Serum Vascular Adhesion Protein-1 and Endothelial Dysfunction in Hepatic Cirrhosis: Searching for New Prognostic Markers
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
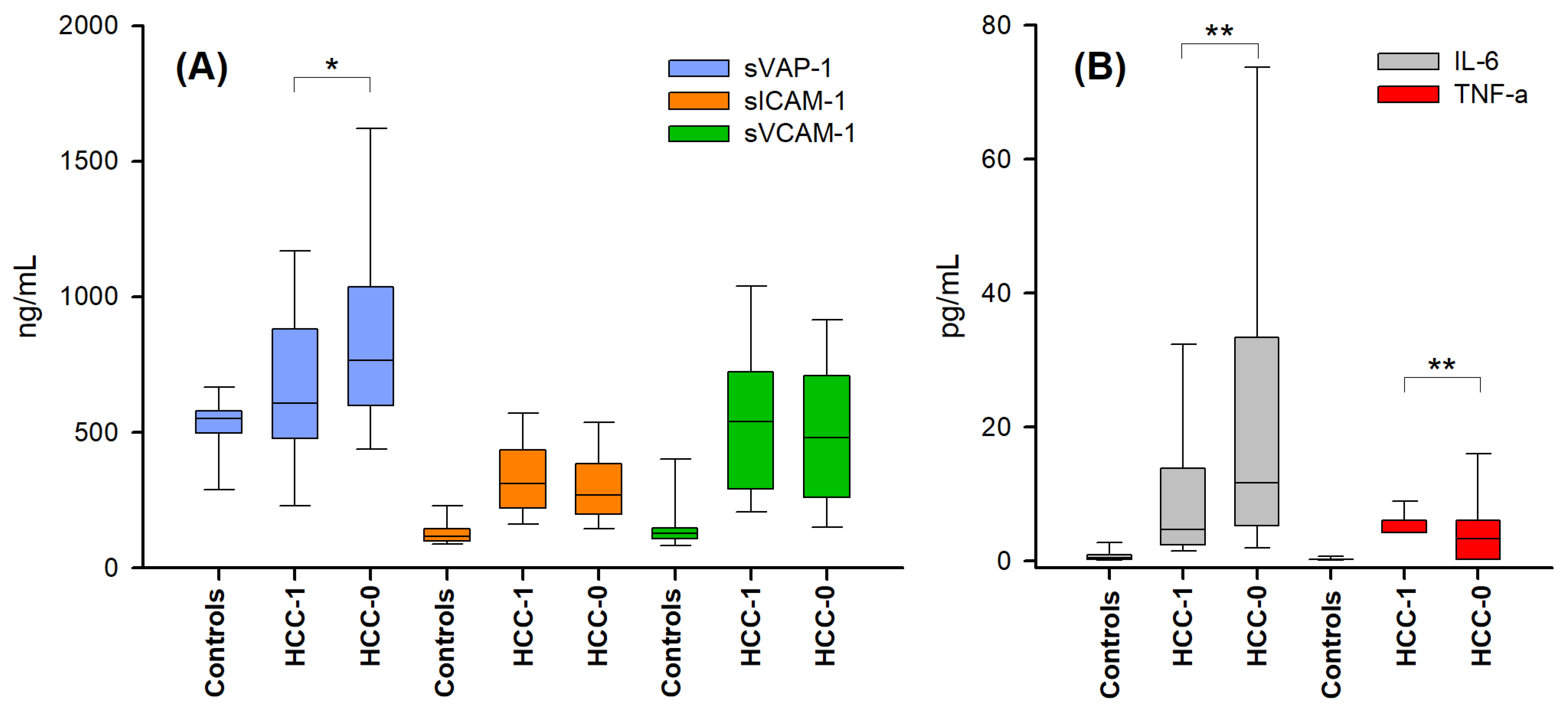
2.2. Markers of Endothelial Activation and Inflammation in the Presence and Absence of Hepatocellular Carcinoma
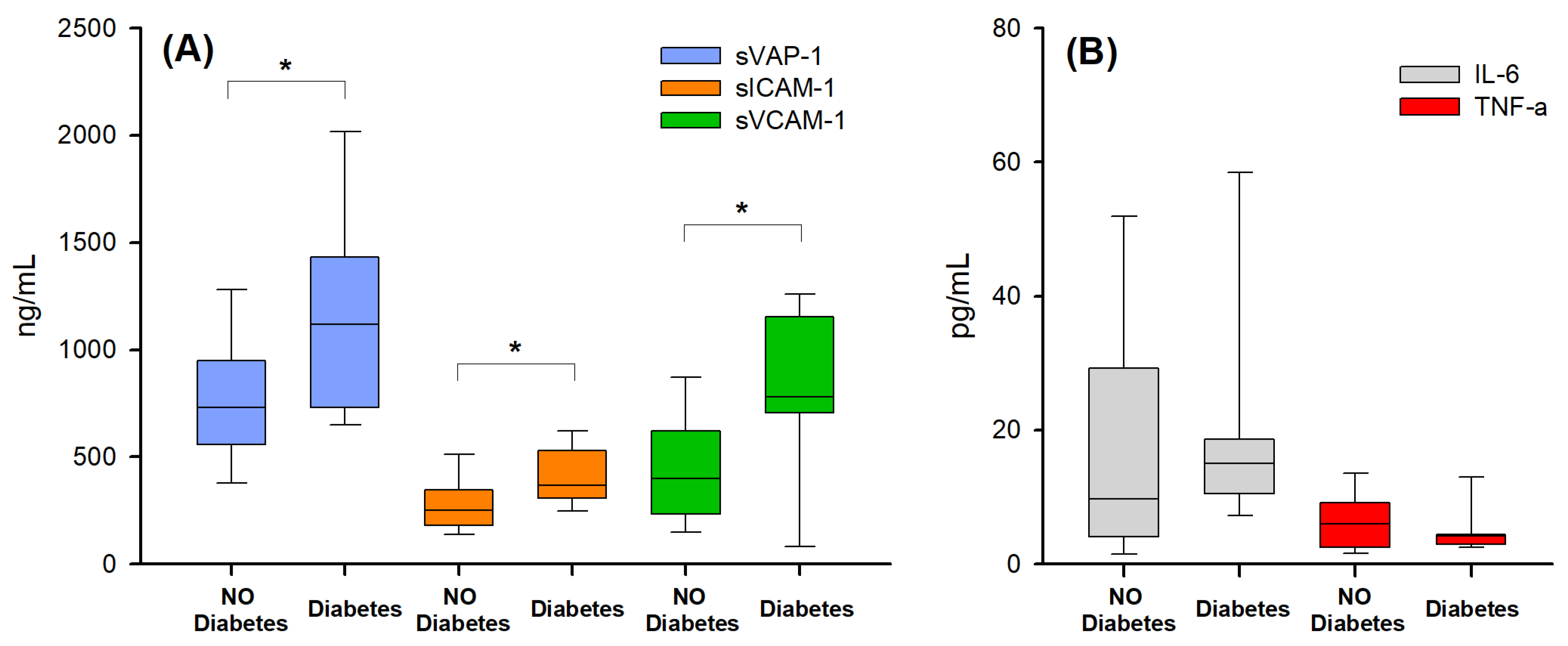
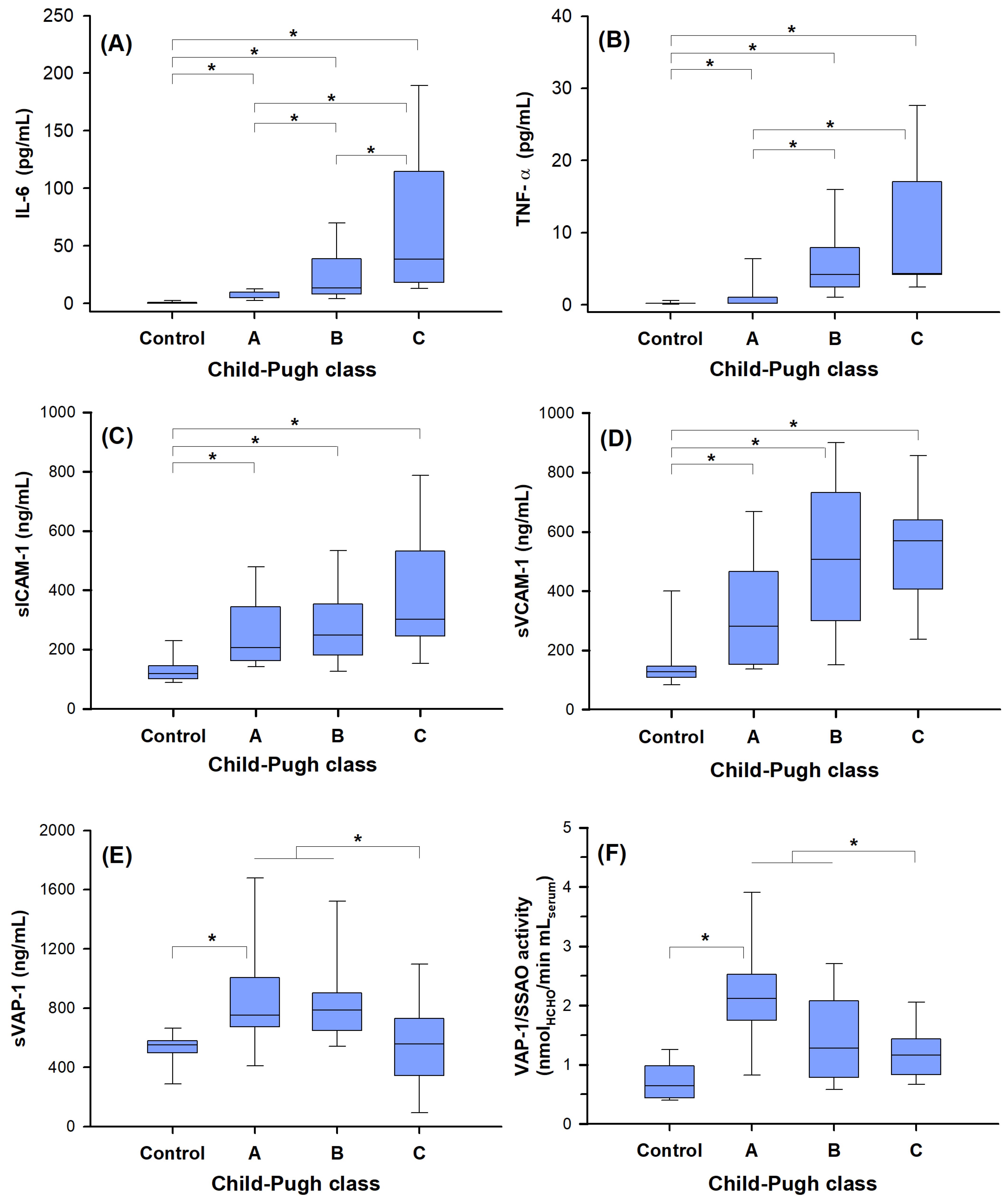
2.3. Biomarker Correlation with Disease Severity
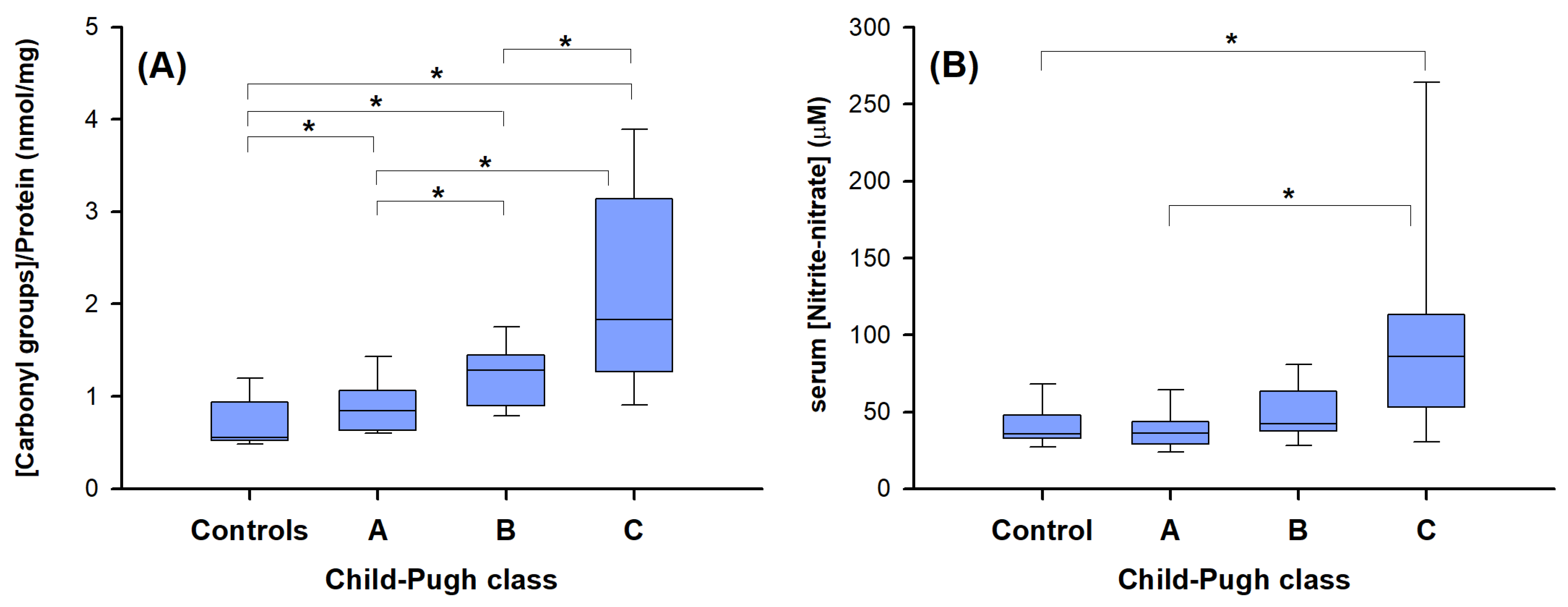
2.4. “Redox” Biomarkers of Child–Pugh Score in Cirrhotic Patients without HCC
2.5. Potential Correlations between the Various Biomarkers
3. Discussion and Conclusions
4. Materials and Methods
4.1. Patient Selection and Plasma Collection
4.2. Methods
4.2.1. sICAM-1, sVCAM-1, TNF-α and IL-6 Quantification
4.2.2. sVAP-1 Protein Quantification and Amine Oxidase Activity Assays
4.2.3. Nitrite, Nitrate, and Oxidatively Modified Protein Determination
4.2.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Shuppan, D.; Kim, Y.O. Evolving therapies for liver fibrosis. J. Clin. Investig. 2013, 123, 1887–1901. [Google Scholar] [CrossRef]
- Zhangdi, H.-J.; Su, S.-B.; Wang, F.; Liang, Z.-Y.; Yan, Y.-D.; Qin, S.-Y.; Jiang, H.-X. Crosstalk network among multiple inflammatory mediators in liver fibrosis. World J. Gastroenterol. 2019, 25, 4835–4849. [Google Scholar] [CrossRef]
- Weston, C.J.; Shepherd, E.L.; Claridge, L.C.; Rantakari, P.; Curbishley, S.M.; Tomlinson, J.W.; Hubscher, S.G.; Reynolds, G.M.; Aalto, K.; Anstee, Q.M.; et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J. Clin. Investig. 2015, 12, 501–520. [Google Scholar] [CrossRef]
- Lafoz, E.; Ruart, M.; Anton, A.; Oncins, A.; Hernández-Gea, V. The endothelium as a driver of liver fibrosis and regeneration. Cells 2020, 9, 929. [Google Scholar] [CrossRef]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.-E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef]
- Ramaiah, S.K.; Jaeschke, H. Role of Neutrophils in the Pathogenesis of Acute Inflammator Liver Injury. Toxicol. Pathol. 2007, 35, 757–766. [Google Scholar] [CrossRef]
- Lalor, P.F.; Tuncer, C.; Weston, C.; Martin-Santos, A.; Smith, D.J.; Adams, D.H. Vascular adhesion protein-1 as a potential therapeutic target in liver disease. Ann. N. Y. Acad. Sci. 2007, 1110, 485–496. [Google Scholar] [CrossRef]
- Aspinall, A.I.; Curbishley, S.M.; Lalor, P.F.; Weston, C.J.; Blahova, M.; Liaskou, E.; Adams, R.M.; Holt, A.P.; Adams, D.H. CX3CR1 and vascular adhesion protein-1-dependent recruitment of CD16+ monocytes across human liver sinusoidal endothelium. Hepatology 2010, 51, 2030–2039. [Google Scholar] [CrossRef]
- Girón-González, J.; Martínez-Sierra, C.; Rodriguez-Ramos, C.; Rendón, P.; Macías, M.; Fernández-Gutiérrez, C.; Díaz, F.; Martín-Herrera, L. Adhesion molecules as a prognostic marker of liver cirrhosis. Scand. J. Gastroenterol. 2005, 40, 217–224. [Google Scholar] [CrossRef]
- Simons, N.; Bijnen, M.; Wouters, K.A.M.; Rensen, S.S.; Beulens, J.W.J.; van Greevenbroek, M.M.J.; Hart, L.M.; Greve, J.W.M.; van der Kallen, C.J.H.; Schaper, N.C.; et al. The endothelial function biomarker soluble E-selectin is associated with nonalcoholic fatty liver disease. Liver Int. 2020, 40, 1079–1088. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. Vascular Adhesion Protein-1: A Cell Surface Amine Oxidase in Translation. Antioxid. Redox Signal. 2019, 30, 314–332. [Google Scholar] [CrossRef]
- McNab, G.; Reeves, J.; Salmi, M.; Hubscher, S.; Jalkanen, S.; Adams, D. Vascular adhesion protein 1 mediates binding of T cells to human hepatic endothelium. Gastroenterology 1996, 110, 522–528. [Google Scholar] [CrossRef]
- Jalkanen, S.; Karikoski, M.; Mercier, N.; Koskinen, K.; Henttinen, T.; Elima, K.; Salmivirta, K.; Salmi, M. The oxidase activity of vascular adhesion protein-1 (VAP-1) induces endothelial E- and P-selectins and leukocyte binding. Blood 2007, 110, 1864–1870. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. VAP-1: An adhesin and an enzyme. Trends Immunol. 2001, 22, 211–216. [Google Scholar] [CrossRef]
- Kurkijarvi, R.; Yegutkin, G.G.; Gunson, B.K.; Jalkanen, S.; Salmi, M.; Adams, D.H. Circulating soluble vascular adhesion protein 1 accounts for the increased serum monoamine oxidase activity in chronic liver disease. Gastroenterology 2000, 119, 1096–1103. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Tickle, J.; Vesterhus, M.N.; Eddowes, P.J.; Bruns, T.; Vainio, J.; Parker, R.; Smith, D.; Liaskou, E.; Thorbjørnsen, L.W.; et al. Vascular adhesion protein-1 is elevated in primary sclerosing cholangitis, is predictive of clinical outcome and facilitates recruitment of gut-tropic lymphocytes to liver in a substrate-dependent manner. Gut 2018, 67, 1135–1145. [Google Scholar] [CrossRef]
- Boomsma, F.; Meiracker, A.H.v.D.; Winkel, S.; Aanstoot, H.J.; Batstra, M.R.; Veld, A.J.M.I.; Bruining, G.J. Circulating Semicarbazide-Sensitive Amine Oxidase is raised both in type I (insulin-dependent), in type II (non-insulin-dependent) diabetes mellitus and even in childhood type I diabetes at first clinical diagnosis. Diabetologia 1999, 42, 233–237. [Google Scholar] [CrossRef]
- Yanaba, K.; Yoshizaki, A.; Muroi, E.; Ogawa, F.; Shimizu, K.; Sato, S. Increased circulating soluble vascular adhesion protein-1 levels in systemic sclerosis: Association with lower frequency and severity of interstitial lung disease. Int. J. Rheum. Dis. 2013, 16, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Unzeta, M.; Solé, M.; Boada, M.; Hernandez, M. Semicarbazide sensitive amine oxidase (SSAO) and its possible contribution to vascular damage in Alzheimer’s disease. J. Neural Transm. 2007, 114, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Kurkijarvi, R.; Adams, D.H.; Leino, R.; Mottonen, T.; Jalkanen, S.; Salmi, M. Circulating form of human vascular adhesion protein-1 (VAP-1): Increased serum levels in inflammatory liver diseases. J. Immunol. 1998, 161, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Somfai, G.M.; Knippel, B.; Ruzicska, E.; Stadler, K.; Tóth, M.; Salacz, G.; Magyar, K.; Somogyi, A. Soluble semicarbazide-sensitive amine oxidase (SSAO) activity is related to oxidative stress and subchronic inflammation in streptozotocin-induced diabetic rats. Neurochem. Int. 2006, 48, 746–752. [Google Scholar] [CrossRef]
- Pannecoeck, R.; Serruys, D.; Benmeridja, L.; Delanghe, J.R.; van Geel, N.; Speeckaert, R.; Speeckaert, M.M. Vascular adhesion protein-1: Role in human pathology and application as a biomarker. Crit. Rev. Clin. Lab. Sci. 2015, 52, 284–300. [Google Scholar] [CrossRef]
- Koc-Zorawska, E.; Malyszko, J.; Malyszko, J.S.; Mysliwiec, M. VAP-1, a novel molecule linked to endothelial damage and kidney function in kidney allograft recipients. Kidney Blood Press. Res. 2012, 36, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Irjala, H.; Salmi, M.; Alanen, K.; Grénman, R.; Jalkanen, S. Vascular adhesion protein 1 mediates binding of immunotherapeutic effector cells to tumor endothelium. J. Immunol. 2001, 166, 6937–6943. [Google Scholar] [CrossRef] [PubMed]
- Klein, D. The tumor vascular endothelium as decision maker in cancer therapy. Front. Oncol. 2018, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Miki, C.; Inoue, Y.; Kawamoto, A.; Kusunoki, M. Circulating form of human vascular adhesion protein-1 (VAP-1): Decreased serum levels in progression of colorectal cancer and predictive marker of lymphatic and hepatic metastasis. J. Surg. Oncol. 2009, 99, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.T.; Weston, C.J.; Shepherd, E.L.; Hejmadi, R.; Ismail, T.; Adams, D.H. Evaluation of serum and tissue levels of VAP-1 in colorectal cancer. BMC Cancer 2016, 16, 154–163. [Google Scholar] [CrossRef]
- Yasuda, H.; Toiyama, Y.; Ohi, M.; Mohri, Y.; Miki, C.; Kusunoki, M. Serum soluble vascular adhesion protein-1 is a valuable prognostic marker in gastric cancer. J. Surg. Oncol. 2011, 103, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.A.; Kucukoner, M.; Inal, A.; Urakci, Z.; Evliyaoglu, O.; Firat, U.; Kaya, M.; Isikdogan, A. Relationship between serum soluble vascular adhesion protein-1 level and gastric cancer prognosis. Oncol. Res. Treat. 2014, 37, 340–344. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, P.; Zhang, K.; Zang, L.; Liao, H.; Ma, W. Evaluation of Serum Vascular Adhesion Protein-1 as a Potential Biomarker in Thyroid Cancer. Int. J. Endocrinol. 2016, 2016, 6312529. [Google Scholar] [CrossRef]
- Lai, Y.C.; Chang, S.J.; Kostoro, J.; Kwan, A.L.; Chai, C.Y. Vascular adhesion protein-1 as indicator of breast cancer tumor aggressiveness and invasiveness. APMIS 2018, 126, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xi, J.; Tian, Y.; Bova, G.S.; Zhang, H. Identification, prioritization, and evaluation of glycoproteins for aggressive prostate cancer using quantitative glycoproteomics and antibody-based assays on tissue specimens. Proteomics 2013, 13, 2268–2277. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.A.; Jones, C.T.; Xu, Y.; Fenaux, M.; Halcomb, R.L. The Race to Bash NASH: Emerging Targets and Drug Development in a Complex Liver Disease. J. Med. Chem. 2020, 63, 5031–5073. [Google Scholar] [CrossRef]
- Kemik, O.; Sümer, A.; Kemik, A.S.; İtik, V.; Dulger, A.C.; Purisa, S.; Tuzun, S. Human vascular adhesion proteın-1 (VAP-1): Serum levels for hepatocellular carcinoma in non-alcoholic and alcoholic fatty liver disease. World J. Surg. Oncol. 2010, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.; Krawczyk, M.; Noor, F.; Grünhage, F.; Lammert, F.; Schneider, J.G. Increased Circulating VAP-1 Levels Are Associated with Liver Fibrosis in Chronic Hepatitis C Infection. J. Clin. Med. 2019, 8, 103. [Google Scholar] [CrossRef]
- Yoong, K.F.; McNab, G.; Hübscher, S.G.; Adams, D.H. Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor- infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma. J. Immunol. 1998, 160, 3978–3988. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, H.; Luo, H.J.; Lin, Z.X.; Jiang, Z.W.; Luo, W.H. SSAO inhibitors suppress hepatocellular tumor growth in mice. Cell. Immunol. 2013, 283, 61–69. [Google Scholar] [CrossRef]
- Aalto, K.; Maksimow, M.; Juonala, M.; Viikari, J.; Jula, A.; Kähönen, M.; Jalkanen, S.; Raitakari, O.T.; Salmi, M. Soluble vascular adhesion protein-1 correlates with cardiovascular risk factors and early atherosclerotic manifestations. Arter. Thromb. Vasc. Biol. 2012, 32, 523–532. [Google Scholar] [CrossRef]
- Girón-González, J.A.; Martínez-Sierra, C.; Rodriguez-Ramos, C.; Macías, M.A.; Rendón, P.; Díaz, F.; Fernández-Gutiérrez, C.; Martín-Herrera, L. Implication of inflammation-related cytokines in the natural history of liver cirrhosis. Liver Int. 2004, 24, 437–445. [Google Scholar] [CrossRef]
- Richter, V.; Rassoul, F.; Purschwitz, K.; Hentschel, B.; Reuter, W.; Kuntze, T. Circulating Vascular Cell Adhesion Molecules VCAM-1, ICAM-1, and E-Selectin in Dependence on Aging. Gerontology 2003, 49, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, G.A.; Awan, Z.; Hashem, E.; Al-Ama, N.; Abunaji, A.B. Levels of soluble cell adhesion molecules in type 2 diabetes mellitus patients with macrovascular complications. J. Int. Med. Res. 2020, 48, 300060519893858. [Google Scholar] [CrossRef]
- Lefere, S.; Van De Velde, F.; Devisscher, L.; Bekaert, M.; Raevens, S.; Verhelst, X.; Van Nieuwenhove, Y.; Praet, M.; Hoorens, A.; Van Steenkiste, C.; et al. Serum vascular cell adhesion molecule-1 predicts significant liver fibrosis in non-alcoholic fatty liver disease. Int. J. Obes. 2017, 41, 1207–1213. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Wadkin, J.C.R.; Patten, D.A.; Kamarajah, S.K.; Shepherd, E.L.; Novitskaya, V.; Berditchevski, F.; Adams, D.H.; Weston, C.J.; Shetty, S. CD151 supports VCAM-1-mediated lymphocyte adhesion to liver endothelium and is upregulated in chronic liver disease and hepatocellular carcinoma. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G138–G149. [Google Scholar] [CrossRef]
- Goyal, R.; Faizy, A.F.; Siddiqui, S.S.; Singhai, M. Evaluation of TNF-α and IL-6 Levels in Obese and Non-obese Diabetics: Pre- and Postinsulin Effects. N. Am. J. Med. Sci. 2012, 4, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Yen, I.W.; Li, H.Y. The role of vascular adhesion protein-1 in diabetes and diabetic complications. J. Diabetes Investig. 2024. Ahead of print. [Google Scholar] [CrossRef]
- Karim, S.; Liaskou, E.; Fear, J.; Garg, A.; Reynolds, G.; Claridge, L.; Adams, D.H.; Newsome, P.N.; Lalor, P.F. Dysregulated hepatic expression of glucose transporters in chronic disease: Contribution of semicarbazide-sensitive amine oxidase to hepatic glucose uptake. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G1180–G1190. [Google Scholar] [CrossRef]
- Bai, X.P.; Fan, Y.M.; Zhang, L.; Yang, G.H.; Li, X. Influence of Liver Cirrhosis on Blood Glucose, Insulin Sensitivity and Islet Function in Mice. Am. J. Med. Sci. 2021, 362, 403–417. [Google Scholar] [CrossRef]
- Yoshida, S.; Murata, M.; Noda, K.; Matsuda, T.; Saito, M.; Saito, W.; Kanda, A.; Ishida, S. Proteolytic cleavage of vascular adhesion protein-1 induced by vascular endothelial growth factor in retinal capillary endothelial cells. Jpn. J. Ophthalmol. 2018, 62, 256–264. [Google Scholar] [CrossRef]
- Zhu, P.P.; Yuan, S.G.; Liao, Y.; Qin, L.L.; Liao, W.J. High level of intercellular adhesion molecule-1 affects prognosis of patients with hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 7254–7263. [Google Scholar] [CrossRef]
- Ho, J.W.; Poon, R.T.; Tong, C.S.; Fan, S.T. Clinical significance of serum vascular cell adhesion molecule-1 levels in patients with hepatocellular carcinoma. World J. Gastroenterol. 2004, 10, 2014–2018. [Google Scholar] [CrossRef]
- Jang, J.W.; Oh, B.S.; Kwon, J.H.; You, C.R.; Chung, K.W.; Kay, C.S.; Jung, H.S. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine 2012, 60, 686–693. [Google Scholar] [CrossRef]
- Zhu, X.W.; Gong, J.P. Expression and role of ICAM-1 in the occurrence and development of hepatocellular carcinoma. Asian Pac. J. Cancer Prev. 2013, 14, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef]
- Lizcano, J.M.; Unzeta, M.; Tipton, K.F. A spectrophotometric method for determination of the oxidative deamination of methylamine by the amine oxidases. Anal. Biochem. 2000, 286, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Holt, A.; Smith, J.; Cendron, L.; Zanotti, G.; Rigo, A.; Di Paolo, M.L. Multiple binding sites for substrates and modulators of semicarbazide-sensitive amine oxidase: Kinetic consequences. Mol. Pharm. 2008, 73, 525–538. [Google Scholar]

| Variable | Controls | (HCC-0) | (HCC-1) |
|---|---|---|---|
| Number (n) | 28 | 59 | 56 |
| Sex (male/female) | 28/0 | 50/9 | 45/11 |
| Age (y) | 34 (22–52) | 61 (41–80) | 62 (35–89) |
| CHILD A/B/C 1 (n) | - | 23/20/16 | 33/16/7 |
| Diabetes (n) | 0 | 11 | 21 |
| ALT (U/L) | 18 (10–35) | 26 (7–71) | 62 (14–482) |
| AST (U/L) | 26 (10–35) | 69 (18–566) | 73 (14–482) |
| αFP (ng/mL) | n.d 2 | 2.6 (2.0–4.7) | 710 (0.9–18372) 3 |
| Biomarker 1 | (HCC-0)’ (n = 48) | (HCC-1)’ (n = 35) |
|---|---|---|
| IL-6 (pg/mL) | 18 ± 20 | 14 ± 17 |
| TNF-α (pg/mL) | 3.2 ± 3.8 | 5.0 ± 1.6 |
| sVCAM-1 (ng/mL) | 433± 238 | 546 ± 266 |
| sICAM-1 (ng/mL) | 290± 161 | 300 ± 94 |
| sVAP-1 (ng/mL) | 762 ± 354 | 698 ± 320 |
| sVCAM-1 (ng/mL) | IL-6 (pg/mL) | TNF-α (pg/mL) | sICAM-1 (ng/mL) | ||
|---|---|---|---|---|---|
| sVAP-1 (ng/mL) | All cases | 0.293 ** | 0.089 | 0.040 | 0.193 |
| HCC-0 | 0.403 ** | −0.030 | 0.020 | 0.298 | |
| HCC-1 | 0.220 | 0.063 | 0.177 | 0.085 | |
| sVCAM-1 (ng/mL) | All cases | 0.172 | 0.137 | 0.569 ** | |
| HCC-0 | −0.024 | 0.166 | 0.479 ** | ||
| HCC-1 | 0.533 ** | 0.047 | 0.716 ** | ||
| IL-6 (pg/mL) | All cases | 0.234 * | 0.153 | ||
| HCC-0 | 0.485 ** | 0.075 | |||
| HCC-1 | −0.141 | 0.304 * | |||
| TNF-α (pg/mL) | All cases | 0.135 | |||
| HCC-0 | 0.169 | ||||
| HCC-1 | 0.097 |
| Standardized Coefficients (Beta) | p-Value | 95% Confidence Interval | |
|---|---|---|---|
| Constant | 0.000 | 410.237–1013.815 | |
| HCC diabetes | −0.106 | 0.440 | −114.799–50.575 |
| IL 6 (pg/mL) | −0.065 | 0.631 | −7.161–4.375 |
| TNF-α (pg/mL) | 0.014 | 0.914 | −27.551–30.706 |
| sICAM-1 (ng/mL) | −0.051 | 0.748 | −1.095–0.791 |
| sVCAM-1 (ng/mL) | 0.374 | 0.021 | 0.073–0.877 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasolato, S.; Bonaiuto, E.; Rossetto, M.; Vanzani, P.; Ceccato, F.; Vittadello, F.; Zennaro, L.; Rigo, A.; Mammano, E.; Angeli, P.; et al. Serum Vascular Adhesion Protein-1 and Endothelial Dysfunction in Hepatic Cirrhosis: Searching for New Prognostic Markers. Int. J. Mol. Sci. 2024, 25, 7309. https://doi.org/10.3390/ijms25137309
Fasolato S, Bonaiuto E, Rossetto M, Vanzani P, Ceccato F, Vittadello F, Zennaro L, Rigo A, Mammano E, Angeli P, et al. Serum Vascular Adhesion Protein-1 and Endothelial Dysfunction in Hepatic Cirrhosis: Searching for New Prognostic Markers. International Journal of Molecular Sciences. 2024; 25(13):7309. https://doi.org/10.3390/ijms25137309
Chicago/Turabian StyleFasolato, Silvano, Emanuela Bonaiuto, Monica Rossetto, Paola Vanzani, Fabio Ceccato, Fabio Vittadello, Lucio Zennaro, Adelio Rigo, Enzo Mammano, Paolo Angeli, and et al. 2024. "Serum Vascular Adhesion Protein-1 and Endothelial Dysfunction in Hepatic Cirrhosis: Searching for New Prognostic Markers" International Journal of Molecular Sciences 25, no. 13: 7309. https://doi.org/10.3390/ijms25137309






