Proteomic Characterization of a 3D HER2+ Breast Cancer Model Reveals the Role of Mitochondrial Complex I in Acquired Resistance to Trastuzumab
Abstract
1. Introduction
2. Results
2.1. Three-Dimensional Architecture and Growth Kinetics of BT-474 Spheroids
2.2. Proteomic Characterization of BT-474 Spheroids
2.3. Identification of Differentially Expressed Proteins and Network Analysis
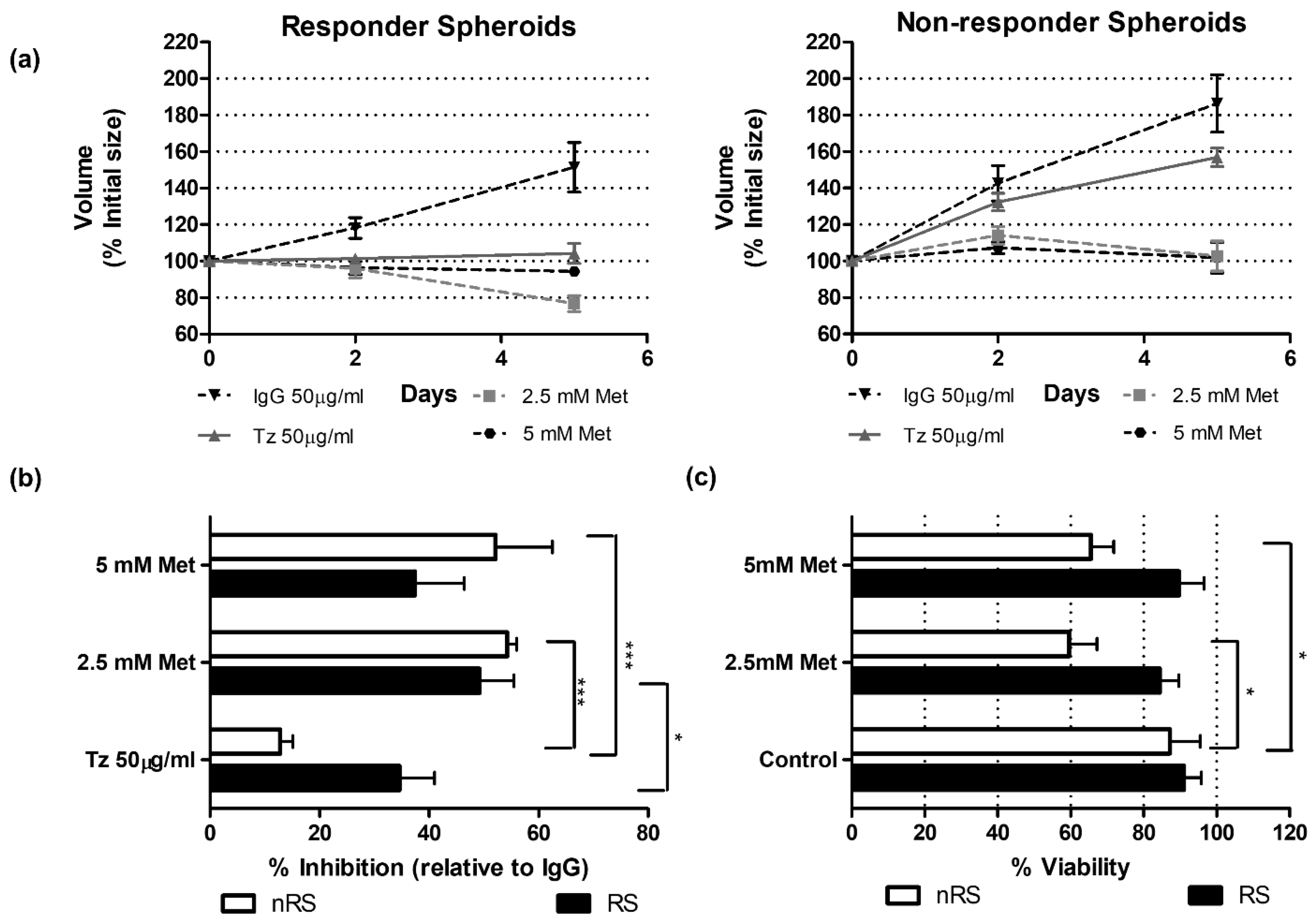
2.4. Functional Validations Suggest Mitochondrial Complex I Involvement
3. Discussion
4. Materials and Methods
4.1. BT-474 Tumor Spheroid Preparation and Treatment
4.1.1. Drugs and Treatments
4.1.2. Cell Cultures and Generation of Tumor Spheroids
4.1.3. Tumor Spheroid Growth
4.1.4. Tumor Spheroid Histology
4.1.5. Tumor Spheroid Viability
4.1.6. Glucose Consumption and Lactate Production Determinations
4.2. LC-MS/MS Analysis of Responder and Non-Responder BT-474 Tumor Spheroids
4.2.1. In-Solution Digestion
4.2.2. Liquid Chromatography
4.2.3. Mass Spectrometry
4.2.4. Proteomic Data Processing and Data Mining
4.2.5. Network Analysis
4.3. Analysis of Clinical Data
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolff, A.C.; Hammond, M.E. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Tebbutt, N.; Pedersen, M.W. Targeting the ERBB family in cancer: Couples therapy. Nat. Rev. Cancer 2013, 13, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Norton, L. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998, 58, 2825–2831, Erratum in: Cancer Res. 1999, 59, 2020. [Google Scholar]
- Baselga, J. Treatment of HER2-overexpressing breast cancer. Ann. Oncol. 2010, 21 (Suppl. S7), vii36–vii40. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Swain, S.M. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Lewis Phillips, G.D.; Li, G. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [PubMed]
- Konecny, G.E.; Pegram, M.D. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006, 66, 1630–1639. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Giordano, S.H.; Franzoi, M.A.B. Systemic Therapy for Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 2612–2635. [Google Scholar] [CrossRef]
- O’Shaughnessy, J.; Robert, N. Recurrence rates in patients with HER2+ breast cancer who achieved a pathological complete response after neoadjuvant pertuzumab plus trastuzumab followed by adjuvant trastuzumab: A real-world evidence study. Breast Cancer Res. Treat. 2021, 187, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Chaganty, B.K.R.; Qiu, S. Trastuzumab Upregulates PD-L1 as a Potential Mechanism of Trastuzumab Resistance through Engagement of Immune Effector Cells and Stimulation of IFNγ Secretion. Cancer Lett. 2018, 430, 47–56. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, Y. Cancer-associated fibroblasts induce trastuzumab resistance in her2 positive breast cancer cells. Mol. Biosyst. 2015, 11, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.; Silvestri, A. Paralysis of the cytotoxic granule machinery is a new cancer immune evasion mechanism mediated by chitinase 3-like-1. J. Immunother. Cancer 2021, 9, e003224. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, M.; Rojo, F. Expression of p95HER2, a Truncated Form of the HER2 Receptor, and Response to Anti-HER2 Therapies in Breast Cancer. J. Natl. Cancer Inst. 2007, 99, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Friedlander, E. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptinresistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005, 65, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Ritter, C.A.; Perez-Torres, M. Human Breast Cancer Cells Selected for Resistance to Trastuzumab in Vivo Overexpress Epidermal Growth Factor Receptor and ErbB Ligands and Remain Dependent on the ErbB Receptor Network. Clin. Cancer Res. 2007, 13, 4909–4919. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zi, X. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J. Natl. Cancer Inst. 2001, 93, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- de Melo Gagliato, D.; Jardim, D.L. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget 2016, 7, 64431–64446. [Google Scholar] [CrossRef]
- Fiszman, G.L.; Jasnis, M.A. Molecular Mechanisms of Trastuzumab Resistance in HER2 Overexpressing Breast Cancer. Int. J. Breast Cancer. 2011, 2011, 352182. [Google Scholar] [CrossRef]
- Rodríguez, C.E.; Berardi, D.E. Breast cancer stem cells are involved in Trastuzumab resistance through the HER2 modulation in 3D culture. J. Cell. Biochem. 2018, 119, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guadarrama, G.; Garcia-Becerra, R. Vasculogenic Mimicry in Breast Cancer: Clinical Relevance and Drivers. Cells 2021, 10, 1758. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, S.; Yazdanparast, R. Activation of STAT3/HIF-1α/Hes-1 axis promotes trastuzumab resistance in HER2-overexpressing breast cancer cells via down-regulation of PTEN. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.E.; Reidel, S.I. Autophagy Protects from Trastuzumab-Induced Cytotoxicity in HER2 Overexpressing Breast Tumor Spheroids. PLoS ONE 2015, 10, e0137920. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res. 2011, 71, 4585–4597. [Google Scholar] [CrossRef] [PubMed]
- Gale, M.; Li, Y. Acquired Resistance to HER2-Targeted Therapies Creates Vulnerability to ATP Synthase Inhibition. Cancer Res. 2020, 80, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Z. Receptor tyrosine kinase ErbB2 translocates into mitochondria and regulates cellular metabolism. Nat. Commun. 2012, 3, 1271. [Google Scholar] [CrossRef]
- Cortese, K.; Ponassi, M. Lipid Metabolism Reprogramming and Trastuzumab Resistance in Breast Cancer Cell Lines Overexpressing the ERBB2 Membrane Receptor. Membranes 2023, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, A.S.; Eetezadi, S. Multicellular tumor spheroids for evaluation of cytotoxicity and tumor growth inhibitory effects of nanomedicines in vitro: A comparison of docetaxel-loaded block copolymer micelles and Taxotere®. PLoS ONE 2013, 8, e62630. [Google Scholar] [CrossRef]
- Muñoz, L.; Espinosa, M. Paradoxial changes in the expression of estrogen receptor alpha in breast cancer multicellular spheroids. Tissue Cell 2010, 42, 334–337. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef]
- Wolkenhauer, O.; Auffray, C. The road from systems biology to systems medicine. Pediatr. Res. 2013, 73 Pt 2, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Chen, Z. Systems medicine: The future of medical genomics and healthcare. Genome Med. 2009, 1, 2. [Google Scholar] [CrossRef]
- Anderson, N.L.; Anderson, N.G. Proteome and proteomics: New technologies, new concepts, and new words. Electrophoresis 1998, 11, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.J.; Jacobs, J.M. Advances and challenges in liquid chromatography-mass spectrometry-based proteomics profiling for clinical applications. Mol. Cell. Proteom. 2006, 5, 1727–1744. [Google Scholar] [CrossRef]
- Jain, K.K. Role of Proteomics in the Development of Personalized Medicine. Adv. Protein Chem. Struct. Biol. 2016, 102, 41–52. [Google Scholar] [PubMed]
- Kowalczyk, T.; Ciborowski, M. Mass spectrometry based proteomics and metabolomics in personalized oncology. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165690. [Google Scholar] [CrossRef]
- Simpson, K.L.; Whetton, A.D. Quantitative mass spectrometry-based techniques for clinical use: Biomarker identification and quantification. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 1240–1249. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, L. Proteomic profiling of human plasma for cancer biomarker discovery. Proteomics 2017, 17, 1600240. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Hilario, M.; Kalousis, A. Approaches to dimensionality reduction in proteomic biomarker studies. Brief. Bioinform. 2007, 9, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.-X. ImageGP: An easy-to-use data visualization web server for scientific researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Smoot, M.E. A travel guide to Cytoscape plugins. Nat. Methods 2012, 9, 1069–1076. [Google Scholar] [CrossRef]
- Burgner, J.W., 2nd; Ray, W.J., Jr. On the origin of the lactate dehydrogenase induced rate effect. Biochemistry 1984, 23, 3636–3648. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fu, J. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4101–4109. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhou, M. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene 2009, 28, 3689–3701. [Google Scholar] [CrossRef]
- Cheyne, R.W.; Trembleau, L. Changes in 2-fluoro-2-deoxy-D-glucose incorporation, hexokinase activity and lactate production by breast cancer cells responding to treatment with the anti-HER-2 antibody trastuzumab. Nucl. Med. Biol. 2011, 38, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y. An Overview: The Diversified Role of Mitochondria in Cancer Metabolism. Int. J. Biol. Sci. 2023, 19, 897–915. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Álvarez, M.; Luque, M. Generation and Characterization of Trastuzumab/Pertuzumab-Resistant HER2-Positive Breast Cancer Cell Lines. Int. J. Mol. Sci. 2023, 25, 207. [Google Scholar] [CrossRef]
- Jeon, J.H.; Kim, D.K. Migration and invasion of drug-resistant lung adenocarcinoma cells are dependent on mitochondrial activity. Exp. Mol. Med. 2016, 48, e277. [Google Scholar] [CrossRef]
- Urra, F.A.; Muñoz, F. The Mitochondrial Complex(I)ty of Cancer. Front. Oncol. 2017, 7, 118. [Google Scholar] [CrossRef]
- Gottlieb, E.; Armour, S.M. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [CrossRef]
- Li, B.; Chauvin, C. Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochim. Biophys. Acta 2012, 1817, 1628–1634. [Google Scholar] [CrossRef]
- Batandier, C.; Leverve, X. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J. Biol. Chem. 2004, 279, 17197–17204. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.N.; Fan, T.W. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Wang, T. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, M.Y. Synergistic Effects of Metformin and Trastuzumab on HER2 Positive Gastroesophageal Adenocarcinoma Cells In Vitro and In Vivo. Cancers 2023, 15, 4768. [Google Scholar] [CrossRef] [PubMed]
- Vancura, A.; Bu, P. Metformin as an Anticancer Agent. Trends Pharmacol. Sci. 2018, 39, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.; Segaran, A. Targeting OXPHOS and the electron transport chain in cancer; Molecular and therapeutic implications. Semin. Cancer Biol. 2022, 86 Pt 2, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Martin-Castillo, B.; Pernas, S. A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: The METTEN study. Oncotarget 2018, 9, 35687–35704. [Google Scholar] [CrossRef]
- Del Duca, D.; Werbowetski, T. Spheroid preparation from hanging drops: Characterization of a model of brain tumor invasion. J. Neurooncol. 2004, 67, 295–303. [Google Scholar] [CrossRef]
- Nomura, E.; Katsuta, K. Acid-labile surfactant improves in-sodium dodecyl sulfate-polyacrylamide gel protein digestion for matrix-assisted laser desorption/ionization mass spectrometric peptide mapping. J. Mass Spectrom. 2004, 39, 202–207. [Google Scholar] [CrossRef]
- Käll, L.; Canterbury, J.D. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 2007, 4, 923–925. [Google Scholar] [CrossRef]
- Mauri, P.; Dehò, G. A Proteomic Approach to the Analysis of RNA Degradosome Composition in Escherichia coli. Methods Enzymol. 2008, 447, 99–117. [Google Scholar] [PubMed]
- De Palma, A.; Fanelli, G. Gcn5p and Ubp8p Affect Protein Ubiquitylation and Cell Proliferation by Altering the Fermentative/Respiratory Flux Balance in Saccharomyces cerevisiae. mBio 2020, 11, e01504-20. [Google Scholar] [CrossRef] [PubMed]
- Liebermeister, W.; Noor, E. Visual account of protein investment in cellular functions. Proc. Natl. Acad. Sci. USA 2014, 111, 8488–8493. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia, I.J.; Perico, D.; Wolos, V.J.; Villaverde, M.S.; Abrigo, M.; Di Silvestre, D.; Mauri, P.; De Palma, A.; Fiszman, G.L. Proteomic Characterization of a 3D HER2+ Breast Cancer Model Reveals the Role of Mitochondrial Complex I in Acquired Resistance to Trastuzumab. Int. J. Mol. Sci. 2024, 25, 7397. https://doi.org/10.3390/ijms25137397
Tapia IJ, Perico D, Wolos VJ, Villaverde MS, Abrigo M, Di Silvestre D, Mauri P, De Palma A, Fiszman GL. Proteomic Characterization of a 3D HER2+ Breast Cancer Model Reveals the Role of Mitochondrial Complex I in Acquired Resistance to Trastuzumab. International Journal of Molecular Sciences. 2024; 25(13):7397. https://doi.org/10.3390/ijms25137397
Chicago/Turabian StyleTapia, Ivana J., Davide Perico, Virginia J. Wolos, Marcela S. Villaverde, Marianela Abrigo, Dario Di Silvestre, Pierluigi Mauri, Antonella De Palma, and Gabriel L. Fiszman. 2024. "Proteomic Characterization of a 3D HER2+ Breast Cancer Model Reveals the Role of Mitochondrial Complex I in Acquired Resistance to Trastuzumab" International Journal of Molecular Sciences 25, no. 13: 7397. https://doi.org/10.3390/ijms25137397
APA StyleTapia, I. J., Perico, D., Wolos, V. J., Villaverde, M. S., Abrigo, M., Di Silvestre, D., Mauri, P., De Palma, A., & Fiszman, G. L. (2024). Proteomic Characterization of a 3D HER2+ Breast Cancer Model Reveals the Role of Mitochondrial Complex I in Acquired Resistance to Trastuzumab. International Journal of Molecular Sciences, 25(13), 7397. https://doi.org/10.3390/ijms25137397








