Influence of Drought Stress on the Rhizosphere Bacterial Community Structure of Cassava (Manihot esculenta Crantz)
Abstract
:1. Introduction
2. Results
2.1. Effects of Drought and Variety on Soil Chemical Properties and Enzyme Activities
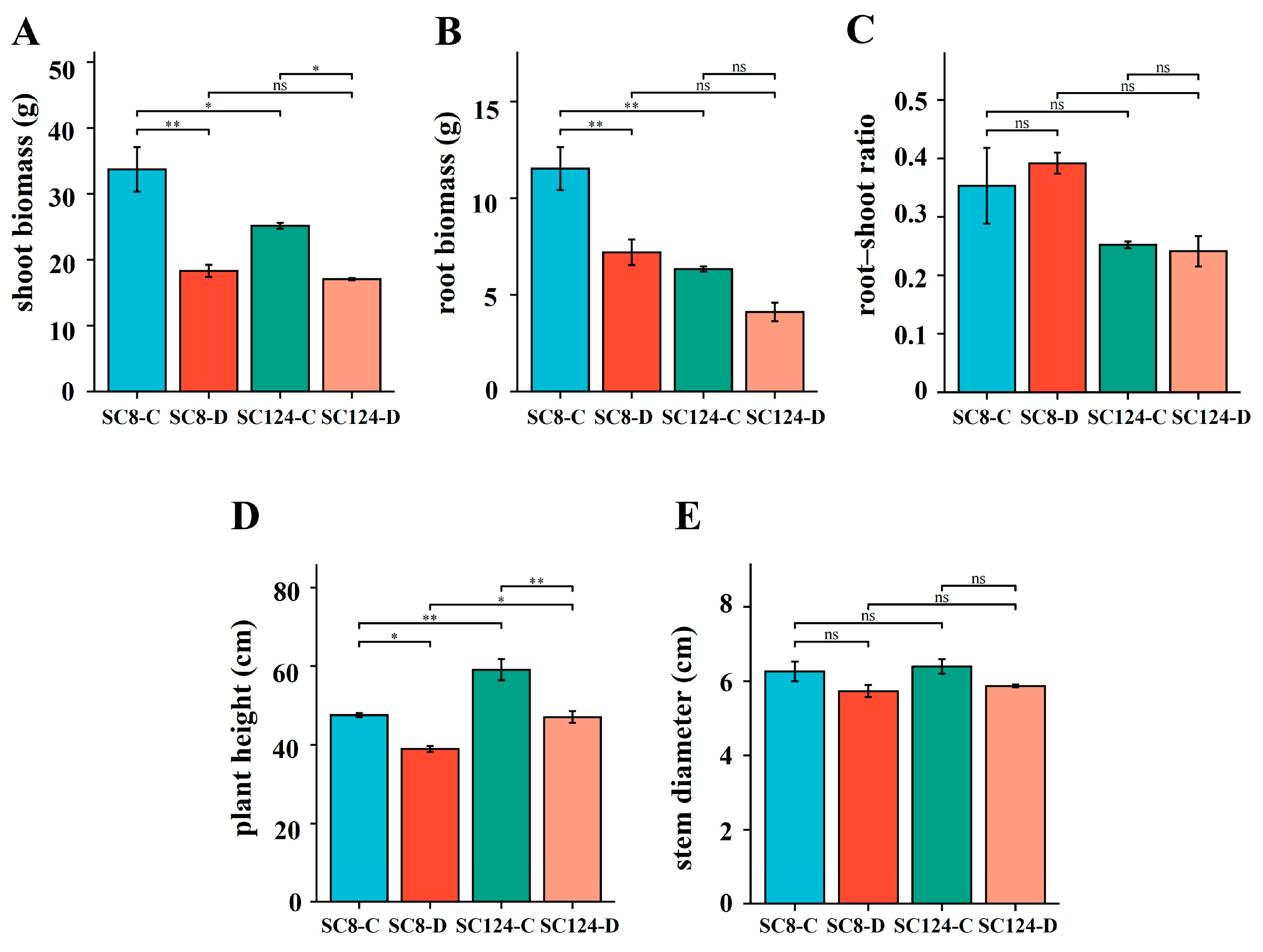
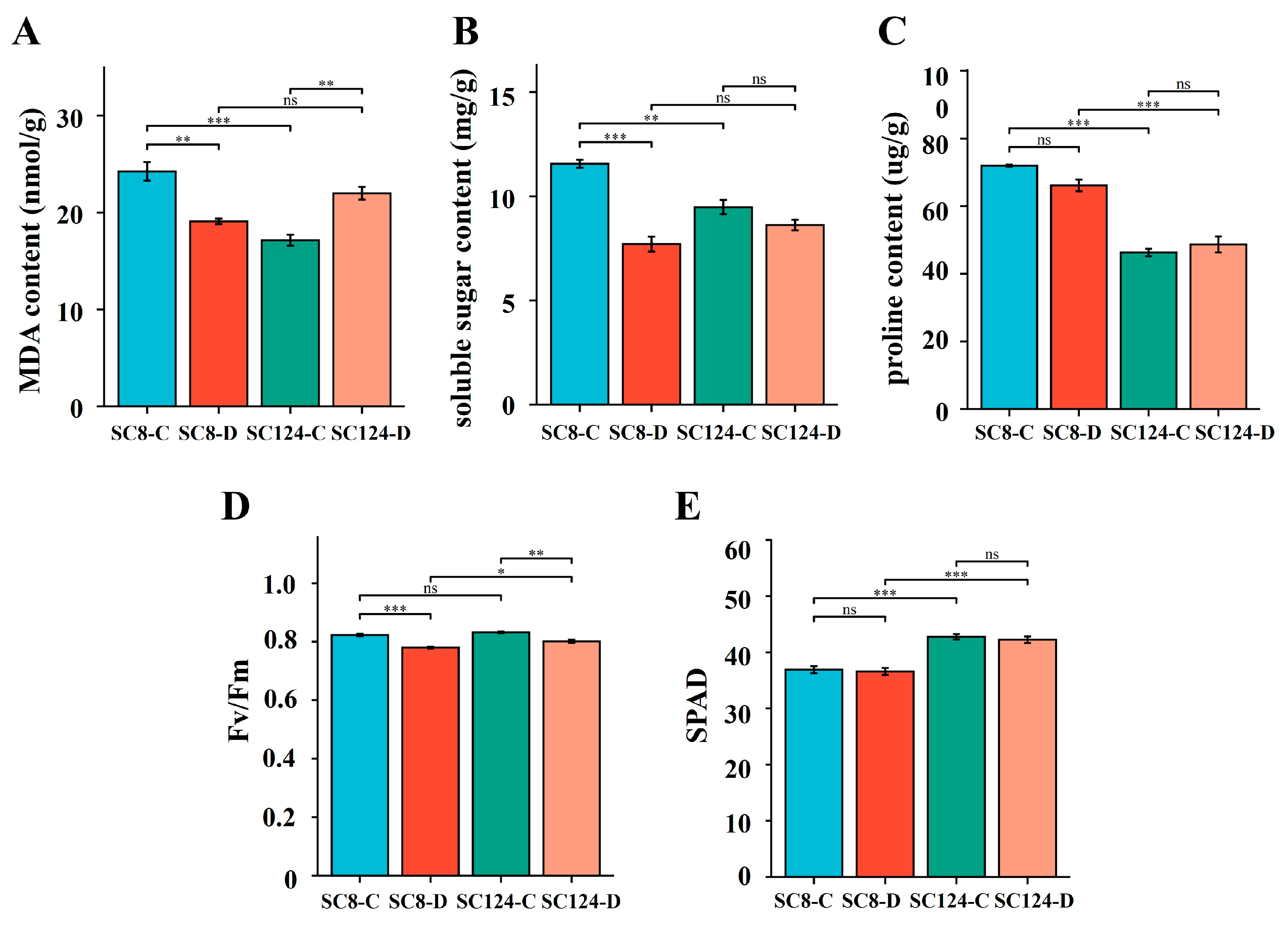
2.2. Effects of Drought and Variety on Plant Phenotypes and Physiological State
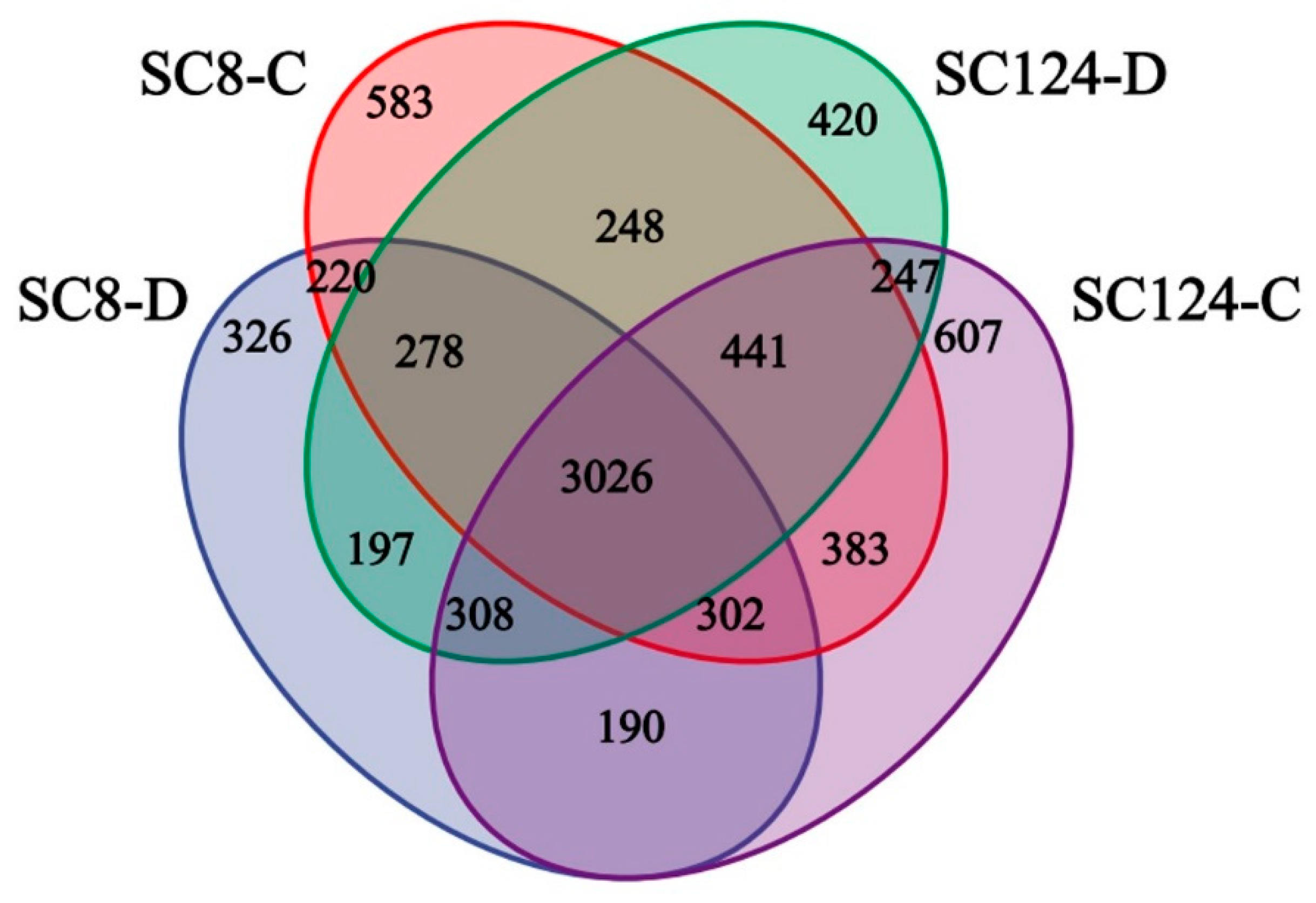
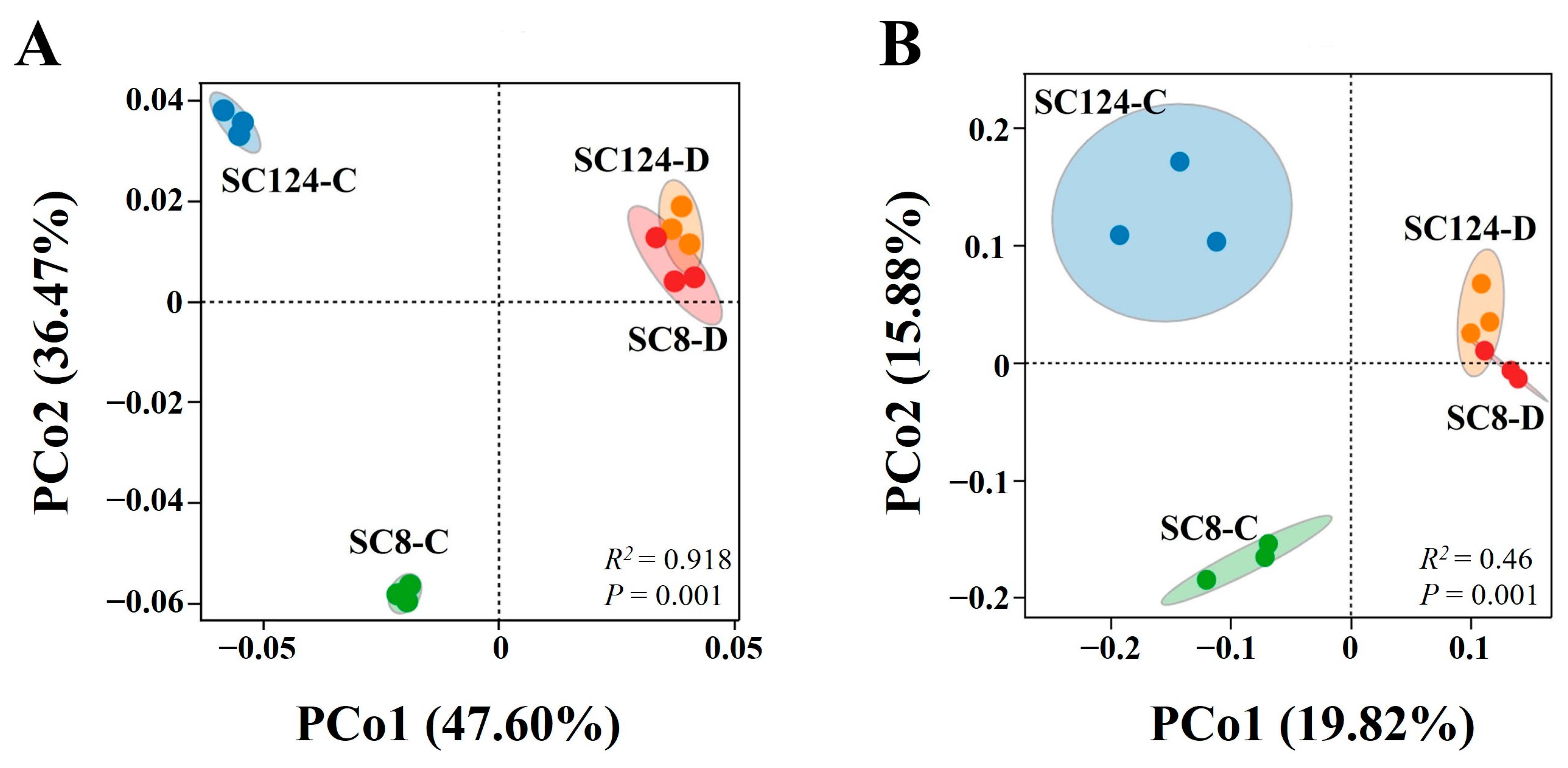
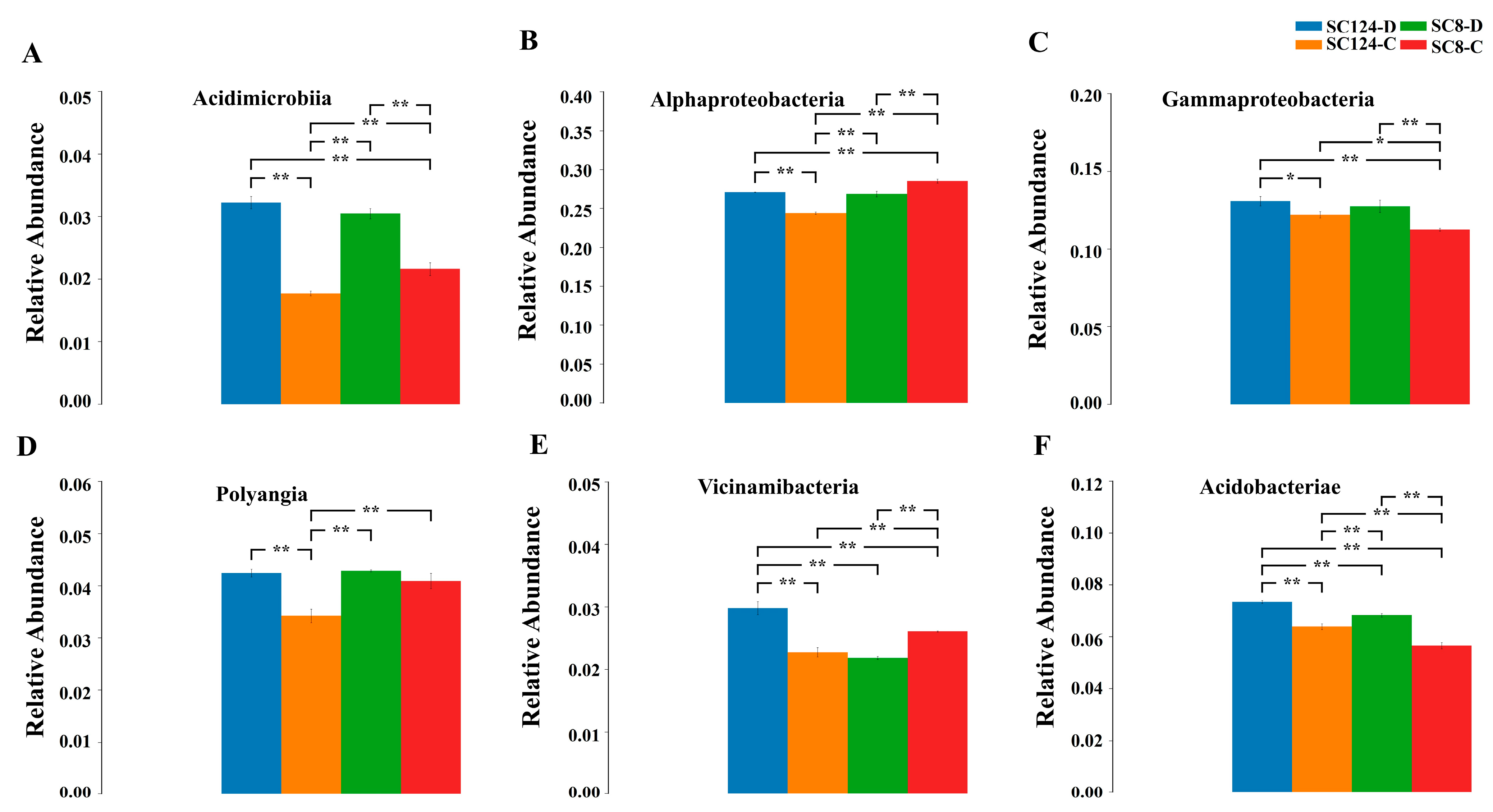
2.3. Effects of Drought and Variety on Rhizosphere Bacterial Communities
2.4. Correlation Analysis between Rhizosphere Bacterial Community and Physicochemical Properties
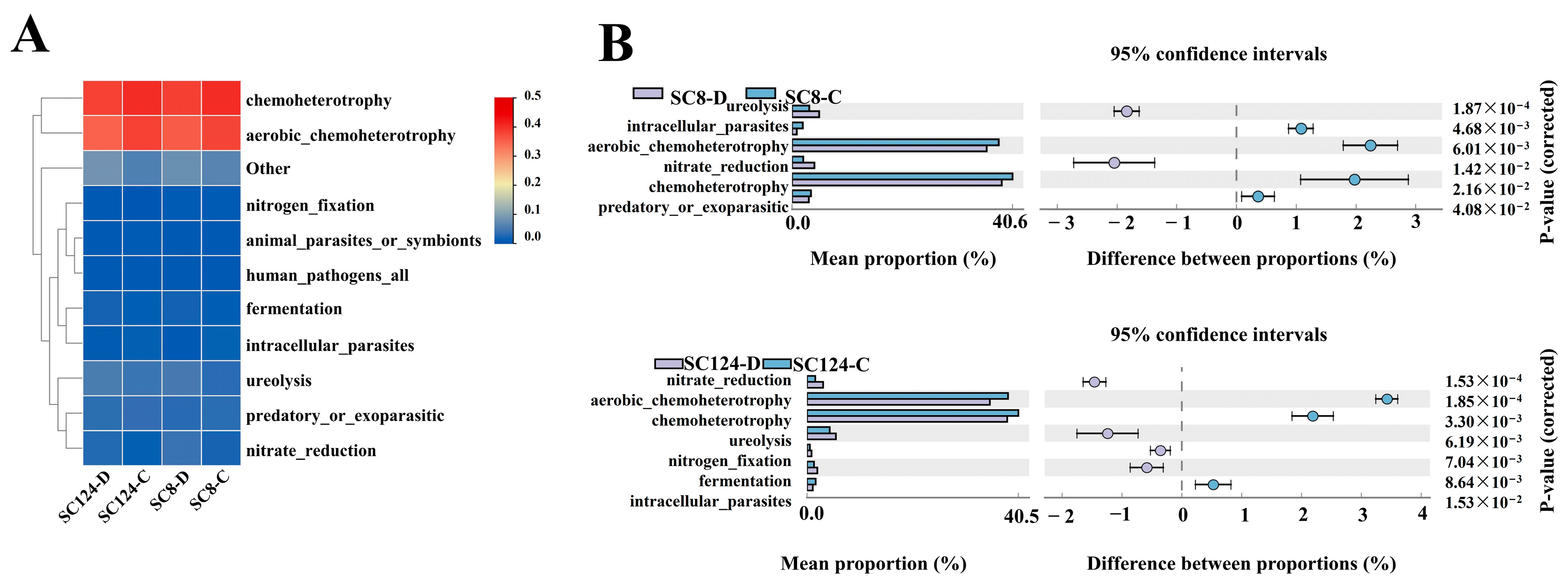
2.5. Functional Prediction of Rhizosphere Bacterial Community
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Soil Sampling and Physicochemical Assessment
4.3. DNA Extraction and Sequencing
4.4. Processing of Sequence Data
4.5. Statistical and Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Ault, T.R. On the essentials of drought in a changing climate. Science 2020, 368, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Sun, X.; Wang, T.; Zhang, A.; Han, D.; Wei, G.; Song, W.; Shu, D. Host genotype-specific rhizosphere fungus enhances drought resistance in wheat. Microbiome 2024, 12, 44. [Google Scholar]
- Varshney, R.K.; Tuberosa, R.; Tardieu, F. Progress in understanding drought tolerance: From alleles to cropping systems. J. Exp. Bot. 2018, 69, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Dong, Z.; Chiniquy, D.; Pierroz, G.; Deng, S.; Gao, C.; Diamond, S.; Simmons, T.; Wipf, H.M.; Caddell, D.; et al. Genome-resolved metagenomics reveals role of iron metabolism in drought-induced rhizosphere microbiome dynamics. Nat. Commun. 2021, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Naylor, D.; Dong, Z.; Simmons, T.; Pierroz, G.; Hixson, K.K.; Kim, Y.M.; Zink, E.M.; Engbrecht, K.M.; Wang, Y.; et al. Drought delays development of the sorghum root microbiome and enriches for monoderm bacteria. Proc. Natl. Acad Sci. USA. 2018, 115, E4284–E4293. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Müller, V. CHEMICAL SYNTHESIS. Microbes in a knight’s armor. Science 2016, 351, 34. [Google Scholar] [CrossRef] [PubMed]
- Zachow, C.; Müller, H.; Tilcher, R.; Berg, G. Differences between the rhizosphere microbiome of Beta vulgaris ssp. maritima-ancestor of all beet crops-and modern sugar beets. Front Microbiol. 2014, 5, 415. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.; Fan, X.; Zhang, Z.; Fu, Z.; Sun, L.; Gillings, M.; Peñuelas, J.; et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 231. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, Y.; Shi, Z.; Wang, S.; Wang, Z.; Liu, Y.; Wen, X.; Mo, F.; Han, J.; Liao, Y. Crop development has more influence on shaping rhizobacteria of wheat than tillage practice and crop rotation pattern in an arid agroecosystem. Appl. Soil Ecol. 2021, 165, 104016. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.X.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.; Qin, Y.; Yan, P.; Zhang, X.; Guo, X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, M.; Wei, G.; Tian, R.; Li, C.; Wang, B.; Lin, R.; Shi, C.; Chi, X.; Zhou, B.; et al. The occurrence of potato common scab correlates with the community composition and function of the geocaulosphere soil microbiome. Microbiome 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wei, Z.; Shao, Z.; Friman, V.P.; Cao, K.; Yang, T.; Kramer, J.; Wang, X.; Li, M.; Mei, X.; et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat. Microbiol. 2020, 5, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Krohn, C.; Franks, A.E.; Wang, X.; Wood, J.L.; Petrovski, S.; McCaskill, M.; Batinovic, S.; Xie, Z.; Tang, C. Elevated atmospheric CO2 alters the microbial community composition and metabolic potential to mineralize organic phosphorus in the rhizosphere of wheat. Microbiome 2022, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Jabborova, D.; Annapurna, K.; Azimov, A.; Tyagi, S.; Pengani, K.R.; Sharma, P.; Vikram, K.V.; Poczai, P.; Nasif, O.; Ansari, M.J.; et al. Co-inoculation of biochar and arbuscular mycorrhizae for growth promotion and nutrient fortification in soybean under drought conditions. Front Plant Sci. 2022, 13, 947547. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xia, K.; Feng, Z.; Qin, Y.; Zhou, Y.; Feng, G.; Zhu, H.; Yao, Q. Tomato plant growth promotion and drought tolerance conferred by three arbuscular mycorrhizal fungi is mediated by lipid metabolism. Plant Physiol. Biochem. 2024, 208, 108478. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Nataraj, K.; Udayashankar, A.C.; Amruthesh, K.N.; Murali, M.; Poczai, P.; Gafur, A.; et al. Insight into Recent progress and perspectives in improvement of antioxidant machinery upon PGPR augmentation in plants under drought stress: A review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, S.N.; Tiwari, A.K.; Pathak, S.K.; Singh, A.K.; Srivastava, S.; Mohan, N. Integration of sugarcane production technologies for enhanced cane and sugar productivity targeting to increase farmers’ income: Strategies and prospects. 3 Biotech. 2019, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. Camb. Philos. Soc. 2016, 91, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Santos-Medellín, C.; Edwards, J.; Liechty, Z.; Nguyen, B.; Sundaresan, V. Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. mBio 2017, 8, e00764-17. [Google Scholar] [CrossRef] [PubMed]
- Naylor, D.; Coleman-Derr, D. Drought stress and root-associated bacterial communities. Front Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Coleman-Derr, D. Causes and consequences of a conserved bacterial root microbiome response to drought stress. Curr. Opin. Microbiol. 2019, 49, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant-microbiome interactions under a changing world: Responses, consequences and perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Bazany, K.E.; Wang, J.T.; Delgado-Baquerizo, M.; Singh, B.K.; Trivedi, P. Water deficit affects inter-kingdom microbial connections in plant rhizosphere. Environ. Microbiol. 2022, 24, 3722–3734. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.H.M.; Pieterse, C.M.J.; de Jonge, R.; Berendsen, R.L. The soil-borne legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef] [PubMed]
- Rizaludin, M.S.; Stopnisek, N.; Raaijmakers, J.M.; Garbeva, P. The chemistry of stress: Understanding the ‘Cry for Help’ of plant roots. Metabolites 2021, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Gheewala, S.H.; Garivait, S. Full chain energy analysis of fuel ethanol from cassava in Thailand. Env. Sci. Technol. 2007, 41, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, M.A. Cassava biology and physiology. Plant Mol. Biol. 2004, 56, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.J.; Ren, M.Y.; Lu, L.F.; Peng, M.; Guan, X.; Zhou, D.B.; Zhang, M.Y.; Qi, D.F.; Li, K.; Tang, W.; et al. Involvement of abscisic acid-responsive element-binding factors in cassava (Manihot esculenta) dehydration stress response. Sci. Rep. 2019, 9, 12661. [Google Scholar] [CrossRef] [PubMed]
- Orek, C.; Gruissem, W.; Ferguson, M.; Vanderschuren, H. Morpho-physiological and molecular evaluation of drought tolerance in cassava (Manihot esculenta Crantz). Field Crops Res. 2020, 255, 107861. [Google Scholar] [CrossRef]
- Li, S.; Cheng, Z.; Li, Z.; Dong, S.; Yu, X.; Zhao, P.; Liao, W.; Yu, X.; Peng, M. MeSPL9 attenuates drought resistance by regulating JA signaling and protectant metabolite contents in cassava. Theor. Appl. Genet. 2022, 135, 817–832. [Google Scholar] [CrossRef]
- Guo, X.; Yu, X.; Xu, Z.; Zhao, P.; Zou, L.; Li, W.; Geng, M.; Zhang, P.; Peng, M.; Ruan, M. CC-type glutaredoxin, MeGRXC3, associates with catalases and negatively regulates drought tolerance in cassava (Manihot esculenta Crantz). Plant Biotechnol. J. 2022, 20, 2389–2405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, P.; Shao, J.; Li, C.; Wang, B.; Guo, X.; Yan, B.; Xia, Y.; Peng, M. Analysis of different strategies adapted by two cassava cultivars in response to drought stress: Ensuring survival or continuing growth. J. Exp. Bot. 2015, 66, 1477–1488. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, X.; Nawaz, G.; Yin, J.; Yang, J. Physiological and biochemical responses of four cassava cultivars to drought stress. Sci. Rep. 2020, 10, 6968. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Gao, Y.; Zhang, R.; Li, K.; Zhang, Y.; Niu, X.; Chen, X.; Luo, K.; Chen, Y. Diversity of the bacterial microbiome associated with the endosphere and rhizosphere of different Cassava (Manihot esculenta Crantz) genotypes. Front Microbiol. 2021, 12, 729022. [Google Scholar] [CrossRef] [PubMed]
- Na, X.; Cao, X.; Ma, C.; Ma, S.; Xu, P.; Liu, S.; Wang, J.; Wang, H.; Chen, L.; Qiao, Z. Plant stage, not drought stress, determines the effect of cultivars on bacterial community diversity in the rhizosphere of broomcorn millet (Panicum miliaceum L.). Front Microbiol. 2019, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, K.; Qin, Q.; Li, Q.; Mo, F.; Nangia, V.; Liu, Y. Integrated microbiome and metabolomic analysis reveal responses of rhizosphere bacterial communities and root exudate composition to drought and genotype in rice (Oryza sativa L.). Rice (N. Y.) 2023, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Zoppellari, F.; Malusà, E.; Chitarra, W.; Lovisolo, C.; Bardi, L. Improvement of drought tolerance in maize (Zea mays L.) by selected rhizospheric microorganisms. Ital. J. Agrometeorol. 2014, 1, 5–18. [Google Scholar]
- Vidal, C.; González, F.; Santander, C.; Pérez, R.; Gallardo, V.; Santos, C.; Aponte, H.; Ruiz, A.; Cornejo, P. Management of rhizosphere microbiota and plant production under drought stress: A comprehensive review. Plants 2022, 11, 2437. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.; Dai, M. Improving crop drought resistance with plant growth regulators and rhizobacteria: Mechanisms, applications, and perspectives. Plant Commun. 2021, 3, 100228. [Google Scholar] [CrossRef]
- Xing, J.; Fan, W.; Wang, J.; Shi, F. Variety-driven effect of rhizosphere microbial-specific recruitment on drought tolerance of Medicago ruthenica (L.). Microorganisms 2023, 11, 2851. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Homayoun, H.; Daliri, M.S.; Mehrabi, P. Effect of drought stress on leaf chlorophyll in corn cultivars (Zea mays). IDOSI Publications 2011, 3, 418–420. [Google Scholar]
- Mathobo, R.; Marais, D.; Steyn, J.M. The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef]
- Shaibu, A.S.; Motagi, B.N.; Muhammad, K.S. Peanut genotypes with high chlorophyll content and low leaf temperature are preferred in breeding program for drought prone areas. Legume Res. 2019, 42, 763–767. [Google Scholar] [CrossRef]
- Nikolaeva, M.K.; Maevskaya, S.N.; Shugaev, A.G.; Bukhov, N.G. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ. J. Plant Physiol. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- Shun, W.; Xueqin, Z.; Yan, C. Effects of drought stress on chlorophyll contents and photosynthetic characteristics of cucumber seedlings. Chin. Agric. Sci. Bull. 2014, 30, 133–137. [Google Scholar]
- Angelini, G.; Ragni, P.; Esposito, D.; Giardi, P.; Pompili, M.L.; Moscardelli, R.; Giardi, M.T. A device to study the effect of space radiation on photosynthetic organisms. Phys. Med. 2001, 17, 267–268. [Google Scholar] [PubMed]
- Souza, R.P.; Machado, E.C.; Silva, J.A.B.; Lagôa, A.M.M.A.; Silveira, J.A.G. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot. 2004, 51, 45–56. [Google Scholar] [CrossRef]
- Paliwoda, D.; Mikiciuk, G.; Mikiciuk, M.; Kisiel, A.; Sas-Paszt, L.; Miller, T. Effects of rhizosphere bacteria on strawberry plants (Fragaria × ananassa Duch.) under water deficit. Int. J. Mol. Sci. 2022, 23, 10449. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Bai, N.; Zhang, Y.; Zhang, X.H.; Zhang, Y.J.; Wang, L.; Wang, E.J.; Tian, Y.Y.; Guo, Y.Y.; Yan, F.; et al. Drought stress alters gas exchange, chlorophyll fluorescence, and antioxidant enzyme activities in Glycyrrhiza uralensisin the Hexi Corridor, China. Russ. J. Plant Physiol. 2022, 69, 123. [Google Scholar] [CrossRef]
- Sherstneva, O.; Khlopkov, A.; Gromova, E.; Yudina, L.; Vetrova, Y.; Pecherina, A.; Kuznetsova, D.; Krutova, E.; Sukhov, V.; Vodeneev, V. Analysis of chlorophyll fluorescence parameters as predictors of biomass accumulation and tolerance to heat and drought stress of wheat (Triticum aestivum) plants. Funct. Plant Biol. 2022, 49, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.N.; Aspinall, D.; Paleg, L.G. Proline accumulation and varietal adaptability to drought in barley: A potential metabolic measure of drought resistance. Nat. New Biol. 1972, 236, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.M.; Campos, S.P.; Passarinho, A.J.; Semedo, N.J.; Marques, N.M.; Ramalho, C.J.; Ricardo, P.C. Drought effect on photosynthetic activity, osmolyte accumulation and membrane integrity of two Cicer genotypes. Photosynthetica 2010, 48, 303–312. [Google Scholar] [CrossRef]
- Naylor, D.; DeGraaf, S.; Purdom, E.; Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017, 11, 2691–2704. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wilson, A.J.; Zhang, X.C.; Thoms, D.; Sohrabi, R.; Song, S.; Geissmann, Q.; Liu, Y.; Walgren, L.; He, S.Y.; et al. FERONIA restricts Pseudomonas in the rhizosphere microbiome via regulation of reactive oxygen species. Nat. Plant 2021, 7, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Liljeroth, E.; Bååth, E. Bacteria and fungi on roots of different barley varieties (Hordeum vulgare L.). Biol. Fertil. Soils 1988, 7, 53–57. [Google Scholar] [CrossRef]
- Babu, S.; Bidyarani, N.; Chopra, P.; Monga, D.; Kumar, R.; Prasanna, R.; Kranthi, S.; Saxena, A.K. Evaluating microbe-plant interactions and varietal differences for enhancing biocontrol efficacy in root rot disease challenged cotton crop. Eur. J. Plant Pathol. 2015, 142, 345–362. [Google Scholar] [CrossRef]
- Fang, J.; Shi, G.; Wei, S.; Ma, J.; Zhang, X.; Wang, J.; Chen, L.; Liu, Y.; Zhao, X.; Lu, Z. Drought sensitivity of spring wheat cultivars shapes rhizosphere microbial community patterns in response to drought. Plants 2023, 12, 3650. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Sun, Y.; Qin, R.L.; Yang, S.D.; Tan, H.W. Comparison of composition and function of soil bacteria in rhizosphere between two different drought-tolerant sugarcane varieties. Southwest China J. Agric. Sci. 2021, 34, 1923–1931. [Google Scholar]
- Wang, S.; Cheng, J.; Liao, Y. Response of rhizosphere microecology to plants, soil and microbes. Acta Microsc. 2020, 29, 43150. [Google Scholar]
- Bokhari, S.S.; Farhat, H.; Ali, S.A.; Urooj, F.; Rahman, A.; Ara, J.; Irfan, M.; Ehteshamul Haque, S. Role of mycorrhizospheric fluorescent Pseudomonas in suppressing the root rot disease, enhancement of vesicular arbuscular mycorrhizal (VAM) population and phosphorus uptake in sunflower. Pak. J. Bot. 2023, 55, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, F.; Zhang, Z.; Chao, H.; He, H.; Hu, W.; Zeng, Y.; Duan, C.; Liu, J.; Fang, L. Influences of arbuscular mycorrhizal fungi on crop growth and potentially toxic element accumulation in contaminated soils: A meta-analysis. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1795–1816. [Google Scholar] [CrossRef]
- Zhu, E.; Cao, Z.; Jia, J.; Liu, C.; Zhang, Z.; Wang, H.; Dai, G.; He, J.-S.; Feng, X. Inactive and inefficient: Warming and drought effect on microbial carbon processing in alpine grassland at depth. Glob. Chang. Biol. 2021, 27, 2241–2253. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Jiang, F.; Lin, W.; Tian, Z.; Wu, N.; Feng, X.; Chen, T.; Nan, Z. Effects of drought on the growth of lespedeza davurica through the alteration of soil microbial communities and nutrient availability. J. Fungi 2022, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Zhao, H.C.; Dong, W.; Shi, B.L.; Mao, Q.W.; Song, S.P.; Yan, L.B. Effects of different fertilizer application on soil enzyme activity of spring corn. Soil Fertil. Sci. China 2016, 5, 18–24. [Google Scholar]
- van der Molen, M.K.; Dolman, A.J.; Ciais, P.; Eglin, T.; Gobron, N.; Law, B.E.; Meir, P.; Peters, W.; Phillips, O.L.; Reichstein, M.; et al. Drought and ecosystem carbon cycling. Agric. For. Meteorol. 2011, 151, 765–773. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Zhang, Z.; Li, W.; Liu, W.; Xiao, N.; Liu, H.; Wang, L.; Li, Z.; Ma, J.; et al. Decreased soil multifunctionality is associated with altered microbial network properties under precipitation reduction in a semiarid grassland. iMeta 2023, 2, e106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xie, S.; Zhao, X.; Liu, Y.; Xing, Y.; Dao, J.; Wei, B.; Peng, Y.; Duan, W.; Wang, Z. Drought sensitivity of sugarcane cultivars shapes rhizosphere bacterial community patterns in response to water stress. Front. Microbiol. 2021, 12, 732989. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, F.A.; Carrillo, Y.; Pendall, E.; Morgan, J.A. Rhizosphere priming: A nutrient perspective. Front. Microbiol. 2013, 4, 216. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mu, Y.; Cheng, Y.; Liu, X.; Nian, H. Effects of intercropping sugarcane and soybean on growth, rhizosphere soil microbes, nitrogen and phosphorus availability. Acta. Physiol. Plant. 2013, 35, 1113–1119. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Cao, H.; Sun, H.; Yang, H.; Sun, B.; Zhao, Q. A review: Soil enzyme activity and its indication for soil quality. Chinese J. Appl. Environ. Biol. 2003, 9, 105–109. [Google Scholar]
- Hueso, S.; García, C.; Hernández, T. Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biol. Biochem. 2012, 50, 167–173. [Google Scholar] [CrossRef]
- Tarkka, M.; Schrey, S.; Hampp, R. Plant associated soil microorganisms. In Molecular Mechanisms of Plant and Microbe Coexistence; Nautiyal, C.S., Dion, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–51. [Google Scholar]
- Lan, X.; Ning, Z.; Jia, Y.; Lin, W.; Xiao, E.; Cheng, Q.; Cai, Q.; Xiao, T. The rhizosphere microbiome reduces the uptake of arsenic and tungsten by blechnum orientale by increasing nutrient cycling in historical tungsten mining area soils. Sci. Total Environ. 2024, 924, 171429. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, Q.; Pang, Z.; Fallah, N.; Liu, Y.; Hu, C.; Lin, W. Sugarcane rhizosphere bacteria community migration correlates with growth stages and soil nutrient. Int. J. Mol. Sci. 2022, 23, 10303. [Google Scholar] [CrossRef] [PubMed]
- Mijangos, I.; Pérez, R.; Albizu, I.; Garbisu, C. Effects of fertilization and tillage on soil biological parameters. Enzyme Microb. Technol. 2006, 40, 100–106. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.G.; Kim, S.W.; Yadav, D.R.; Hyum, U.; Adhikari, M.; Lee, Y.S. Penicillium menonorum: A novel fungus to promote growth and nutrient management in cucumber plants. Mycobiology 2015, 43, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Canarini, A.; Andrew, M.; Dijkstra, F.A. Drought effects on Helianthus annuus and Glycine max metabolites: From phloem to root exudates. Rhizosphere 2016, 2, 85–97. [Google Scholar] [CrossRef]
- Durán, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemen, E.; Schulze-Lefert, P.; Hacquard, S. Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell 2018, 175, 973–983.e14. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Luo, Y.; Wei, Y.; Huang, Y.; Zhang, H.; He, W.; Sheng, H.; An, L. Effect of aridity and dune type on rhizosphere soil bacterial communities of caragana microphylla in desert regions of northern China. PLoS ONE 2019, 14, e0224195. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, T.; Halden, R.U. Can stress enhance phytoremediation of polychlorinated biphenyls? Environ Eng. Sci. 2012, 29, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defense system in maize (Zea mays L.). Physiol Plant 2021, 172, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Yandigeri, M.S.; Meena, K.K.; Singh, D.; Malviya, N.; Singh, D.P.; Solanki, M.K.; Yadav, A.K.; Arora, D.K. Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul. 2012, 68, 411–420. [Google Scholar] [CrossRef]
- Vurukonda, S.S.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, D.P.; Dias, A.C.; Cotta, S.R.; Vilela, D.; De Andrade, P.A.; Pellizari, V.H.; Andreote, F.D. Changes of bacterial communities in the rhizosphere of sugarcane under elevated concentration of atmospheric CO2. GCB Bioenergy 2018, 10, 137–145. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.; Bagchi, R. Biodiversity and ecosystem multifunctionality. Nature 2007, 448, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.W.; Kuramae, E.E.; Navarrete, A.A.; van Veen, J.A.; Tsai, S.M. Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J. 2014, 8, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, H.; Sun, M.; Peng, Q.; Li, A. Photosynthetic physiological performance and proteomic profiling of the oleaginous algae Scenedesmus acuminatus reveal the mechanism of lipid accumulation under low and high nitrogen supplies. Photosynth. Res. 2018, 138, 73–102. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.; Philippot, L. A plant perspective on nitrogen cyclingin the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Karimi, N.; Goltapeh, E.M.; Amini, J.; Mehnaz, S.; Zarea, M.J. Effect of Azospirillum zeae and seed priming with zinc manganese and auxin on growth and yield parameters of Wheat under dryland farming. Agric. Res. 2020, 10, 44–55. [Google Scholar] [CrossRef]
- Liu, H.; Macdonald, C.A.; Cook, J.; Anderson, I.C.; Singh, B.K. An ecological loop: Host microbiomes across multitrophic interactions. Trends Ecol. Evol. 2019, 34, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, S.; Zou, L.; Guo, X.; Liang, J.; Liao, W.; Peng, M. Natural variation MeMYB108 associated with tolerance to stress-induced leaf abscission linked to enhanced protection against reactive oxygen species in cassava. Plant Cell Rep. 2022, 41, 1573–1587. [Google Scholar] [CrossRef]
- Xing, Y.; Dao, J.; Chen, M.; Chen, C.; Li, B.; Wang, Z. Multi-omics reveals the sugarcane rhizosphere soil metabolism-microbiota interactions affected by drought stress. Appl. Soil Ecol. 2023, 190, 104994. [Google Scholar] [CrossRef]
- Qin, Q.; Zhu, J.; Shi, Z.; Yu, T.; Cao, K. Association of leaf anatomical structure characteristics with photosynthetic capacity and drought tolerance in seven sugarcane varieties. Acta Plant Physiol. 2017, 053, 705–712. [Google Scholar]
- Schmitz, L.; Yan, Z.; Schneijderberg, M.; de Roij, M.; Pijnenburg, R.; Zheng, Q.; Franken, C.; Dechesne, A.; Trindade, L.M.; van Velzen, R.; et al. Synthetic bacterial community derived from a desert rhizosphere confers salt stress resilience to tomato in the presence of a soil microbiome. ISME J. 2022, 16, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic. A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Wang, D.; Lin, H.; Ma, Q.; Bai, Y.; Qu, J. Manganese oxides in Phragmites rhizosphere accelerates ammonia oxidation in constructed wetlands. Water Res. 2021, 205, 117688. [Google Scholar] [CrossRef]




| Soil Volume Water Content (%) | pH | TN (mg/g) | TP (mg/g) | AP (mg/g) | TOC (%) | S-CAT (U/g) | S-ACP (μmol/g) | S-SC (U/g) | S-UE (U/g) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SC8 | Control | 24.77 ± 1.66 a | 5.71 ± 0.07 a | 1.54 ± 0.13 a | 0.85 ± 0.01 a | 18.74 ± 1.04 a | 1.52 ± 0.02 a | 5.94 ± 0.39 ab | 48.54 ± 4.81 a | 6.21 ± 0.83 a | 2.70 ± 0.35 a |
| Drought | 3.67 ± 0.31 b | 5.85 ± 0.34 a | 0.72 ± 0.08 c | 0.79 ± 0.01 b | 16.25 ± 0.42 b | 1.09 ± 0.12 b | 5.36 ± 0.53 b | 47.49 ± 3.30 a | 4.43 ± 1.12 a | 1.98 ± 0.17 bc | |
| SC124 | Control | 24.58 ± 0.83 a | 5.50 ± 0.26 a | 1.41 ± 0.07 a | 0.85 ± 0.01 a | 20.40 ± 0.92 a | 1.52 ± 0.02 a | 7.26 ± 1.26 a | 44.20 ± 3.38 a | 6.18 ± 0.66 a | 2.36 ± 0.25 ab |
| Drought | 4.57 ± 0.05 b | 5.58 ± 0.14 a | 0.98 ± 0.08 b | 0.79 ± 0.01 b | 16.87 ± 0.65 b | 1.35 ± 0.10 a | 5.37 ± 0.51 b | 52.39 ± 1.57 a | 4.64 ± 1.16 a | 1.70 ± 0.11 c | |
| Kruskal-Wallice Test | |||||||||||
| H | 9.495 | 2.077 | 9.667 | 8.687 | 8.333 | 9.012 | 3.923 | 6.897 | 4.385 | 8.744 | |
| p | 0.023 | 0.557 | 0.022 | 0.034 | 0.040 | 0.029 | 0.270 | 0.075 | 0.223 | 0.033 | |
| Treatment | ACE Index | Chao1 Index | Simpson Index | Shannon Index |
|---|---|---|---|---|
| SC124-C | 4464 ± 15 b | 4403 ± 28 b | 0.991 d | 9.175 ± 0.019 d |
| SC124-D | 4184 ± 25 c | 4150 ± 31 c | 0.995 a | 9.497 ± 0.014 a |
| SC8-C | 4606 ± 22 a | 4524 ± 23 a | 0.992 c | 9.287 ± 0.007 c |
| SC8-D | 3786 ± 20 d | 3757 ± 20 d | 0.995 b | 9.338 ± 0.030 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Li, M.; Guo, Q.; Zhang, R.; Zhang, Y.; Luo, K.; Chen, Y. Influence of Drought Stress on the Rhizosphere Bacterial Community Structure of Cassava (Manihot esculenta Crantz). Int. J. Mol. Sci. 2024, 25, 7326. https://doi.org/10.3390/ijms25137326
Huang H, Li M, Guo Q, Zhang R, Zhang Y, Luo K, Chen Y. Influence of Drought Stress on the Rhizosphere Bacterial Community Structure of Cassava (Manihot esculenta Crantz). International Journal of Molecular Sciences. 2024; 25(13):7326. https://doi.org/10.3390/ijms25137326
Chicago/Turabian StyleHuang, Huling, Mingchao Li, Qiying Guo, Rui Zhang, Yindong Zhang, Kai Luo, and Yinhua Chen. 2024. "Influence of Drought Stress on the Rhizosphere Bacterial Community Structure of Cassava (Manihot esculenta Crantz)" International Journal of Molecular Sciences 25, no. 13: 7326. https://doi.org/10.3390/ijms25137326





