Association between Visceral Adipose Tissue Metabolism and Cerebral Glucose Metabolism in Patients with Cognitive Impairment
Abstract
:1. Introduction
2. Results
2.1. Population Characteristics
2.2. Association of VAT Metabolism with Cerebral Glucose Metabolism
2.3. Association between VAT Metabolism and Cognitive Function
3. Discussion
4. Materials and Methods
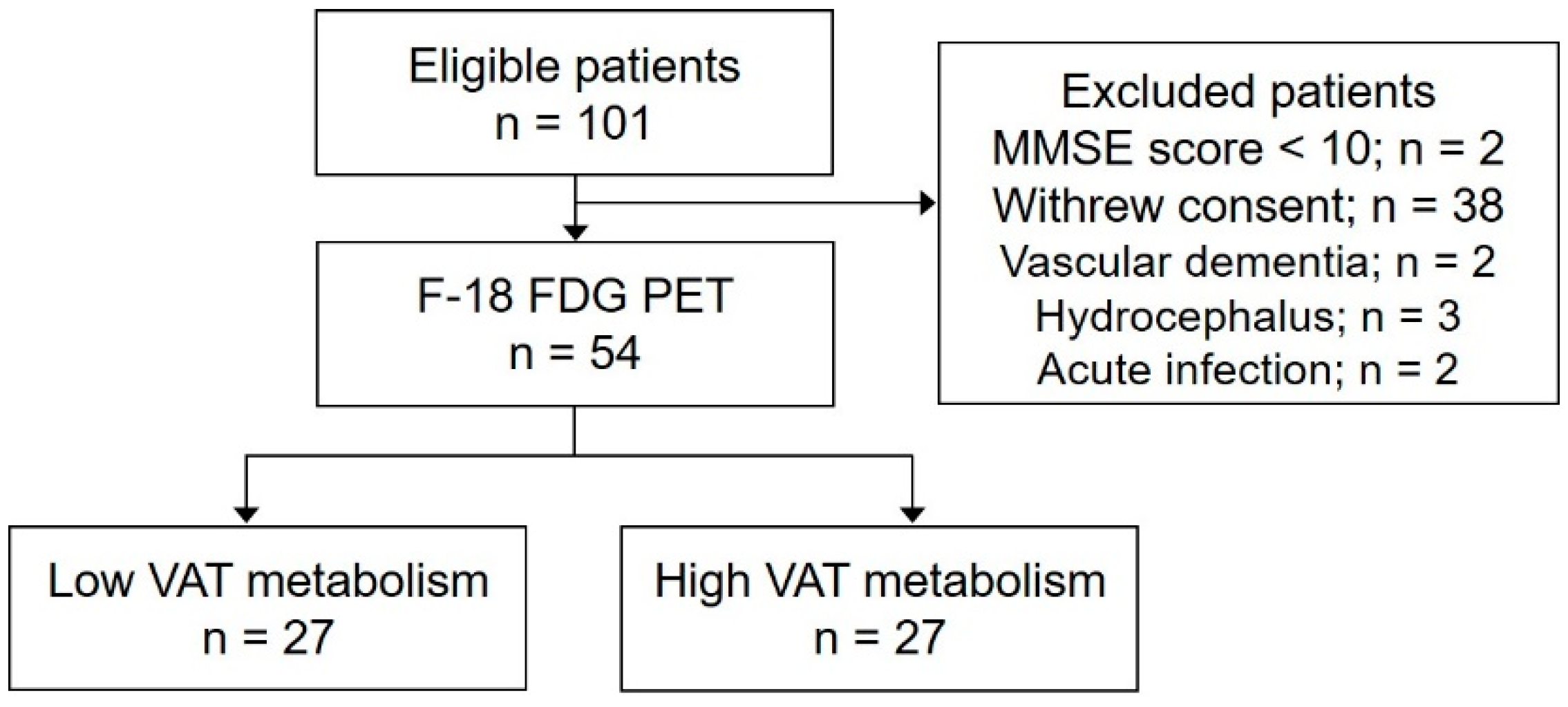
4.1. Study Design and Participants
4.2. 18F-FDG Torso PET/CT Imaging
4.3. 18F-FDG Brain PET/CT Imaging
4.4. Neuropsychological Assessment
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, Y.; Lutz, M.W.; Xing, Y. A systems-based model of Alzheimer’s disease. Alzheimer’s Dement. 2019, 15, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Ezkurdia, A.; Ramírez, M.J.; Solas, M. Metabolic Syndrome as a Risk Factor for Alzheimer’s Disease: A Focus on Insulin Resistance. Int. J. Mol. Sci. 2023, 24, 4354. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lv, J.; Huang, X.; Wisniewski, T.; Zhang, W. High-fat diet-induced atherosclerosis promotes neurodegeneration in the triple transgenic (3 × Tg) mouse model of Alzheimer’s disease associated with chronic platelet activation. Alzheimer’s Res. Ther. 2021, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Capucho, A.M.; Chegão, A.; Martins, F.O.; Vicente Miranda, H.; Conde, S.V. Dysmetabolism and Neurodegeneration: Trick or Treat? Nutrients 2022, 14, 1425. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Hulme, J.; Vo, T.K.; Van Vo, G. The Potential Crosstalk Between the Brain and Visceral Adipose Tissue in Alzheimer’s Development. Neurochem. Res. 2022, 47, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, K.H.; Cho, S.G.; Kang, S.R.; Yoo, S.W.; Kwon, S.Y.; Min, J.J.; Bom, H.S.; Song, H.C. Association of muscle and visceral adipose tissues with the probability of Alzheimer’s disease in healthy subjects. Sci. Rep. 2019, 9, 949. [Google Scholar] [CrossRef]
- Kiliaan, A.J.; Arnoldussen, I.A.; Gustafson, D.R. Adipokines: A link between obesity and dementia? Lancet Neurol. 2014, 13, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yi, H.A.; Won, K.S.; Lee, J.S.; Kim, H.W. Association between Visceral Adipose Tissue Metabolism and Alzheimer’s Disease Pathology. Metabolites 2022, 12, 258. [Google Scholar] [CrossRef]
- Fakih, W.; Zeitoun, R.; AlZaim, I.; Eid, A.H.; Kobeissy, F.; Abd-Elrahman, K.S.; El-Yazbi, A.F. Early metabolic impairment as a contributor to neurodegenerative disease: Mechanisms and potential pharmacological intervention. Obesity 2022, 30, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Iadecola, C. Adipocyte-derived factors in age-related dementia and their contribution to vascular and Alzheimer pathology. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Letra, L.; Matafome, P.; Rodrigues, T.; Duro, D.; Lemos, R.; Baldeiras, I.; Patrício, M.; Castelo-Branco, M.; Caetano, G.; Seiça, R. Association between adipokines and biomarkers of Alzheimer’s disease: A cross-sectional study. J. Alzheimer’s Dis. 2019, 67, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, A.; Crandall, J.P.; Fraum, T.J.; Wahl, R.L. Repeatability of Radiomic Features of Brown Adipose Tissue. J. Nucl. Med. 2021, 62, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Reijrink, M.; de Boer, S.A.; Antunes, I.F.; Spoor, D.S.; Heerspink, H.J.L.; Lodewijk, M.E.; Mastik, M.F.; Boellaard, R.; Greuter, M.J.W.; Benjamens, S.; et al. [18F]FDG Uptake in Adipose Tissue Is Not Related to Inflammation in Type 2 Diabetes Mellitus. Mol. Imaging Biol. 2021, 23, 117–126. [Google Scholar] [CrossRef]
- Pahk, K.; Kim, E.J.; Lee, Y.J.; Kim, S.; Seo, H.S. Characterization of glucose uptake metabolism in visceral fat by 18F-FDG PET/CT reflects inflammatory status in metabolic syndrome. PLoS ONE 2020, 15, e0228602. [Google Scholar] [CrossRef] [PubMed]
- Bucerius, J.; Mani, V.; Wong, S.; Moncrieff, C.; Izquierdo-Garcia, D.; Machac, J.; Fuster, V.; Farkouh, M.E.; Rudd, J.H.; Fayad, Z.A. Arterial and fat tissue inflammation are highly correlated: A prospective 18F-FDG PET/CT study. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, S.; Lee, H.S.; Park, E.J.; Baik, S.H.; Jeon, T.J.; Lee, K.Y.; Ryu, Y.H.; Kang, J. Different prognostic impact of glucose uptake in visceral adipose tissue according to sex in patients with colorectal cancer. Sci. Rep. 2021, 11, 21556. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-E.; Joung, C.; Pahk, K.J.; Kim, H.; Pahk, K. Metabolic activity of visceral adipose tissue is associated with age-related macular degeneration: A pilot 18F-FDG PET/CT study. Front. Endocrinol. 2024, 14, 1322326. [Google Scholar] [CrossRef] [PubMed]
- Pahk, K.; Kwon, Y.; Kim, M.-K.; Park, S.; Kim, S. Visceral fat metabolic activity evaluated by 18F-FDG PET/CT is associated with osteoporosis in healthy postmenopausal Korean women. Obes. Res. Clin. Pract. 2020, 14, 339–344. [Google Scholar] [CrossRef]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Chételat, G.; Arbizu, J.; Barthel, H.; Garibotto, V.; Law, I.; Morbelli, S.; van de Giessen, E.; Agosta, F.; Barkhof, F.; Brooks, D.J.; et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020, 19, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Minoshima, S.; Cross, D.; Thientunyakit, T.; Foster, N.L.; Drzezga, A. 18F-FDG PET Imaging in Neurodegenerative Dementing Disorders: Insights into Subtype Classification, Emerging Disease Categories, and Mixed Dementia with Copathologies. J. Nucl. Med. 2022, 63, 2s–12s. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.; Bae, S.J.; Ahn, S.G.; Jeong, J.; Park, W.C.; Ryu, Y.H.; Jeon, T.J.; Lee, Y.; Cha, Y.J.; et al. 18F-FDG uptake of visceral adipose tissue on preoperative PET/CT as a predictive marker for breast cancer recurrence. Sci. Rep. 2022, 12, 21109. [Google Scholar] [CrossRef]
- Pahk, K.; Ryu, K.J.; Joung, C.; Kwon, H.W.; Lee, S.; Park, H.; Kim, T.; Song, J.Y.; Kim, S. Metabolic Activity of Visceral Adipose Tissue Is Associated with Metastatic Status of Lymph Nodes in Endometrial Cancer: A 18F-FDG PET/CT Study. Int. J. Environ. Res. Public Health 2021, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, P.; Łukaszewicz-Zając, M.; Mroczko, P.; Kornhuber, J. Clinical significance of fluid biomarkers in Alzheimer’s Disease. Pharmacol. Rep. 2020, 72, 528–542. [Google Scholar] [CrossRef]
- Arjunan, A.; Song, J. Pharmacological and physiological roles of adipokines and myokines in metabolic-related dementia. Biomed. Pharmacother. 2023, 163, 114847. [Google Scholar] [CrossRef] [PubMed]
- Dezonne, R.S.; Pereira, C.M.; de Moraes Martins, C.J.; de Abreu, V.G.; Francischetti, E.A. Adiponectin, the adiponectin paradox, and Alzheimer’s Disease: Is this association biologically plausible? Metab. Brain Dis. 2023, 38, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Puig, K.L.; Floden, A.M.; Adhikari, R.; Golovko, M.Y.; Combs, C.K. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS ONE 2012, 7, e30378. [Google Scholar] [CrossRef]
- Furiya, Y.; Ryo, M.; Kawahara, M.; Kiriyama, T.; Morikawa, M.; Ueno, S. Renin-angiotensin system blockers affect cognitive decline and serum adipocytokines in Alzheimer’s disease. Alzheimer’s Dement. 2013, 9, 512–518. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Boccara, E.; Golan, S.; Beeri, M.S. The association between regional adiposity, cognitive function, and dementia-related brain changes: A systematic review. Front. Med. 2023, 10, 1160426. [Google Scholar] [CrossRef]
- Guo, D.H.; Yamamoto, M.; Hernandez, C.M.; Khodadadi, H.; Baban, B.; Stranahan, A.M. Visceral adipose NLRP3 impairs cognition in obesity via IL-1R1 on CX3CR1+ cells. J. Clin. Investig. 2020, 130, 1961–1976. [Google Scholar] [CrossRef]
- Al-Roub, A.; Al Madhoun, A.; Akhter, N.; Thomas, R.; Miranda, L.; Jacob, T.; Al-Ozairi, E.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. IL-1β and TNFα Cooperativity in Regulating IL-6 Expression in Adipocytes Depends on CREB Binding and H3K14 Acetylation. Cells 2021, 10, 3228. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Zelicha, H.; Kloting, N.; Kaplan, A.; Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Chassidim, Y.; Bluher, M.; Ceglarek, U.; Isermann, B. The effect of high-polyphenol Mediterranean diet on visceral adiposity: The DIRECT PLUS randomized controlled trial. BMC Med. 2022, 20, 327. [Google Scholar] [CrossRef]
- Pahk, K.; Kim, E.J.; Joung, C.; Seo, H.S.; Kim, S. Exercise training reduces inflammatory metabolic activity of visceral fat assessed by 18F-FDG PET/CT in obese women. Clin. Endocrinol. 2020, 93, 127–134. [Google Scholar] [CrossRef]
- Ziyrek, M.; Kahraman, S.; Ozdemir, E.; Dogan, A. Metformin monotherapy significantly decreases epicardial adipose tissue thickness in newly diagnosed type 2 diabetes patients. Rev. Port. Cardiol. 2019, 38, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Pan, X.; Zhang, X.; Tong, N. The effect of thiazolidinediones on body fat redistribution in adults: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2024, 25, e13675. [Google Scholar] [CrossRef]
- Liu, F.; Yang, Q.; Zhang, H.; Zhang, Y.; Yang, G.; Ban, B.; Li, Y.; Zhang, M. The effects of glucagon-like peptide-1 receptor agonists on adipose tissues in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0270899. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Tanner, E.A.; Hill, D.W.; Vingren, J.L. Prebiotic/probiotic supplementation resulted in reduced visceral fat and mRNA expression associated with adipose tissue inflammation, systemic inflammation, and chronic disease risk. Genes Nutr. 2022, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Barthel, H.; Gertz, H.J.; Dresel, S.; Peters, O.; Bartenstein, P.; Buerger, K.; Hiemeyer, F.; Wittemer-Rump, S.M.; Seibyl, J.; Reininger, C.; et al. Cerebral amyloid-beta PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: A multicentre phase 2 diagnostic study. Lancet Neurol. 2011, 10, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Jeong, Y.J.; Park, K.W.; Kang, D.-Y. Correlation of early-phase F-18 florapronal PET with F-18 FDG PET in Alzheimer’s disease and normal brain. Nucl. Med. Mol. Imaging 2019, 53, 328–333. [Google Scholar] [CrossRef]
- Li, Y.; Ng, Y.L.; Paranjpe, M.D.; Ge, Q.; Gu, F.; Li, P.; Yan, S.; Lu, J.; Wang, X.; Zhou, Y.; et al. Tracer-specific reference tissues selection improves detection of 18F-FDG, 18F-florbetapir, and 18F-flortaucipir PET SUVR changes in Alzheimer’s disease. Hum. Brain Mapp. 2022, 43, 2121–2133. [Google Scholar] [CrossRef]
- Lee, J.J.; Earnest, T.; Ha, S.M.; Bani, A.; Kothapalli, D.; Liu, P.; Sotiras, A. Patterns of Glucose Metabolism in [18F] FDG PET Indicate Regional Variability and Neurodegeneration in the Progression of Alzheimer’s Dementia. medRxiv 2023. [Google Scholar] [CrossRef]

| Characteristics | Total (n = 54) | Low-VAT-Metabolism Group (n = 27) | High-VAT-Metabolism Group (n = 27) | p |
|---|---|---|---|---|
| Age, years (SD) | 66.4 (8.4) | 65.4 (8.1) | 67.5 (8.8) | 0.181 |
| Sex, female, n (%) | 34 (63.0) | 16 (59.3) | 18 (66.7) | 0.291 |
| Body mass index (SD) | 23.3 (3.4) | 23.6 (2.8) | 23 (4.0) | 0.263 |
| Education, years (SD) | 11.5 (6.1) | 11.4 (6.0) | 11.5 (6.3) | 0.474 |
| Diabetes, n (%) | 8 (14.8) | 4 (14.8) | 4 (14.8) | 0.500 |
| Hypertension, n (%) | 16 (29.6) | 10 (37.0) | 6 (22.2) | 0.121 |
| Cardiovascular disease, n (%) | 6 (11.1) | 4 (14.8) | 2 (7.4) | 0.198 |
| Hyperlipidemia, n (%) | 9 (16.7) | 5 (18.5) | 4 (14.8) | 0.361 |
| WMH volume (SD) | 3.4 (5.1) | 2.4 (3.1) | 4.4 (6.4) | 0.083 |
| VAT SUVmax | 0.7 (0.2) | 0.6 (0.1) | 0.8 (0.1) | <0.001 |
| Regions | Brodmann Area | Size | MINI Coordinates | T Value | p | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Right parietal lobe, postcentral gyrus | BA40 | 228 | 60 | −22 | 20 | 4.14 | <0.001 |
| Right parietal lobe, superior parietal lobule | BA7 | 393 | 24 | −62 | 50 | 3.70 | <0.001 |
| Right parietal lobe, superior parietal lobule | BA7 | 30 | −52 | 52 | 3.35 | 0.001 | |
| Right parietal lobe, inferior parietal lobule | BA40 | 38 | −32 | 46 | 3.30 | 0.001 | |
| Left parietal lobe, superior parietal lobule | BA7 | 161 | −10 | −64 | 52 | 3.65 | <0.001 |
| Left parietal lobe, precuneus | BA7 | −12 | −74 | 38 | 3.01 | 0.002 | |
| Left parietal lobe, superior parietal lobule | BA7 | −26 | −66 | 50 | 2.77 | 0.004 | |
| Left temporal lobe, superior temporal gyrus | BA38 | 182 | −42 | 6 | −10 | 3.36 | 0.001 |
| Left temporal lobe, middle temporal gyrus | BA21 | −48 | 4 | −18 | 2.96 | 0.002 | |
| Cognitive Domain | Neuropsychological Variables | Univariable Model | Multivariable Model | |||
|---|---|---|---|---|---|---|
| r | p | Adjusted R2 | Standardized β * | p | ||
| Attention | Digit span forward | −0.140 | 0.313 | 0.430 | −0.145 | 0.178 |
| Working Memory | Digit span backward | −0.182 | 0.191 | 0.416 | −0.180 | 0.090 |
| Language Ability | K-BNT | −0.297 | 0.034 | 0.684 | −0.271 | 0.012 |
| Visuospatial Ability | RCFT copy | −0.098 | 0.513 | 0.498 | −0.170 | 0.138 |
| Memory | SVLT immediate recall | −0.122 | 0.383 | 0.570 | −0.135 | 0.182 |
| Memory | SVLT delayed recall | −0.195 | 0.161 | 0.457 | −0.206 | 0.068 |
| Memory | SVLT recognition | −0.212 | 0.131 | 0.323 | −0.225 | 0.071 |
| Memory | RCFT immediate recall | −0.302 | 0.047 | 0.287 | −0.239 | 0.097 |
| Memory | RCFT delayed recall | −0.234 | 0.127 | 0.397 | −0.156 | 0.266 |
| Verbal Fluency | COWAT animal | −0.137 | 0.331 | 0.442 | −0.161 | 0.163 |
| Verbal Fluency | COWAT supermarket | −0.396 | 0.010 | 0.488 | −0.275 | 0.025 |
| Verbal Fluency | COWAT phonemic | −0.300 | 0.060 | 0.574 | −0.253 | 0.024 |
| Executive Function | Stroop color reading | −0.273 | 0.077 | 0.502 | −0.243 | 0.034 |
| Global Cognition | K-MMSE score | −0.232 | 0.091 | 0.619 | −0.183 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.-H.; Lim, J.S.; Yi, H.-A.; Won, K.S.; Kim, H.W. Association between Visceral Adipose Tissue Metabolism and Cerebral Glucose Metabolism in Patients with Cognitive Impairment. Int. J. Mol. Sci. 2024, 25, 7479. https://doi.org/10.3390/ijms25137479
Yu M-H, Lim JS, Yi H-A, Won KS, Kim HW. Association between Visceral Adipose Tissue Metabolism and Cerebral Glucose Metabolism in Patients with Cognitive Impairment. International Journal of Molecular Sciences. 2024; 25(13):7479. https://doi.org/10.3390/ijms25137479
Chicago/Turabian StyleYu, Mi-Hee, Ji Sun Lim, Hyon-Ah Yi, Kyoung Sook Won, and Hae Won Kim. 2024. "Association between Visceral Adipose Tissue Metabolism and Cerebral Glucose Metabolism in Patients with Cognitive Impairment" International Journal of Molecular Sciences 25, no. 13: 7479. https://doi.org/10.3390/ijms25137479
APA StyleYu, M.-H., Lim, J. S., Yi, H.-A., Won, K. S., & Kim, H. W. (2024). Association between Visceral Adipose Tissue Metabolism and Cerebral Glucose Metabolism in Patients with Cognitive Impairment. International Journal of Molecular Sciences, 25(13), 7479. https://doi.org/10.3390/ijms25137479






