The Role of Lutheran/Basal Cell Adhesion Molecule in Hematological Diseases and Tumors
Abstract
:1. Introduction
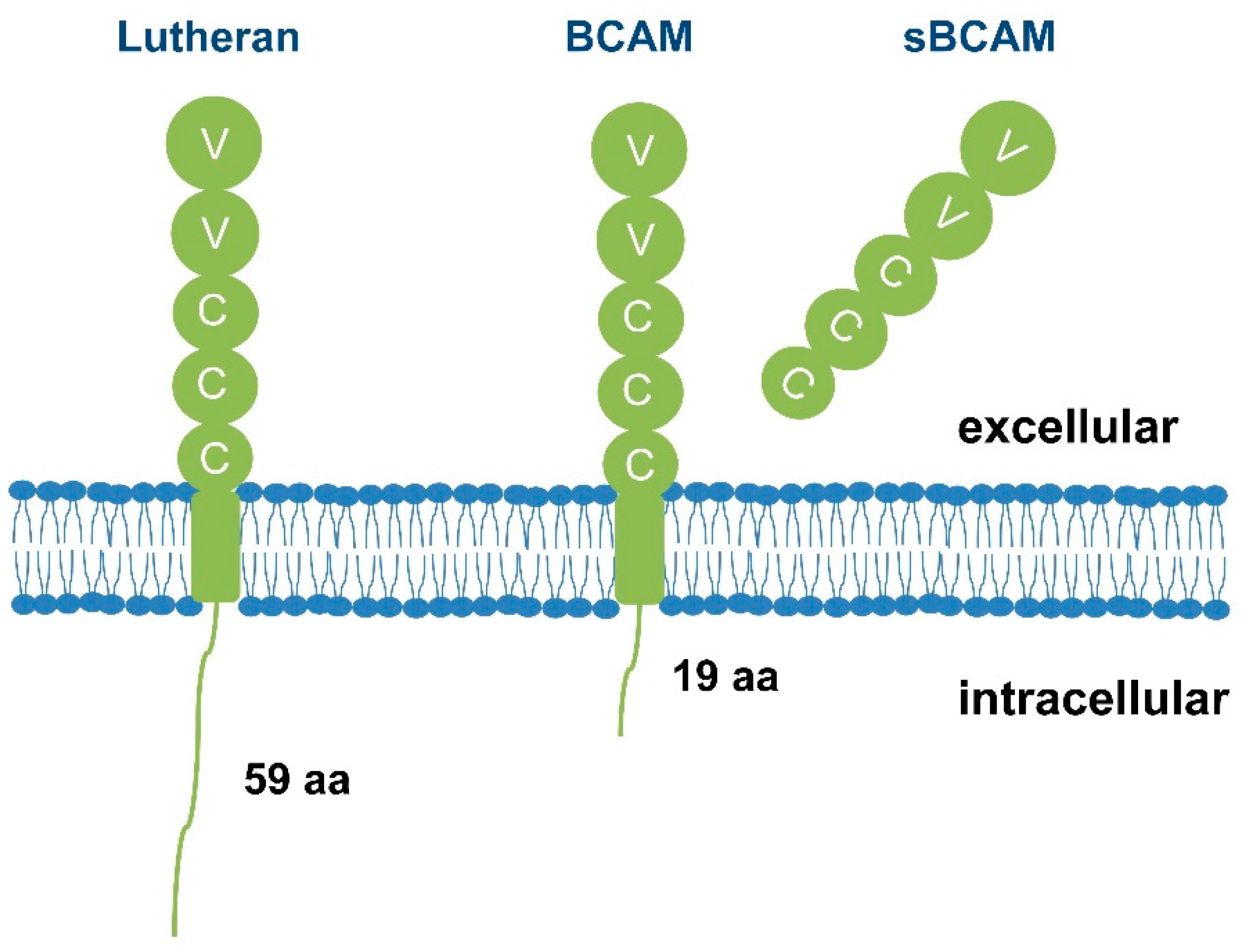
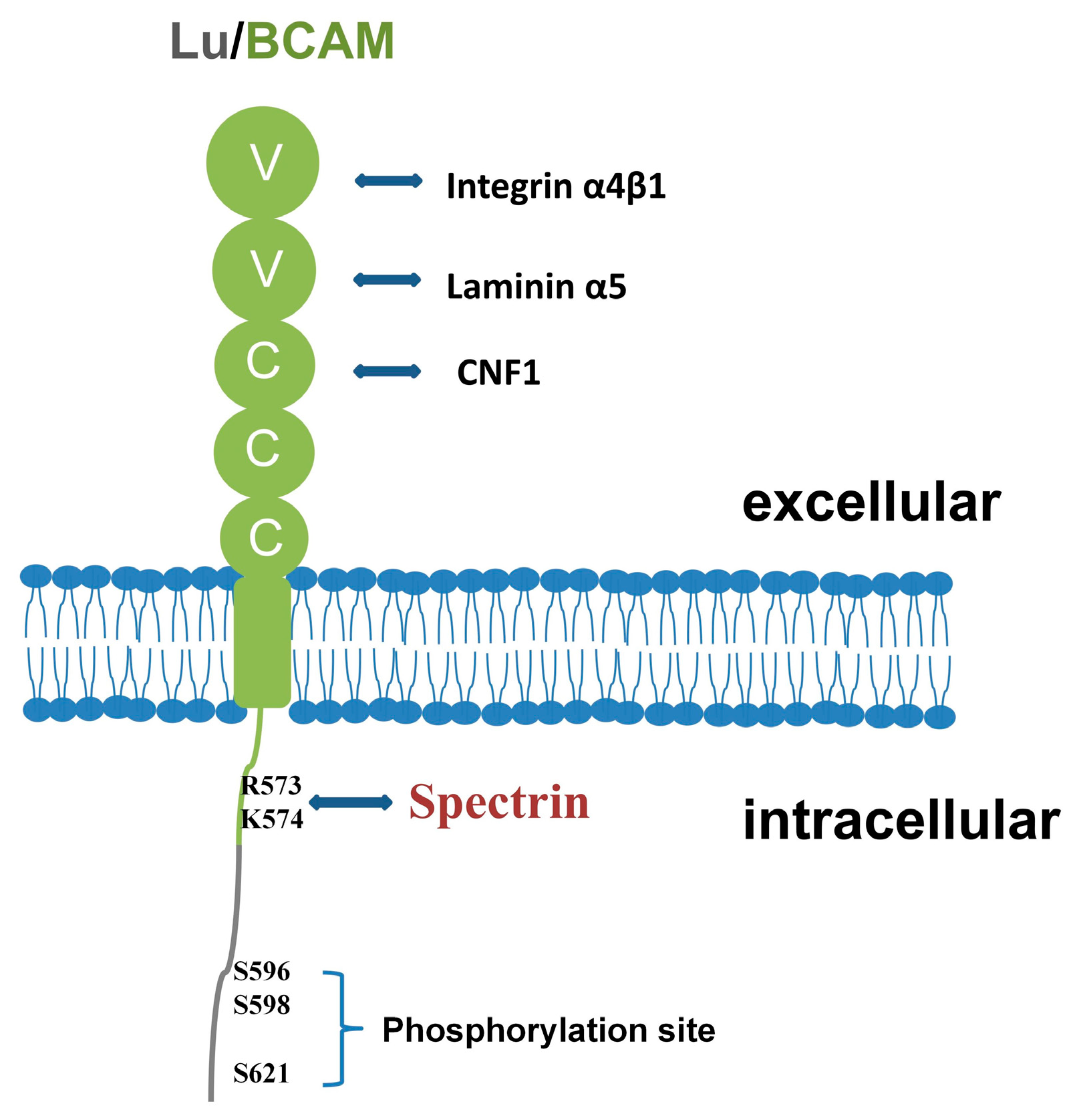
2. The Role of Lu/BCAM
2.1. Lu/BCAM Can Serve as a Receptor for Laminin
2.2. Lu/BCAM Can Interact with Integrin
2.3. Lu/BCAM Can Serve as a Receptor for Cytotoxic Necrosis Factor CNF1
2.4. Lu/BCAM Can Be Connected to Spectrin
3. Regulation of Lu/BCAM
3.1. Expression Regulation of Lu/BCAM
3.2. Phosphorylation Modification Regulation of Lu/BCAM
3.3. Distribution Modification of Lu/BCAM
3.4. Lu/BCAM Can Be Cut by MT1-MMP
3.5. Activation of Lu/BCAM
4. Lu/BCAM in Human Diseases
4.1. Lu/BCAM and Hematological Diseases
4.1.1. Polycythemia Vera
4.1.2. SCA
4.1.3. Severe Malarial Anemia
4.1.4. HIV-Related Atherosclerotic Lesions
4.2. Lu/BCAM and Tumors
4.2.1. Skin Tumor
4.2.2. Liver Cancer
4.2.3. Bladder Cancer
4.2.4. Colorectal Cancer
4.2.5. Ovarian Cancer
4.2.6. Gastric Cancer
4.2.7. Thyroid Cancer
5. Potential Clinical Applications of Lu/BCAM
5.1. Biomarkers
5.2. Therapeutic Targets
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Honig, B.; Shapiro, L. Adhesion Protein Structure, Molecular Affinities, and Principles of Cell-Cell Recognition. Cell 2020, 181, 520–535. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Stevens, A.J.; Harris, A.R.; Gerdts, J.; Kim, K.H.; Trentesaux, C.; Ramirez, J.T.; McKeithan, W.L.; Fattahi, F.; Klein, O.D.; Fletcher, D.A.; et al. Programming multicellular assembly with synthetic cell adhesion molecules. Nature 2023, 614, 144–152. [Google Scholar] [CrossRef] [PubMed]
- El Nemer, W.; Gane, P.; Colin, Y.; Bony, V.; Rahuel, C.; Galactéros, F.; Cartron, J.P.; Le Van Kim, C. The Lutheran blood group glycoproteins, the erythroid receptors for laminin, are adhesion molecules. J. Biol. Chem. 1998, 273, 16686–16693. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.G.; Foulkes, W.D.; Senger, G.; Trowsdale, J.; Garin-Chesa, P.; Rettig, W.J. Molecular cloning of the B-CAM cell surface glycoprotein of epithelial cancers: A novel member of the immunoglobulin superfamily. Cancer Res. 1994, 54, 5761–5765. [Google Scholar]
- El Nemer, W.; Rahuel, C.; Colin, Y.; Gane, P.; Cartron, J.P.; Le Van Kim, C. Organization of the human LU gene and molecular basis of the Lu(a)/Lu(b) blood group polymorphism. Blood 1997, 89, 4608–4616. [Google Scholar] [CrossRef]
- Burton, N.M.; Brady, R.L. Molecular structure of the extracellular region of Lutheran blood group glycoprotein and location of the laminin binding site. Blood Cells Mol. Dis. 2008, 40, 446–448. [Google Scholar] [CrossRef]
- Rahuel, C.; Le Van Kim, C.; Mattei, M.G.; Cartron, J.P.; Colin, Y. A unique gene encodes spliceoforms of the B-cell adhesion molecule cell surface glycoprotein of epithelial cancer and of the Lutheran blood group glycoprotein. Blood 1996, 88, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, E.; Rahuel, C.; Wautier, M.P.; El Nemer, W.; Gane, P.; Wautier, J.L.; Cartron, J.P.; Colin, Y.; Le Van Kim, C. Protein kinase A-dependent phosphorylation of Lutheran/basal cell adhesion molecule glycoprotein regulates cell adhesion to laminin alpha5. J. Biol. Chem. 2005, 280, 30055–30062. [Google Scholar] [CrossRef]
- Finkernagel, F.; Reinartz, S.; Schuldner, M.; Malz, A.; Jansen, J.M.; Wagner, U.; Worzfeld, T.; Graumann, J.; von Strandmann, E.P.; Müller, R. Dual-platform affinity proteomics identifies links between the recurrence of ovarian carcinoma and proteins released into the tumor microenvironment. Theranostics 2019, 9, 6601–6617. [Google Scholar] [CrossRef]
- Sivakumar, S.; Lieber, S.; Librizzi, D.; Keber, C.; Sommerfeld, L.; Finkernagel, F.; Roth, K.; Reinartz, S.; Bartsch, J.W.; Graumann, J.; et al. Basal cell adhesion molecule promotes metastasis-associated processes in ovarian cancer. Clin. Transl. Med. 2023, 13, e1776. [Google Scholar] [CrossRef]
- Kikkawa, Y.; Miner, J.H. Review: Lutheran/B-CAM: A laminin receptor on red blood cells and in various tissues. Connect. Tissue Res. 2005, 46, 193–199. [Google Scholar] [CrossRef]
- Udani, M.; Zen, Q.; Cottman, M.; Leonard, N.; Jefferson, S.; Daymont, C.; Truskey, G.; Telen, M.J. Basal cell adhesion molecule/lutheran protein. The receptor critical for sickle cell adhesion to laminin. J. Clin. Investig. 1998, 101, 2550–2558. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, Y.; Ogawa, T.; Sudo, R.; Yamada, Y.; Katagiri, F.; Hozumi, K.; Nomizu, M.; Miner, J.H. The Lutheran/Basal Cell Adhesion Molecule Promotes Tumor Cell Migration by Modulating Integrin-mediated Cell Attachment to Laminin-511 Protein. J. Biol. Chem. 2013, 288, 30990–31001. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, A.; Cardaci, S.; Lamba, S.; Oddo, D.; Marchiò, C.; Cassoni, P.; Amoreo, C.A.; Corti, G.; Testori, A.; Bussolino, F.; et al. BCAM and LAMA5 Mediate the Recognition between Tumor Cells and the Endothelium in the Metastatic Spreading of KRAS-Mutant Colorectal Cancer. Clin. Cancer Res. 2016, 22, 4923–4933. [Google Scholar] [CrossRef]
- Chang, H.Y.; Chang, H.M.; Wu, T.J.; Chaing, C.Y.; Tzai, T.S.; Cheng, H.L.; Raghavaraju, G.; Chow, N.H.; Liu, H.S. The role of Lutheran/basal cell adhesion molecule in human bladder carcinogenesis. J. Biomed. Sci. 2017, 24, 61. [Google Scholar] [CrossRef]
- Aumailley, M.; Smyth, N. The role of laminins in basement membrane function. J. Anat. 1998, 193, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Colognato, H.; Yurchenco, P.D. Form and function: The laminin family of heterotrimers. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2000, 218, 213–234. [Google Scholar] [CrossRef]
- Kikkawa, Y.; Sanzen, N.; Fujiwara, H.; Sonnenberg, A.; Sekiguchi, K. Integrin binding specificity of laminin-10/11: Laminin-10/11 are recognized by alpha 3 beta 1, alpha 6 beta 1 and alpha 6 beta 4 integrins. J. Cell Sci. 2000, 113, 869–976. [Google Scholar] [CrossRef]
- Shimizu, H.; Hosokawa, H.; Ninomiya, H.; Miner, J.H.; Masaki, T. Adhesion of cultured bovine aortic endothelial cells to laminin-1 mediated by dystroglycan. J. Biol. Chem. 1999, 274, 11995–12000. [Google Scholar] [CrossRef]
- Luo, D.; Ruan, S.; Liu, A.; Kong, X.; Lee, I.S.; Chen, C. Laminin functionalized biomimetic apatite to regulate the adhesion and proliferation behaviors of neural stem cells. Int. J. Nanomed. 2018, 13, 6223–6233. [Google Scholar] [CrossRef]
- Miner, J.H.; Patton, B.L.; Lentz, S.I.; Gilbert, D.J.; Snider, W.D.; Jenkins, N.A.; Copeland, N.G.; Sanes, J.R. The laminin alpha chains: Expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alpha3 isoform. J. Cell Biol. 1997, 137, 685–701. [Google Scholar] [CrossRef]
- El Nemer, W.; Gane, P.; Colin, Y.; D’Ambrosio, A.M.; Callebaut, I.; Cartron, J.P.; Van Kim, C.L. Characterization of the laminin binding domains of the Lutheran blood group glycoprotein. J. Biol. Chem. 2001, 276, 23757–23762. [Google Scholar] [CrossRef] [PubMed]
- Zen, Q.; Cottman, M.; Truskey, G.; Fraser, R.; Telen, M.J. Critical factors in basal cell adhesion molecule/lutheran-mediated adhesion to laminin. J. Biol. Chem. 1999, 274, 728–734. [Google Scholar] [CrossRef]
- El Nemer, W.; Colin, Y.; Le Van Kim, C. Role of Lu/BCAM glycoproteins in red cell diseases. Transfus. Clin. Et Biol. 2010, 17, 143–147. [Google Scholar] [CrossRef]
- Belkin, A.M.; Stepp, M.A. Integrins as receptors for laminins. Microsc. Res. Tech. 2000, 51, 280–301. [Google Scholar] [CrossRef] [PubMed]
- El Nemer, W.; Wautier, M.P.; Rahuel, C.; Gane, P.; Hermand, P.; Galactéros, F.; Wautier, J.L.; Cartron, J.P.; Colin, Y.; Le Van Kim, C. Endothelial Lu/BCAM glycoproteins are novel ligands for red blood cell alpha4beta1 integrin: Role in adhesion of sickle red blood cells to endothelial cells. Blood 2007, 109, 3544–3551. [Google Scholar] [CrossRef] [PubMed]
- van der Flier, A.; Sonnenberg, A. Function and interactions of integrins. Cell Tissue Res. 2001, 305, 285–298. [Google Scholar] [CrossRef]
- Hillery, C.A.; Du, M.C.; Montgomery, R.R.; Scott, J.P. Increased adhesion of erythrocytes to components of the extracellular matrix: Isolation and characterization of a red blood cell lipid that binds thrombospondin and laminin. Blood 1996, 87, 4879–4886. [Google Scholar] [CrossRef]
- Ulett, G.C.; Totsika, M.; Schaale, K.; Carey, A.J.; Sweet, M.J.; Schembri, M.A. Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection. Curr. Opin. Microbiol. 2013, 16, 100–107. [Google Scholar] [CrossRef]
- Davis, J.M.; Rasmussen, S.B.; O’Brien, A.D. Cytotoxic necrotizing factor type 1 production by uropathogenic Escherichia coli modulates polymorphonuclear leukocyte function. Infect. Immun. 2005, 73, 5301–5310. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Hong, S.J.; Kim, K.J.; Goti, D.; Stins, M.F.; Shin, S.; Dawson, V.L.; Dawson, T.M.; Kim, K.S. 37-kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J. Biol. Chem. 2003, 278, 16857–16862. [Google Scholar] [CrossRef] [PubMed]
- Piteau, M.; Papatheodorou, P.; Schwan, C.; Schlosser, A.; Aktories, K.; Schmidt, G. Lu/BCAM adhesion glycoprotein is a receptor for Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1). PLoS Pathog. 2014, 10, e1003884. [Google Scholar] [CrossRef]
- de Brevern, A.G. Analysing the Structural Effect of Point Mutations of Cytotoxic Necrotizing Factor 1 (CNF1) on Lu/BCAM Adhesion Glycoprotein Association. Toxins 2018, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Reppin, F.; Cochet, S.; El Nemer, W.; Fritz, G.; Schmidt, G. High Affinity Binding of Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1) to Lu/BCAM Adhesion Glycoprotein. Toxins 2017, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.H. Actin-binding proteins. 1: Spectrin super family. Protein Profile 1995, 2, 703–800. [Google Scholar] [PubMed]
- Stankewich, M.C.; Cianci, C.D.; Stabach, P.R.; Ji, L.; Nath, A.; Morrow, J.S. Cell organization, growth, and neural and cardiac development require αII-spectrin. J. Cell Sci. 2011, 124, 3956–3966. [Google Scholar] [CrossRef] [PubMed]
- Machnicka, B.; Grochowalska, R.; Boguslawska, D.M.; Sikorski, A.F.; Lecomte, M.C. Spectrin-based skeleton as an actor in cell signaling. Cell. Mol. Life Sci. 2012, 69, 191–201. [Google Scholar] [CrossRef]
- Marchesi, V.T.; Steers, E., Jr. Selective solubilization of a protein component of the red cell membrane. Science 1968, 159, 203–204. [Google Scholar] [CrossRef]
- Kroviarski, Y.; El Nemer, W.; Gane, P.; Rahuel, C.; Gauthier, E.; Lecomte, M.C.; Cartron, J.P.; Colin, Y. Direct interaction between the Lu/B-CAM adhesion glycoproteins and erythroid spectrin. Br. J. Haematol. 2004, 126, 255–264. [Google Scholar] [CrossRef]
- An, X.L.; Gauthier, E.; Zhang, X.H.; Guo, X.H.; Anstee, D.J.; Mohandas, N.; Chasis, J.A. Adhesive activity of Lu glycoproteins is regulated by interaction with spectrin. Blood 2008, 112, 5212–5218. [Google Scholar] [CrossRef]
- Collec, E.; Lecomte, M.-C.; El Nemer, W.; Colin, Y.; Le Van Kim, C. Novel role for the Lu/BCAM-spectrin interaction in actin cytoskeleton reorganization. Biochem. J. 2011, 436, 699–708. [Google Scholar] [CrossRef]
- Parsons, S.F.; Lee, G.; Spring, F.A.; Willig, T.N.; Peters, L.L.; Gimm, J.A.; Tanner, M.J.; Mohandas, N.; Anstee, D.J.; Chasis, J.A. Lutheran blood group glycoprotein and its newly characterized mouse homologue specifically bind alpha5 chain-containing human laminin with high affinity. Blood 2001, 97, 312–320. [Google Scholar] [CrossRef]
- Komiya, Y.; Kurabe, N.; Katagiri, K.; Ogawa, M.; Sugiyama, A.; Kawasaki, Y.; Tashiro, F. A novel binding factor of 14-3-3β functions as a transcriptional repressor and promotes anchorage-independent growth, tumorigenicity, and metastasis. J. Biol. Chem. 2008, 283, 18753–18764. [Google Scholar] [CrossRef]
- Akiyama, H.; Iwahana, Y.; Suda, M.; Yoshimura, A.; Kogai, H.; Nagashima, A.; Ohtsuka, H.; Komiya, Y.; Tashiro, F. The FBI1/Akirin2 Target Gene, BCAM, Acts as a Suppressive Oncogene. PLoS ONE 2013, 8, e78716. [Google Scholar] [CrossRef]
- Xu, J.; Wu, K.-J.; Jia, Q.-J.; Ding, X.-F. Roles of miRNA and IncRNA in triple-negative breast cancer. J. Zhejiang Univ. Sci. B 2020, 21, 673–689. [Google Scholar] [CrossRef]
- Kim, B.-K.; Kim, I.; Yoon, S.K. Identification of miR-199a-5p target genes in the skin keratinocyte and their expression in cutaneous squamous cell carcinoma. J. Dermatol. Sci. 2015, 79, 137–147. [Google Scholar] [CrossRef]
- Jin, J.; Xie, S.S.; Sun, Q. Upregulation of BCAM and its sense lncRNA BAN are associated with gastric cancer metastasis and poor prognosis. Mol. Oncol. 2020, 14, 829–845. [Google Scholar] [CrossRef]
- Maciaszek, J.L.; Andemariam, B.; Huber, G.; Lykotrafitis, G. Epinephrine Modulates BCAM/Lu and ICAM-4 Expression on the Sickle Cell Trait Red Blood Cell Membrane. Biophys. J. 2012, 102, 1137–1143. [Google Scholar] [CrossRef]
- De Grandis, M.; Cambot, M.; Wautier, M.-P.; Cassinat, B.; Chomienne, C.; Colin, Y.; Wautier, J.-L.; Le Van Kim, C.; El Nemer, W. JAK2V617F activates Lu/BCAM-mediated red cell adhesion in polycythemia vera through an EpoR-independent Rap1/Akt pathway. Blood 2013, 121, 658–665. [Google Scholar] [CrossRef]
- De Grandis, M.; Cassinat, B.; Kiladjian, J.-J.; Chomienne, C.; El Nemer, W. Lu/BCAM-mediated cell adhesion as biological marker of JAK2V617F activity in erythrocytes of polycythemia vera patients. Am. J. Hematol. 2015, 90, E137–E138. [Google Scholar] [CrossRef]
- Guadall, A.; Cochet, S.; Renaud, O.; Colin, Y.; Le Van Kim, C.; de Brevern, A.G.; El Nemer, W. Dimerization and phosphorylation of Lutheran/basal cell adhesion molecule are critical for its function in cell migration on laminin. J. Biol. Chem. 2019, 294, 14911–14921. [Google Scholar] [CrossRef]
- Lizarralde-Iragorri, M.A.; Lefevre, S.D.; Cochet, S.; El Hoss, S.; Brousse, V.; Filipe, A.; Dussiot, M.; Azouzi, S.; Le Van Kim, C.; Rodrigues-Lima, F.; et al. Oxidative stress activates red cell adhesion to laminin in sickle cell disease. Haematologica 2021, 106, 2478–2488. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorganic Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Niiya, D.; Egawa, N.; Sakamoto, T.; Kikkawa, Y.; Shinkawa, T.; Isobe, T.; Koshikawa, N.; Seiki, M. Identification and characterization of Lutheran blood group glycoprotein as a new substrate of membrane-type 1 matrix metalloproteinase 1 (MT1-MMP): A systemic whole cell analysis of MT1-MMP-associating proteins in A431 cells. J. Biol. Chem. 2009, 284, 27360–27369. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Itoh, Y.; Seiki, M. MT1-MMP: A potent modifier of pericellular microenvironment. J. Cell. Physiol. 2006, 206, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.U.; Bogdanova, A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front. Physiol. 2013, 4, 387. [Google Scholar] [CrossRef] [PubMed]
- Lew, V.L.; Tiffert, T. On the Mechanism of Human Red Blood Cell Longevity: Roles of Calcium, the Sodium Pump, PIEZO1, and Gardos Channels. Front. Physiol. 2017, 8, 977. [Google Scholar] [CrossRef]
- Gallagher, P.G. Disorders of erythrocyte hydration. Blood 2017, 130, 2699–2708. [Google Scholar] [CrossRef]
- Klei, T.R.L.; Dalimot, J.J.; Beuger, B.M.; Veldthuis, M.; Ichou, F.A.; Verkuijlen, P.J.J.H.; Seignette, I.M.; Ligthart, P.C.; Kuijpers, T.W.; van Zwieten, R.; et al. The Gardos effect drives erythrocyte senescence and leads to Lu/BCAM and CD44 adhesion molecule activation. Blood Adv. 2020, 4, 6218–6229. [Google Scholar] [CrossRef]
- Klei, T.R.L.; de Back, D.Z.; Asif, P.J.; Verkuijlen, P.J.J.H.; Veldthuis, M.; Ligthart, P.C.; Berghuis, J.; Clifford, E.; Beuger, B.M.; van den Berg, T.K.; et al. Glycophorin-C sialylation regulates Lu/BCAM adhesive capacity during erythrocyte aging. Blood Adv. 2018, 2, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Wautier, M.P.; El Nemer, W.; Gane, P.; Rain, J.D.; Cartron, J.P.; Colin, Y.; Le Van Kim, C.; Wautier, J.L. Increased adhesion to endothelial cells of erythrocytes from patients with polycythemia vera is mediated by laminin alpha5 chain and Lu/BCAM. Blood 2007, 110, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Brusson, M.; Cochet, S.; Leduc, M.; Guillonneau, F.; Mayeux, P.; Peyrard, T.; Chomienne, C.; Le Van Kim, C.; Cassinat, B.; Kiladjian, J.-J.; et al. Enhanced calreticulin expression in red cells of polycythemia vera patients harboring the JAK2V617F mutation. Haematologica 2017, 102, E241–E244. [Google Scholar] [CrossRef]
- Brusson, M.; De Grandis, M.; Cochet, S.; Bigot, S.; Marin, M.; Leduc, M.; Guillonneau, F.; Mayeux, P.; Peyrard, T.; Chomienne, C.; et al. Impact of hydroxycarbamide and interferon-α on red cell adhesion and membrane protein expression in polycythemia vera. Haematologica 2018, 103, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.-L.; Wautier, M.-P. Wautier, Cellular and Molecular Aspects of Blood Cell-Endothelium Interactions in Vascular Disorders. Int. J. Mol. Sci. 2020, 21, 5315. [Google Scholar] [CrossRef]
- El Hoss, S.; Cochet, S.; Marin, M.; Lapoumeroulie, C.; Dussiot, M.; Bouazza, N.; Elie, C.; de Montalembert, M.; Arnaud, C.; Guitton, C.; et al. Insights into determinants of spleen injury in sickle cell anemia. Blood Adv. 2019, 3, 2328–2336. [Google Scholar] [CrossRef]
- Chaar, V.; Laurance, S.; Lapoumeroulie, C.; Cochet, S.; De Grandis, M.; Colin, Y.; Elion, J.; Le Van Kim, C.; El Nemer, W. Hydroxycarbamide Decreases Sickle Reticulocyte Adhesion to Resting Endothelium by Inhibiting Endothelial Lutheran/Basal Cell Adhesion Molecule (Lu/BCAM) through Phosphodiesterase 4A Activation. J. Biol. Chem. 2014, 289, 11512–11521. [Google Scholar] [CrossRef]
- Nader, E.; Conran, N.; Leonardo, F.C.; Hatem, A.; Boisson, C.; Carin, R.; Renoux, C.; Costa, F.F.; Joly, P.; Brito, P.L.; et al. Piezo1 activation augments sickling propensity and the adhesive properties of sickle red blood cells in a calcium-dependent manner. Br. J. Haematol. 2023, 202, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Dalimot, J.J.; Klei, T.R.L.; Beuger, B.M.; Dikmen, Z.; Bouwman, S.A.M.; Mombo-Ngoma, G.; Zoleko-Manego, R.; Ndzebe-Ndoumba, W.F.; Egee, S.; Kuijpers, T.W.; et al. Malaria-associated adhesion molecule activation facilitates the destruction of uninfected red blood cells. Blood Adv. 2022, 6, 5798–5810. [Google Scholar] [CrossRef]
- Motswaledi, M.S.; Kasvosve, I.; Oguntibeju, O.O. Potential role of Lu/BCAM in HIV-related atherosclerosis. Afr. J. Lab. Med. 2019, 8, 792. [Google Scholar] [CrossRef]
- Motswaledi, M.S.; Kasvosve, I.; Oguntibeju, O.O. Blood Group Antigens C, Lub and P1 May Have a Role in HIV Infection in Africans. PLoS ONE 2016, 11, e0149883. [Google Scholar] [CrossRef]
- Schon, M.; Klein, C.E.; Hogenkamp, V.; Kaufmann, R.; Wienrich, B.G.; Schon, M.P. Basal-cell adhesion molecule (B-CAM) is induced in epithelial skin tumors and inflammatory epidermis, and is expressed at cell-cell and cell-substrate contact sites. J. Investig. Dermatol. 2000, 115, 1047–1053. [Google Scholar] [CrossRef]
- Drewniok, C.; Wienrich, B.G.; Schon, M.; Ulrich, J.; Zen, Q.; Telen, M.J.; Hartig, R.J.; Wieland, I.; Gollnick, H.; Schon, M.P. Molecular interactions of B-CAM (basal-cell adhesion molecule) and laminin in epithelial skin cancer. Arch. Dermatol. Res. 2004, 296, 59–66. [Google Scholar] [CrossRef]
- Kikkawa, Y.; Sudo, R.; Kon, J.; Mizuquchi, T.; Nomizu, M.; Hirata, K.; Mitaka, T. Laminin α5 mediates ectopic adhesion of hepatocellular carcinoma through integrins and/or Lutheran/basal cell adhesion molecule. Exp. Cell Res. 2008, 314, 2579–2590. [Google Scholar] [CrossRef]
- McAllister, S.S.; Weinberg, R.A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 2014, 16, 717–727. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xu, L.; Ding, J.; Song, L.; Yang, W.; Klooster, I.; Pilco-Janeta, D.F.; Serrano, C.; Fang, H.; Jiang, G.; et al. Co-targeting of ACK1 and KIT triggers additive anti-proliferative and -migration effects in imatinib-resistant gastrointestinal stromal tumors. Biochim. Et Biophys. Acta-Mol. Basis Dis. 2023, 1869, 166690. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, L.; Liu, H.; Fang, H.; Wang, C.; Huang, B.; Liu, X.; Zhou, X.; Wang, Y. Oncolytic Adenovirus CD55-Smad4 Suppresses Cell Proliferation, Metastasis, and Tumor Stemness in Colorectal Cancer by Regulating Wnt/β-Catenin Signaling Pathway. Biomedicines 2020, 8, 593. [Google Scholar] [CrossRef]
- Wang, P.; Gao, X.-Y.; Yang, S.-Q.; Sun, Z.-X.; Dian, L.-L.; Qasim, M.; Phyo, A.T.; Liang, Z.-S.; Sun, Y.-F. Jatrorrhizine inhibits colorectal carcinoma proliferation and metastasis through Wnt/β-catenin signaling pathway and epithelial-mesenchymal transition. Drug Des. Dev. Ther. 2019, 13, 2235–2247. [Google Scholar] [CrossRef]
- Webb, C.P.; Van Aelst, L.; Wigler, M.H.; Vande Woude, G.F. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc. Natl. Acad. Sci. USA 1998, 95, 8773–8778. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W.; Dao, F.; Dhir, R.; DiSaia, P.; Gabra, H.; Glenn, P.; et al. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar]
- Bast, R.C., Jr.; Mills, G.B. Dissecting “PI3Kness”: The Complexity of Personalized Therapy for Ovarian Cancer. Cancer Discov. 2012, 2, 16–18. [Google Scholar] [CrossRef]
- Kannan, K.; Coarfa, C.; Chao, P.-W.; Luo, L.; Wang, Y.; Brinegar, A.E.; Hawkins, S.M.; Milosavljevic, A.; Matzuk, M.M.; Yen, L. Recurrent BCAM-AKT2 fusion gene leads to a constitutively activated AKT2 fusion kinase in high-grade serous ovarian carcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, E1272–E1277. [Google Scholar] [CrossRef] [PubMed]
- Moreira Latini, F.R.; Bastos, A.U.; Arnoni, C.P.; Muniz, J.G.; Person, R.M.; Baleotti, W., Jr.; Barreto, J.A.; Castilho, L.; Cerutti, J.M. DARC (Duffy) and BCAM (Lutheran) reduced expression in thyroid cancer. Blood Cells Mol. Dis. 2013, 50, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, Y.; Miwa, T.; Tanimizu, N.; Kadoya, Y.; Ogawa, T.; Katagiri, F.; Hozumi, K.; Nomizu, M.; Mizuguchi, T.; Hirata, K.; et al. Soluble Lutheran/basal cell adhesion molecule is detectable in plasma of hepatocellular carcinoma patients and modulates cellular interaction with laminin-511 in vitro. Exp. Cell Res. 2014, 328, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, L.; Zhang, W.; Liao, Y.; Chen, J.; Shi, Y.; Luo, S. Serum cytokine profile in patients with breast cancer. Cytokine 2017, 89, 173–178. [Google Scholar] [CrossRef]
- Yu, K.H.; Barry, C.G.; Austin, D.; Busch, C.M.; Sangar, V.; Rustgi, A.K.; Blair, I.A. Stable Isotope Dilution Multidimensional Liquid Chromatography-Tandem Mass Spectrometry for Pancreatic Cancer Serum Biomarker Discovery. J. Proteome Res. 2009, 8, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.-Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Martini, M.; Molinari, F.; Veronese, S.; Nichelatti, M.; Artale, S.; Di Nicolantonio, F.; Saletti, P.; De Dosso, S.; Mazzucchelli, L.; et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009, 69, 1851–1857. [Google Scholar] [CrossRef]
- Larsen, F.O.; Pfeiffer, P.; Nielsen, D.; Skougaard, K.; Qvortrup, C.; Vistisen, K.; Fromm, A.-L.; Jorgensen, T.L.; Bjerregaard, J.K.; Hoegdall, E.; et al. Bevacizumab in combination with cetuximab and irinotecan after failure of cetuximab and irinotecan in patients with metastatic colorectal cancer. Acta Oncol. 2011, 50, 574–577. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Guo, Q.; Yan, Z. The Role of Lutheran/Basal Cell Adhesion Molecule in Hematological Diseases and Tumors. Int. J. Mol. Sci. 2024, 25, 7268. https://doi.org/10.3390/ijms25137268
Jin J, Guo Q, Yan Z. The Role of Lutheran/Basal Cell Adhesion Molecule in Hematological Diseases and Tumors. International Journal of Molecular Sciences. 2024; 25(13):7268. https://doi.org/10.3390/ijms25137268
Chicago/Turabian StyleJin, Juan, Qinqin Guo, and Zhibin Yan. 2024. "The Role of Lutheran/Basal Cell Adhesion Molecule in Hematological Diseases and Tumors" International Journal of Molecular Sciences 25, no. 13: 7268. https://doi.org/10.3390/ijms25137268
APA StyleJin, J., Guo, Q., & Yan, Z. (2024). The Role of Lutheran/Basal Cell Adhesion Molecule in Hematological Diseases and Tumors. International Journal of Molecular Sciences, 25(13), 7268. https://doi.org/10.3390/ijms25137268



