The Prognostic and Therapeutic Potential of Fragile X Mental Retardation 1 (FMR1) Gene Expression in Prostate Adenocarcinoma: Insights into Survival Outcomes and Oncogenic Pathway Modulation
Abstract
:1. Introduction
2. Results
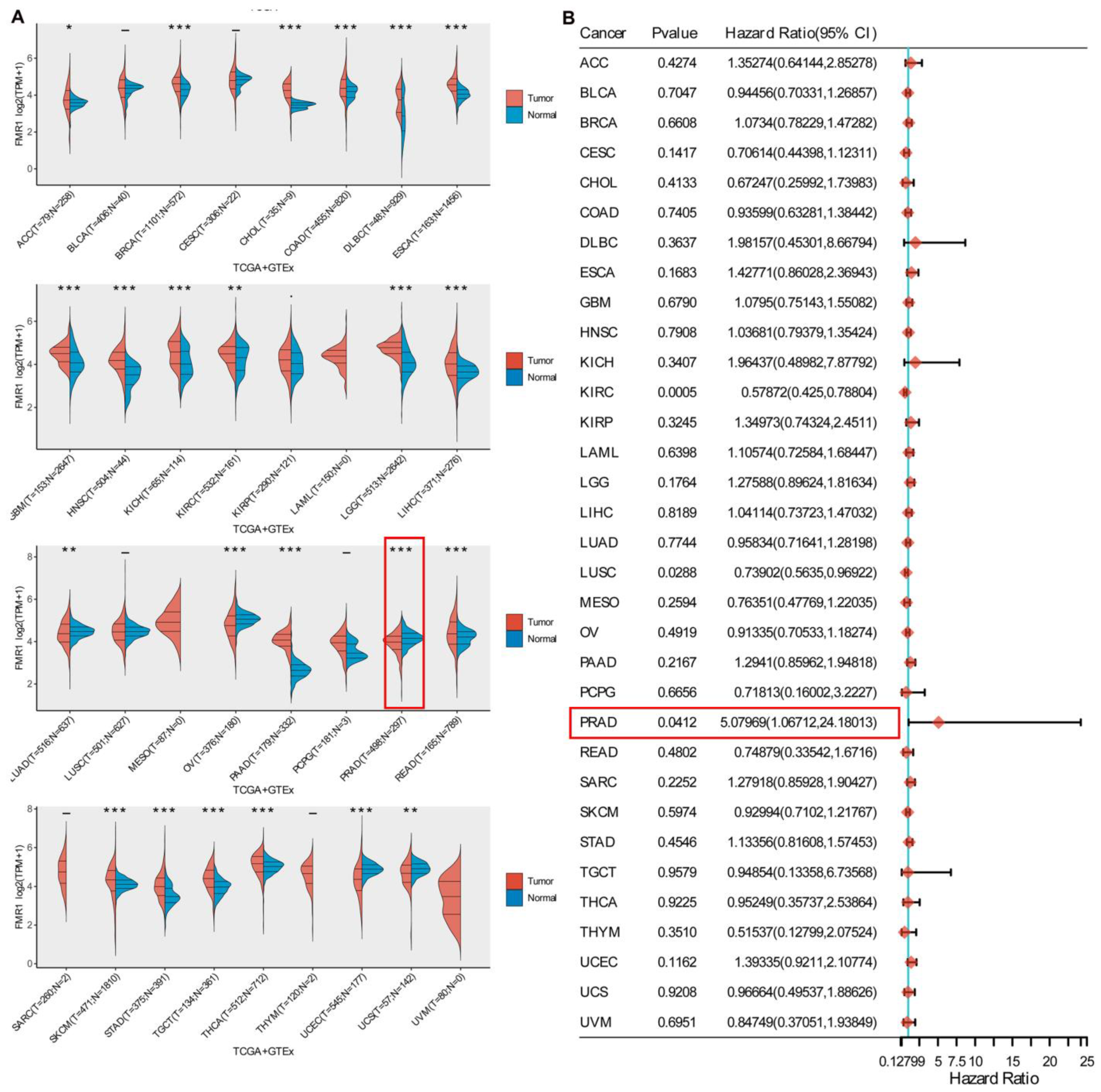
2.1. Gene Expression Profile and Prognostic Implication of FMR1
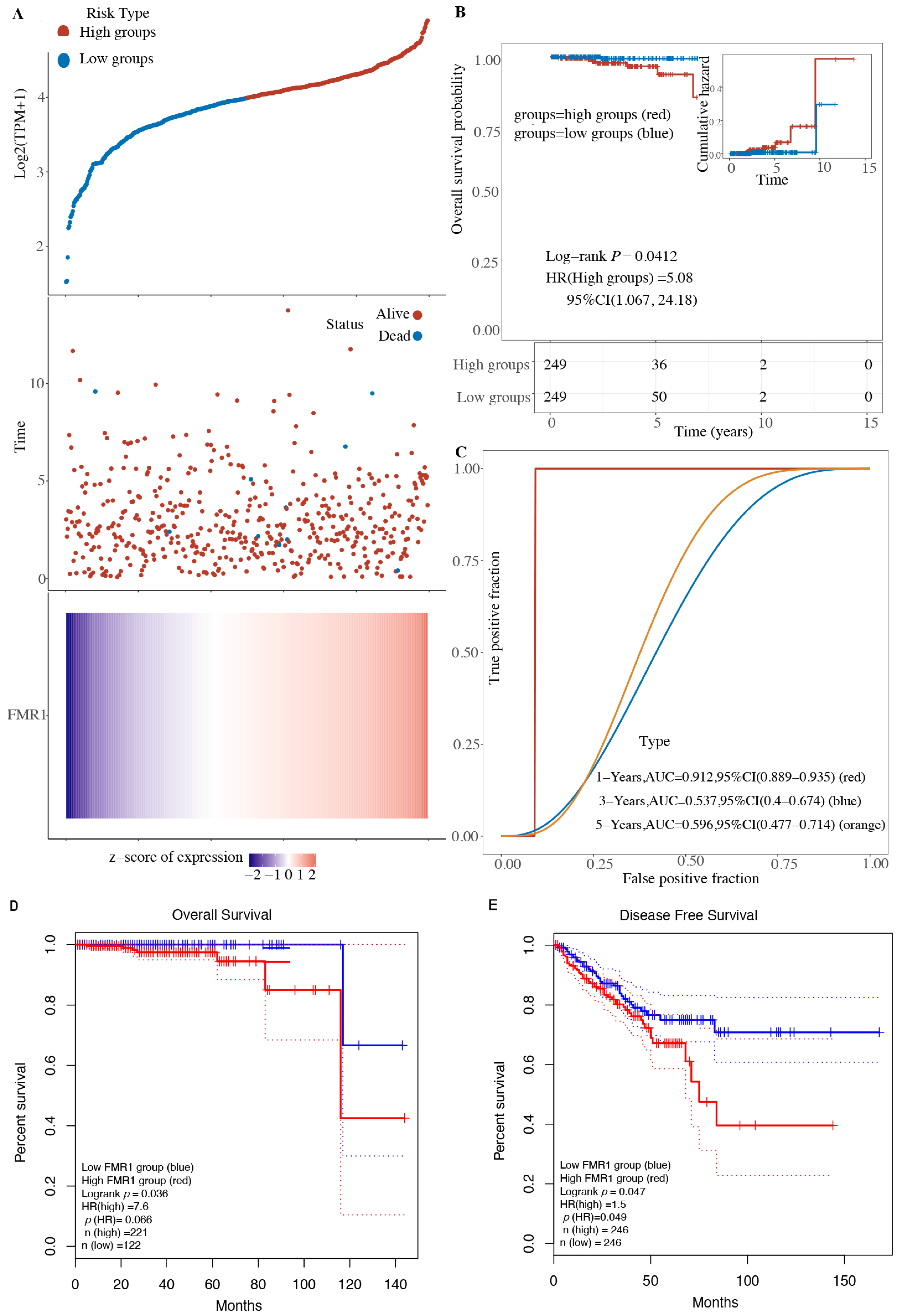
2.2. Expression of FMR1 Decreases Overall Survival in PRAD Patients
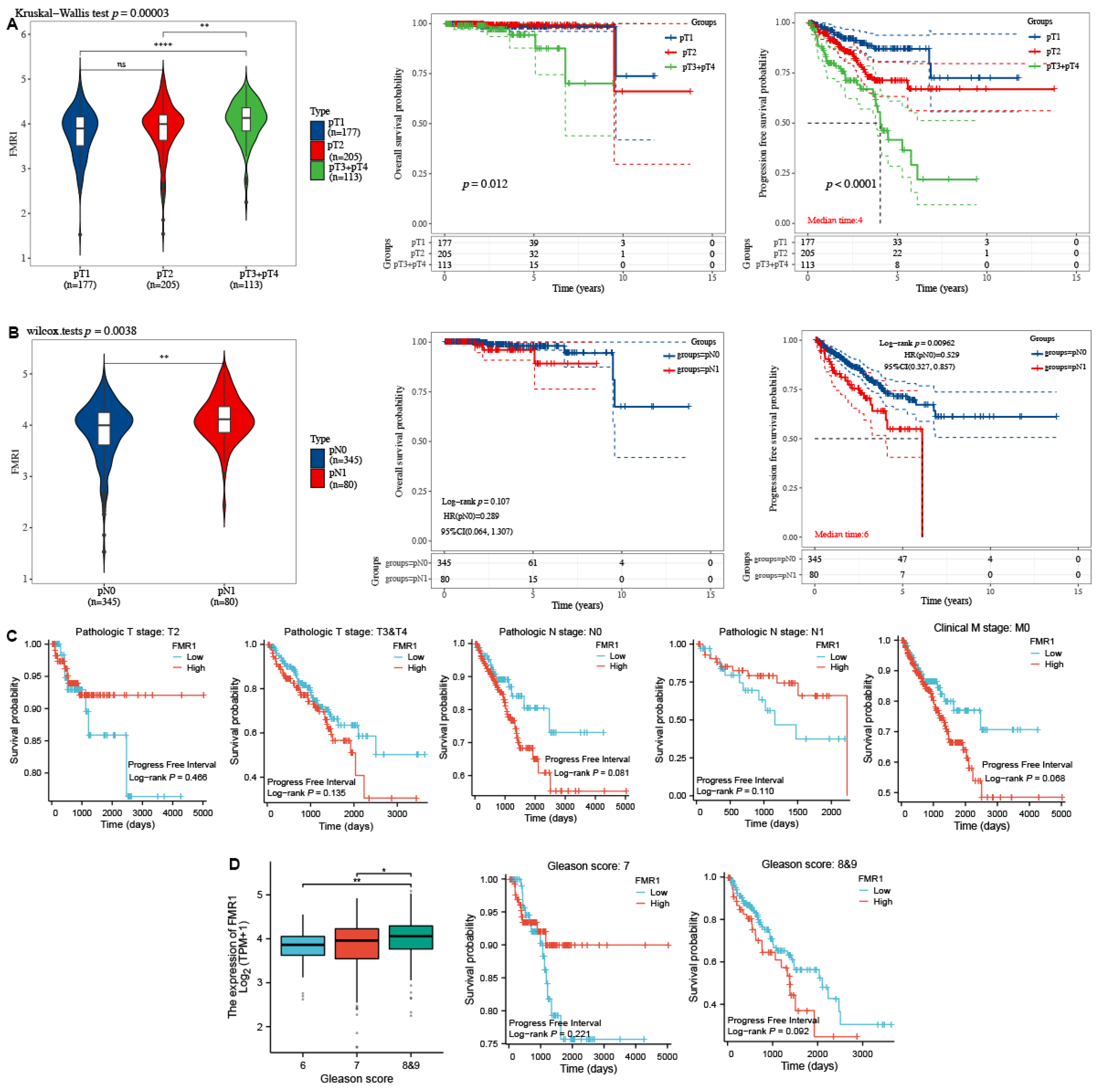
2.3. FMR1 Expression Is Correlated with Advanced Pathological Stages in PRAD
2.4. The Influence of the FMR1 Gene on Cancer Pathways Leads to Enhanced PRAD
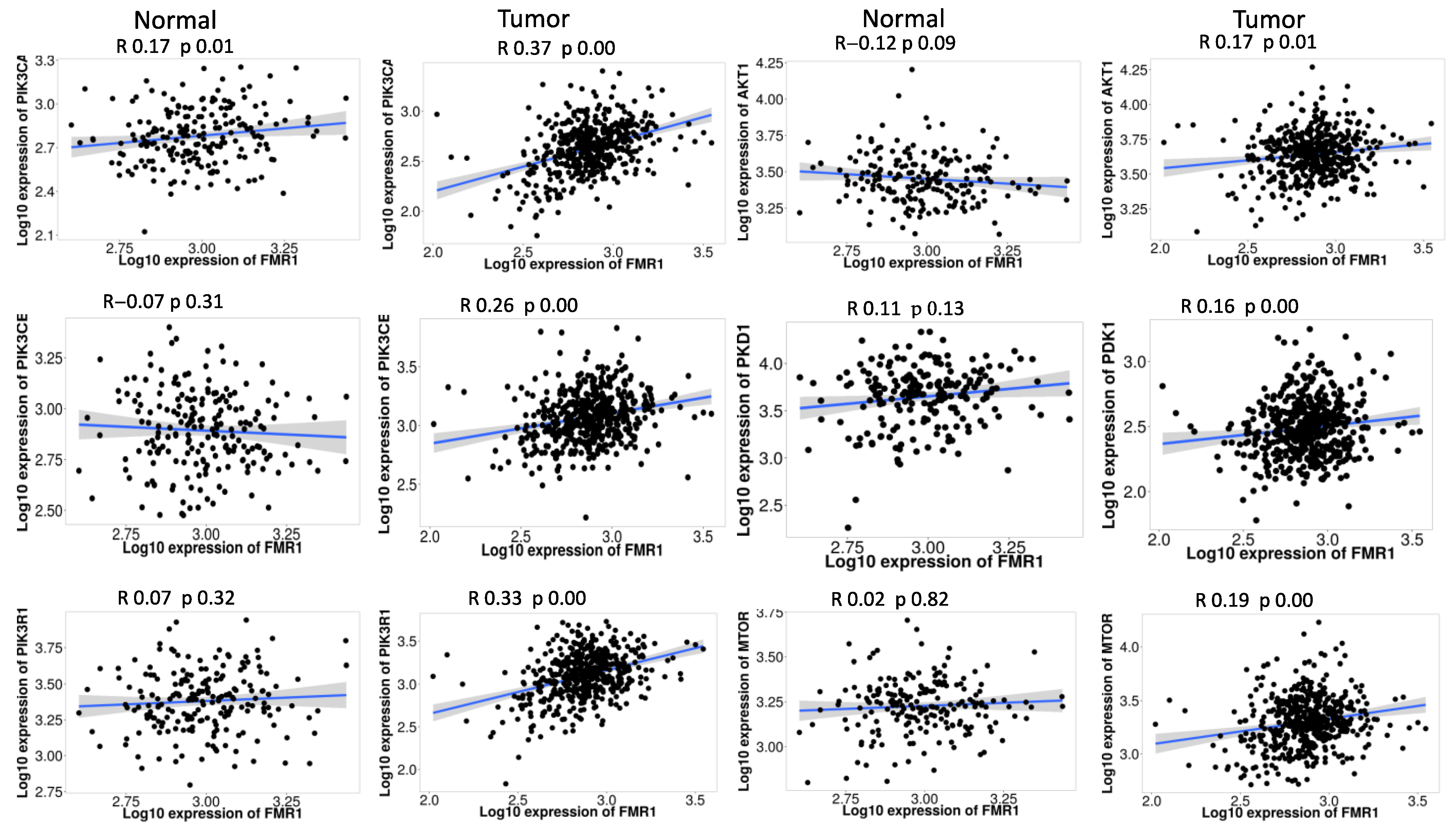
2.5. FMR1 Correlates with PI3K_AKT_mTOR_ Pathway Signature in PRAD
2.6. Analysis of FMR1 Copy Number Variations and Their Impact on mRNA Expression in PRAD
2.7. MicroRNAs and Transcription Factors Networks Regulating FMR1 Gene Expression in PRAD
3. Discussion
4. Materials and Methods
4.1. Pan Cancer Analysis of FMR1 Gene
4.2. Study the Prognostic Impact of FMR1 Gene on PRAD Patients
4.3. Clinicopathology of FMR1 in PRAD
4.4. Analysis of FMR1 Gene Expression and Pathway Signature Correlations in PRAD
4.5. FMR1 Gene Expression and PI3K_AKT_mTOR_Pathway Correlations in PRAD
4.6. Exploring the Complex Regulation of the FMR1 Gene in PRAD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takahashi, S.; Takada, I. Recent advances in prostate cancer: WNT signaling, chromatin regulation, and transcriptional coregulators. Asian J. Androl. 2023, 25, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Sanchez-Carbente, M.d.R.; Lachance, C.; Radzioch, D.; Tremblay, S.; Khandjian, E.W.; DesGroseillers, L.; Murai, K.K. Fragile X Related Protein 1 Clusters with Ribosomes and Messenger RNAs at a Subset of Dendritic Spines in the Mouse Hippocampus. PLoS ONE 2011, 6, e26120. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.R.; Barron, T.; Hui, A.; Spitzer, A.; Yalçin, B.; Ivec, A.E.; Geraghty, A.C.; Hartmann, G.G.; Arzt, M.; Gillespie, S.M.; et al. Glioma synapses recruit mechanisms of adaptive plasticity. Nature 2023, 623, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Zongaro, S.; Hukema, R.; D’Antoni, S.; Davidovic, L.; Barbry, P.; Catania, M.V.; Willemsen, R.; Mari, B.; Bardoni, B. The 3’ UTR of FMR1 mRNA is a target of miR-101, miR-129-5p and miR-221: Implications for the molecular pathology of FXTAS at the synapse. Hum. Mol. Genet. 2013, 22, 1971–1982. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gao, Q.; Ma, S.; Yu, P.; Ding, S.; Yao, X.; Zhang, Z.; Lu, S.; Lu, M.; Zhang, J.; et al. FMR1 promotes the progression of colorectal cancer cell by stabilizing EGFR mRNA in an m6A-dependent manner. Cell Death Dis. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Hanson, J.E.; Madison, D.V. PresynapticFmr1Genotype Influences the Degree of Synaptic Connectivity in a Mosaic Mouse Model of Fragile X Syndrome. J. Neurosci. 2007, 27, 4014–4018. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Wei, J.; Chen, Y.; Kang, Y.; Shi, H.; Yang, F.; Shi, Z.; Chen, S.; Zhou, Y.; Sepich-Poore, C.; et al. FMRP phosphorylation modulates neuronal translation through YTHDF1. Mol. Cell 2023, 83, 4304–4317.e8. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, L.; Klann, E.; Nelson, D.L. Altered Hippocampal Synaptic Plasticity in the Fmr1 Gene Family Knockout Mouse Models. J. Neurophysiol. 2009, 101, 2572–2580. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Zhou, K.Q.; Yin, Z.; Gao, Y.L.; Yang, X. Knockdown of FBP1 enhances radiosensitivity in prostate cancer cells by activating autophagy. Neoplasma 2020, 67, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gao, R.; Yu, C.; Chen, L.; Feng, Y. The RNA-binding protein FXR1 modulates prostate cancer progression by regulating FBXO4. Funct. Integr. Genom. 2019, 19, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Enokida, H.; Shiina, H.; Igawa, M.; Ogishima, T.; Kawakami, T.; Bassett, W.W.; Anast, J.W.; Li, L.-C.; Urakami, S.; Terashima, M.; et al. CpG Hypermethylation of MDR1 Gene Contributes to the Pathogenesis and Progression of Human Prostate Cancer. Cancer Res. 2004, 64, 5956–5962. [Google Scholar] [CrossRef] [PubMed]
- Nianyong, Y.; Li, G. Comprehensive analysis reveals the involvement of hsa_circ_0037858/miR-5000-3p/FMR1 axis in malignant metastasis of clear cell renal cell carcinoma. Aging 2023, 15, 5399–5411. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Zhai, Y.; Wu, L.; Liu, L.; Zhang, W.; Jiang, W.; Bi, N.; Song, Y.; Hui, Z.; Wang, L. MiR-323a-3p acts as a tumor suppressor by suppressing FMR1 and predicts better esophageal squamous cell carcinoma outcome. Cancer Cell Int. 2022, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.M.; Koreckij, T.D.; Corey, E. Targeted Therapy for Advanced Prostate Cancer: Inhibition of the PI3K/Akt/mTOR Pathway. Curr. Cancer Drug Targets 2009, 9, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-MTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef] [PubMed]
- Bitting, R.L.; Armstrong, A.J. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr. Relat. Cancer 2013, 20, R83–R99. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Liu, G.; Gao, J.; Cao, M. Unveiling the m6A Methylation Regulator Links between Prostate Cancer and Periodontitis by Transcriptomic Analysis. Dis. Markers 2022, 2022, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lucá, R.; Averna, M.; Zalfa, F.; Vecchi, M.; Bianchi, F.; La Fata, G.; Del Nonno, F.; Nardacci, R.; Bianchi, M.; Nuciforo, P.; et al. The Fragile X Protein binds m RNA s involved in cancer progression and modulates metastasis formation. EMBO Mol. Med. 2013, 5, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Saghafinia, S.; Chryplewicz, A.; Fournier, N.; Christe, L.; Xie, Y.-Q.; Guillot, J.; Yucel, S.; Li, P.; Galván, J.A.; et al. Aberrant hyperexpression of the RNA binding protein FMRP in tumors mediates immune evasion. Science 2022, 378, eabl7207. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Zhang, Q.; Zhang, Z.; Safi, M.; Khamgan, H.; Wu, H.; Zhang, M.; Qian, Y.; Gao, Y.; Shopit, A.; et al. ARID1A downregulation promotes cell proliferation and migration of colon cancer via VIM activation and CDH1 suppression. J. Cell. Mol. Med. 2022, 26, 5984–5997. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yang, Y.; Cheng, C.; Tu, Y.; Chang, H.; Tsai, K. Overexpression of malic enzyme is involved in breast cancer growth and is correlated with poor prognosis. J. Cell. Mol. Med. 2024, 28, e18163. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.E.; Antonarakis, E.S. Surrogate End Points in Early Prostate Cancer Clinical States: Ready for Implementation? Ann. Transl. Med. 2017, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Isbarn, H.; Huland, H.; Graefen, M. Results of Radical Prostatectomy in Newly Diagnosed Prostate Cancer. Dtsch. Aerzteblatt Online 2013, 110, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Zapatero, A.; Banda, L.F.; Büscher, D.; Torres, L.; Conde, A.C.; Adrados, M.; Olivier, C.; Murillo, M. Positive Prostate Biopsy Following Radiation Therapy Can Predict Metastasis-Free Survival In Localized Prostate Cancer. Int. J. Radiat. Oncol. 2018, 102, e150. [Google Scholar] [CrossRef]
- Lundstam, S.; Rosenblad, A.K.; Ljungberg, B. Histopathological upstage, recurrence, and survival in a population-based cohort of surgically treated patients with clinical T1b renal cell carcinoma. J. Clin. Oncol. 2023, 41, 731. [Google Scholar] [CrossRef]
- Sood, A.; Rudzinski, J.K.; Labbate, C.V.; Hensley, P.J.; Bree, K.K.; Guo, C.C.; Alhalabi, O.; Campbell, M.T.; Siefker-Radtke, A.O.; Navai, N.; et al. Long-Term Oncological Outcomes in Patients Diagnosed With Nonmetastatic Plasmacytoid Variant of Bladder Cancer: A 20-Year University of Texas MD Anderson Cancer Center Experience. J. Urol. 2024, 211, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Harju, S.M.; Cambrin, S.M.; Averill-Murray, R.C.; Nafus, M.; Field, K.J.; Allison, L.J. Using incidental mark-encounter data to improve survival estimation. Ecol. Evol. 2019, 10, 360–370. [Google Scholar] [CrossRef]
- Nakajima, K.; Yamashita, T.; Kita, T.; Takeda, M.; Sasaki, N.; Kasahara, K.; Shinohara, M.; Rikitake, Y.; Ishida, T.; Yokoyama, M.; et al. Orally Administered Eicosapentaenoic Acid Induces Rapid Regression of Atherosclerosis Via Modulating the Phenotype of Dendritic Cells in LDL Receptor-Deficient Mice. Arter. Thromb. Vasc. Biol. 2011, 31, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-S.; Wang, W.-B.; Zhang, S.-D.; Zhang, M.-Q.; Qi, L.; Gao, J.-F.; Zang, Y.-J. Expression of Glucocorticoid Receptor in Prostate Cancer and Its Clinical Significance. Zhonghua Nan Ke Xue=Natl. J. Androl. 2022, 28, 211–216. [Google Scholar]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Carvalho, F.; Welbourn, W.; Reid, J.; Humphreys, E.; Han, M.; Lanchbury, J.; Gutin, A.; Stone, S.; Berman, D. 1475 EVIDENCE FOR A CELL CYCLE PROLIFERATION “FIELD EFFECT” IN PROSTATE CANCER. J. Urol. 2013, 189, e605. [Google Scholar] [CrossRef]
- Stoyanova, T.; Cooper, A.R.; Drake, J.M.; Liu, X.; Armstrong, A.J.; Pienta, K.J.; Zhang, H.; Kohn, D.B.; Huang, J.; Witte, O.N.; et al. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. Proc. Natl. Acad. Sci. USA 2013, 110, 20111–20116. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, C.; van der Horst, G.; Cheung, H.; Buijs, J.T.; Lippitt, J.M.; Guzmán-Ramírez, N.; Hamdy, F.C.; Eaton, C.L.; Thalmann, G.N.; Cecchini, M.G.; et al. High Aldehyde Dehydrogenase Activity Identifies Tumor-Initiating and Metastasis-Initiating Cells in Human Prostate Cancer. Cancer Res. 2010, 70, 5163–5173. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.D. PTEN-PI3K pathway alterations in advanced prostate cancer and clinical implications. Prostate 2022, 82, S60–S72. [Google Scholar] [CrossRef] [PubMed]
- Blaine, S.M.; Honeywell, C.; Allanson, J.; Cremin, C.; Gibbons, C.A.; Meschino, W.S.; Permaul, J.; Carroll, J.C. Genetics Genetics Prostate Cancer. Can. Fam. Physician 2009, 55, 710. [Google Scholar] [PubMed]
- Shand, R.L.; Gelmann, E.P. Molecular biology of prostate-cancer pathogenesis. Curr. Opin. Urol. 2006, 16, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Srivastava, S.K. Mutations in Cancer Driver Genes: An Insight into Prostate Cancer Progression. Ann. Urol. Oncol. 2019, 2, 40–46. [Google Scholar] [CrossRef]
- Mazaris, E.; Tsiotras, A. Molecular Pathways in Prostate Cancer. Nephro-Urology Mon. 2013, 5, 792–800. [Google Scholar] [CrossRef]
- Sutherland, S.I.; Benito, R.P.; Henshall, S.M.; Horvath, L.G.; Kench, J.G. Expression of phosphorylated-mTOR during the development of prostate cancer. Prostate 2014, 74, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.B.; Li, J.; Meniel, V.S.; Fennell, C.M.; Waring, P.; Montgomery, K.G.; Rebello, R.J.; Macpherson, A.A.; Koushyar, S.; Furic, L.; et al. Identification of Pik3ca Mutation as a Genetic Driver of Prostate Cancer That Cooperates with Pten Loss to Accelerate Progression and Castration-Resistant Growth. Cancer Discov. 2018, 8, 764–779. [Google Scholar] [CrossRef]
- Alqahtani, A.; Ayesh, H.S.K.; Halawani, H. PIK3CA Gene Mutations in Solid Malignancies: Association with Clinicopathological Parameters and Prognosis. Cancers 2019, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Grădinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.P. A preview of selected articles. Stem Cells 2021, 39, 371–374. [Google Scholar] [CrossRef]
- Dolskiy, A.A.; Yarushkin, A.A.; Grishchenko, I.V.; Lemskaya, N.A.; Pindyurin, A.V.; Boldyreva, L.V.; Pustylnyak, V.O.; Yudkin, D.V. miRNA expression and interaction with the 3′UTR of FMR1 in FRAXopathy pathogenesis. Non-Coding RNA Res. 2021, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Anvari, M.S.; Vasei, H.; Najmabadi, H.; Badv, R.S.; Golipour, A.; Mohammadi-Yeganeh, S.; Salehi, S.; Mohamadi, M.; Goodarzynejad, H.; Mowla, S.J. Identification of microRNAs associated with human fragile X syndrome using next-generation sequencing. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.M.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e411. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; He, Y.; Ivanov, I.; Sun, Y.; Feng, W.; Refat, M.; Mohammed, S.A.D.; Adlat, S.; Tian, Z.; Wang, Y.; et al. Novel characterization discoveries of ferroptosis-associated molecules in COAD microenvironment based TCGA data. Front. Mol. Biosci. 2022, 9, 1102735. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Huang, K.; Chen, Z.; Hu, M.; Bai, Y.; Lin, S.; Du, H. Characterization of Glycolysis-Associated Molecules in the Tumor Microenvironment Revealed by Pan-Cancer Tissues and Lung Cancer Single Cell Data. Cancers 2020, 12, 1788. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Khamgan, H.; Qian, Y.; Wu, H.; Zhang, Z.; Zhang, M.; Gao, Y.; Safi, M.; Al-Radhi, M.; Zuo, Y.-F. Downregulated ARID1A by miR-185 Is Associated With Poor Prognosis and Adverse Outcomes in Colon Adenocarcinoma. Front. Oncol. 2021, 11, 679334. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; He, Y.; Ivanov, I.; Khamgan, H.; Safi, M.; Alradhi, M.; Shopit, A.; Al-Danakh, A.; Al-Nusaif, M.; Gao, Y.; et al. Aberrantly hypermethylated ARID1B is a novel biomarker and potential therapeutic target of colon adenocarcinoma. Front. Genet. 2022, 13, 914354. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Song, C.; Song, S.; Zhang, G.; Yin, M.; Zhang, Y.; Qian, F.; Wang, Q.; Guo, M.; Li, C. KnockTF 2.0: A comprehensive gene expression profile database with knockdown/knockout of transcription (co-)factors in multiple species. Nucleic Acids Res. 2023, 52, D183–D193. [Google Scholar] [CrossRef] [PubMed]

| Pathway | Spearman Correlation Coefficient (r_Spearman) | p-Value (p_Spearman) | |
|---|---|---|---|
| A | Tumor inflammation signature | 0.11 | 0.440 |
| B | Cellular response to hypoxia | 0.34 | 0.018 |
| C | Tumor proliferation signature | 0.22 | 0.126 |
| D | EMT signature | −0.14 | 0.356 |
| E | ECM related genes | −0.29 | 0.048 |
| F | Angiogenesis | −0.01 | 0.949 |
| G | Apoptosis | 0.33 | 0.023 |
| H | DNA repair | −0.04 | 0.806 |
| I | G2M checkpoint | 0.56 | 3.55 × 10−5 |
| J | Inflammatory response | 0.10 | 0.517 |
| K | PI3K_AKT_mTOR pathway | 0.66 | 3.53 × 10−7 |
| L | P35 | 0.06 | 0.680 |
| M | MYC targets | 0.19 | 0.188 |
| N | TGFB | 0.44 | 0.002 |
| O | IL_10_Anti-inflammatory signature | 0.19 | 0.187 |
| P | Gene upregulated by reactive oxygen (ROS) | 0.16 | 0.263 |
| Q | DNA replication | 0.23 | 0.116 |
| R | Collagen formation | −0.16 | 0.290 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldi, S.; Amer, B.; Alnadari, F.; AL-Mogahed, M.; Gao, Y.; Gamallat, Y. The Prognostic and Therapeutic Potential of Fragile X Mental Retardation 1 (FMR1) Gene Expression in Prostate Adenocarcinoma: Insights into Survival Outcomes and Oncogenic Pathway Modulation. Int. J. Mol. Sci. 2024, 25, 7290. https://doi.org/10.3390/ijms25137290
Baldi S, Amer B, Alnadari F, AL-Mogahed M, Gao Y, Gamallat Y. The Prognostic and Therapeutic Potential of Fragile X Mental Retardation 1 (FMR1) Gene Expression in Prostate Adenocarcinoma: Insights into Survival Outcomes and Oncogenic Pathway Modulation. International Journal of Molecular Sciences. 2024; 25(13):7290. https://doi.org/10.3390/ijms25137290
Chicago/Turabian StyleBaldi, Salem, Bushra Amer, Fawze Alnadari, Maged AL-Mogahed, Yaqin Gao, and Yaser Gamallat. 2024. "The Prognostic and Therapeutic Potential of Fragile X Mental Retardation 1 (FMR1) Gene Expression in Prostate Adenocarcinoma: Insights into Survival Outcomes and Oncogenic Pathway Modulation" International Journal of Molecular Sciences 25, no. 13: 7290. https://doi.org/10.3390/ijms25137290






