CrossRef] [PubMed]
- Scoville, W.B.; Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 1957, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; Dong, H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Mikulovic, S.; Restrepo, C.E.; Siwani, S.; Bauer, P.; Pupe, S.; Tort, A.B.; Kullander, K.; Leão, R.N. Ventral hippocampal OLM cells control type 2 theta oscillations and response to predator odor. Nat. Commun. 2018, 9, 3638. [Google Scholar] [CrossRef] [PubMed]
- Barkus, C.; McHugh, S.B.; Sprengel, R.; Seeburg, P.H.; Rawlins, J.N.P.; Bannerman, D.M. Hippocampal NMDA receptors and anxiety: At the interface between cognition and emotion. Eur. J. Pharmacol. 2010, 626, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Otmakhova, N.A.; Lewey, J.; Asrican, B.; Lisman, J.E. Inhibition of perforant path input to the CA1 region by serotonin and noradrenaline. J. Neurophysiol. 2005, 94, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Luján, R.; Watanabe, M.; Adelman, J.P.; Maylie, J. SK2 channel plasticity contributes to LTP at Schaffer collateral–CA1 synapses. Nat. Neurosci. 2008, 11, 170–177. [Google Scholar] [CrossRef]
- Di Prisco, G.V. Hebb synaptic plasticity. Prog. Neurobiol. 1984, 22, 89–102. [Google Scholar]
- Bear, M.F.; Malenka, R.C. Synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol. 1994, 4, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Steele, P.M.; Mauk, M.D. Inhibitory control of LTP and LTD: Stability of synapse strength. J. Neurophysiol. 1999, 81, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E. Presynaptic LTP and LTD of excitatory and inhibitory synapses. Cold Spring Harb. Perspect. Biol. 2012, 4, a005728. [Google Scholar] [CrossRef] [PubMed]
- Huganir, R.L.; Nicoll, R.A. AMPARs and synaptic plasticity: The last 25 years. Neuron 2013, 80, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Dong, L.; Tian, L.; Zhao, L.; Zhao, Y.; Zheng, Y. Changes in intracellular calcium concentration level accompany age-related inhibitions of long-term potentiation in hippocampus induced by extremely low frequency electromagnetic fields. Eur. J. Neurosci. 2023, 58, 2437–2450. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Zheng, Y.; Dong, L.; Tian, C. Short-Term Extremely Low-Frequency Electromagnetic Field Inhibits Synaptic Plasticity of Schaffer Collateral-CA1 Synapses in Rat Hippocampus via the Ca2+/Calcineurin Pathway. ACS Chem. Neurosci. 2021, 12, 3550–3557. [Google Scholar] [CrossRef]
- Yang, S.N.; Tang, Y.G.; Zucker, R.S. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J. Neurophysiol. 1999, 81, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, C.; Dong, L.; Tian, L.; Glabonjat, R.A.; Xiong, C. Effect of arsenic-containing hydrocarbon on the long-term potentiation at Schaffer Collateral-CA1 synapses from infantile male rat. Neurotoxicology 2021, 84, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Lisman, J.; Schulman, H.; Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002, 3, 175–190. [Google Scholar] [CrossRef]
- Lu, W.Y.; Man, H.Y.; Ju, W.; Trimble, W.S.; MacDonald, J.F.; Wang, Y.T. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 2001, 29, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Mulkey, R.M.; Herron, C.E.; Malenka, R.C. An essential role for protein phosphatases in hippocampal long-term depression. Science 1993, 261, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Malenka, R.C.; Bear, M.F. LTP and LTD: An embarrassment of riches. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Peineau, S.; Howland, J.G.; Wang, Y.T. Long-term depression in the CNS. Nat. Rev. Neurosci. 2010, 11, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, X.X.; Dong, L.; Ma, W.; Cheng, J.H. Effects of uninterrupted sinusoidal LF-EMF stimulation on LTP induced by different combinations of TBS/HFS at the Schaffer collateral-CA1 of synapses. Brain Res. 2019, 1725, 146487. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, G.; Gao, Y.; Lin, L.; Zheng, Y.; Cao, X.B. Exploring the form-and time-dependent effect of low-frequency electromagnetic fields on maintenance of hippocampal long-term potentiation. Eur. J. Neurosci. 2020, 52, 3166–3180. [Google Scholar] [CrossRef] [PubMed]
- Kopanitsa, M.V.; Afinowi, N.O.; Grant, S.G. Recording long-term potentiation of synaptic transmission by three-dimensional multi-electrode arrays. BMC Neurosci. 2006, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chafai, M.; Corbani, M.; Guillon, G.; Desarménien, M.G. Vasopressin inhibits LTP in the CA2 mouse hippocampal area. PLoS ONE 2012, 7, e49708. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Yang, Y.; Xiong, Y.; Zhang, Y.J.; Jiang, J.; Zhou, L.P.; Du, X.H.; Wang, C.X.; Zhu, Z.R. Blockade of adenosine A2A receptors reverses early spatial memory defects in the APP/PS1 mouse model of Alzheimer’s disease by promoting synaptic plasticity of adult-born granule cells. Alzheimer’s Res. Ther. 2023, 15, 187. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, C.; Qi, Y.; Zheng, Y.; Xia, P.; Liu, Q.; Luan, M.; Zheng, J.; Song, R.; Wang, M.; Qi, D.; et al. Exploring the Effect of Arsenic-Containing Hydrocarbon on the Bidirectional Synaptic Plasticity of the Dorsal Hippocampus. Int. J. Mol. Sci. 2024, 25, 7223. https://doi.org/10.3390/ijms25137223
Tian C, Qi Y, Zheng Y, Xia P, Liu Q, Luan M, Zheng J, Song R, Wang M, Qi D, et al. Exploring the Effect of Arsenic-Containing Hydrocarbon on the Bidirectional Synaptic Plasticity of the Dorsal Hippocampus. International Journal of Molecular Sciences. 2024; 25(13):7223. https://doi.org/10.3390/ijms25137223
Chicago/Turabian StyleTian, Chunxiao, Yenan Qi, Yu Zheng, Pei Xia, Qiwen Liu, Mengying Luan, Junyao Zheng, Rujuan Song, Meng Wang, Dejiao Qi, and et al. 2024. "Exploring the Effect of Arsenic-Containing Hydrocarbon on the Bidirectional Synaptic Plasticity of the Dorsal Hippocampus" International Journal of Molecular Sciences 25, no. 13: 7223. https://doi.org/10.3390/ijms25137223




