The Expression of Genes Related to Reverse Cholesterol Transport and Leptin Receptor Pathways in Peripheral Blood Mononuclear Cells Are Decreased in Morbid Obesity and Related to Liver Function
Abstract
1. Introduction
2. Results
2.1. Anthropometric Characteristics and Biochemical Study
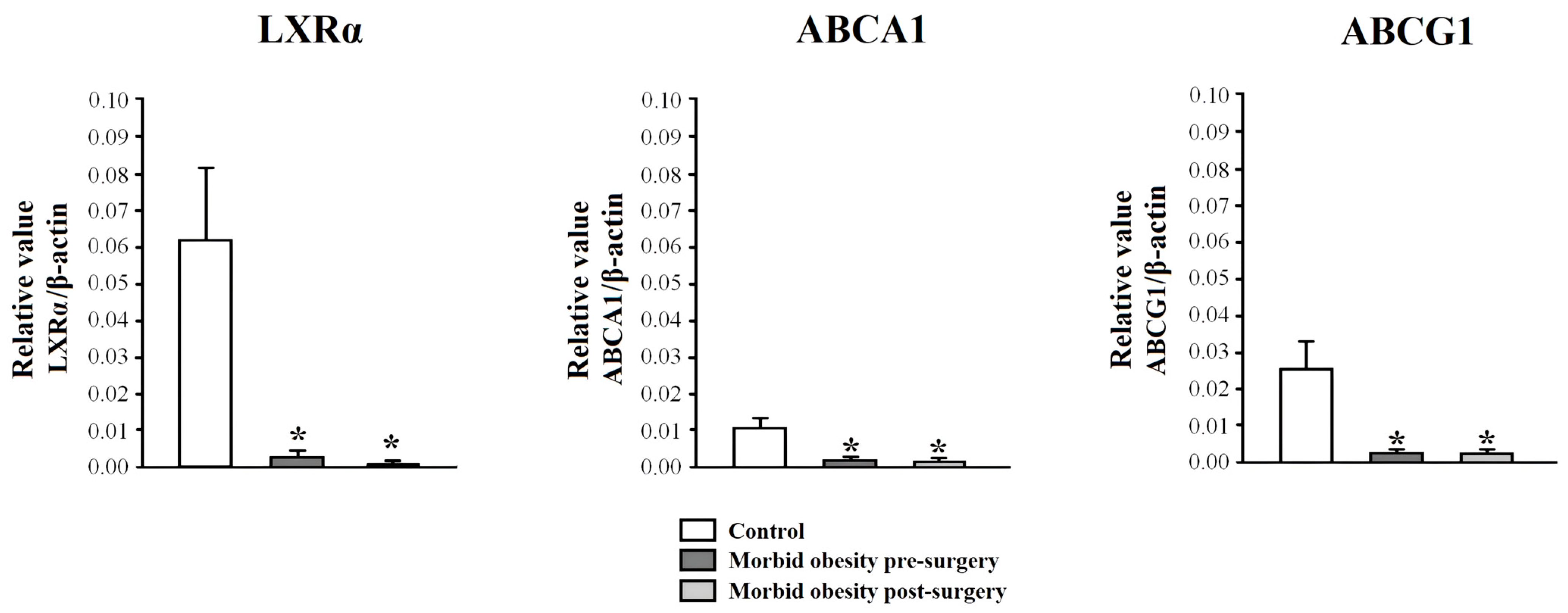
2.2. LXRα, ABCA1, and ABCG1 Expression in PBMCs from Non-Obese Subjects and Patients with MO Pre- and Post-Surgery
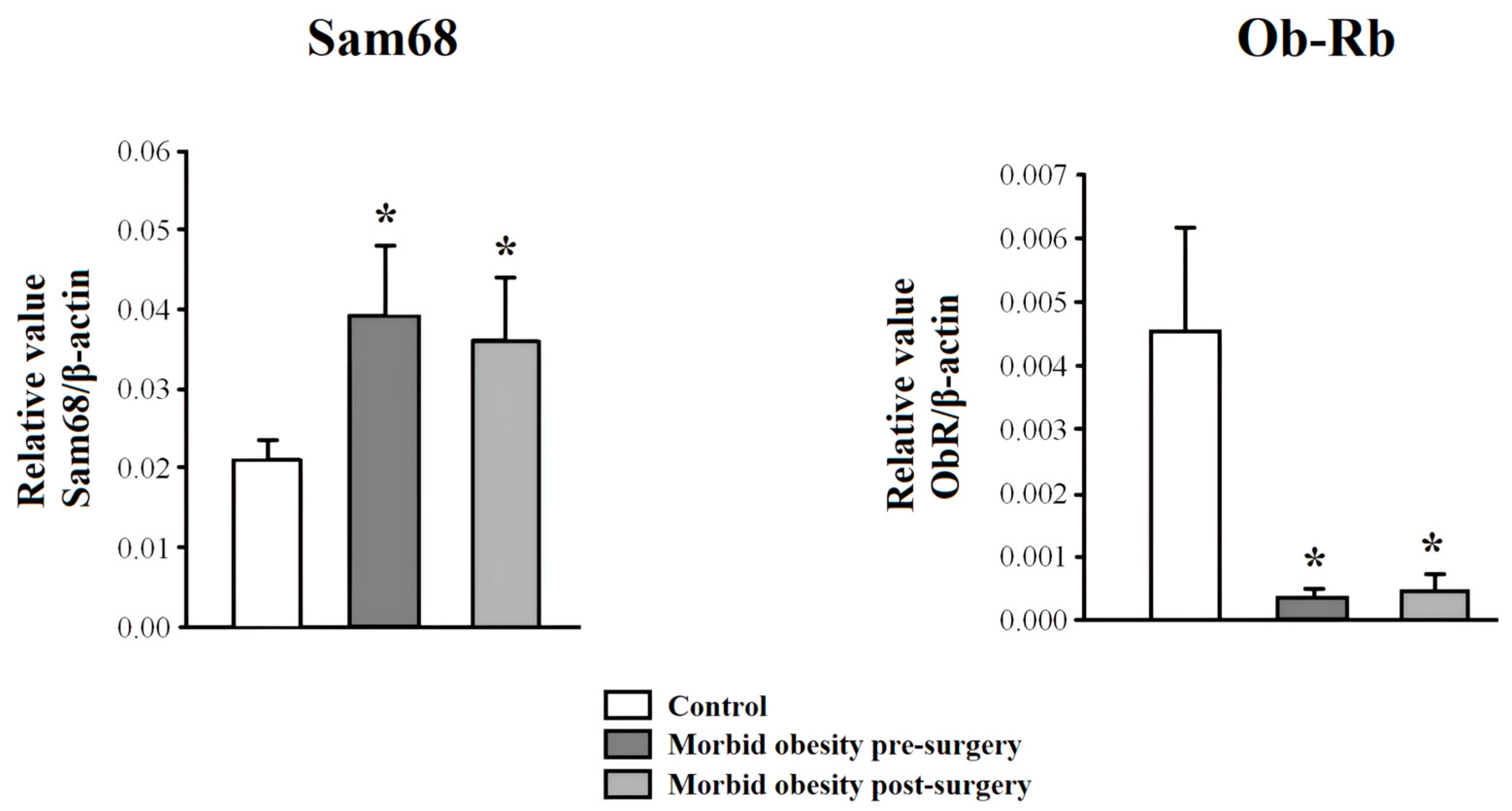
2.3. Ob-Rb and Sam68 Expression in PBMC from Non-Obese Subjects and Patients with MO Pre- and Post-Surgery
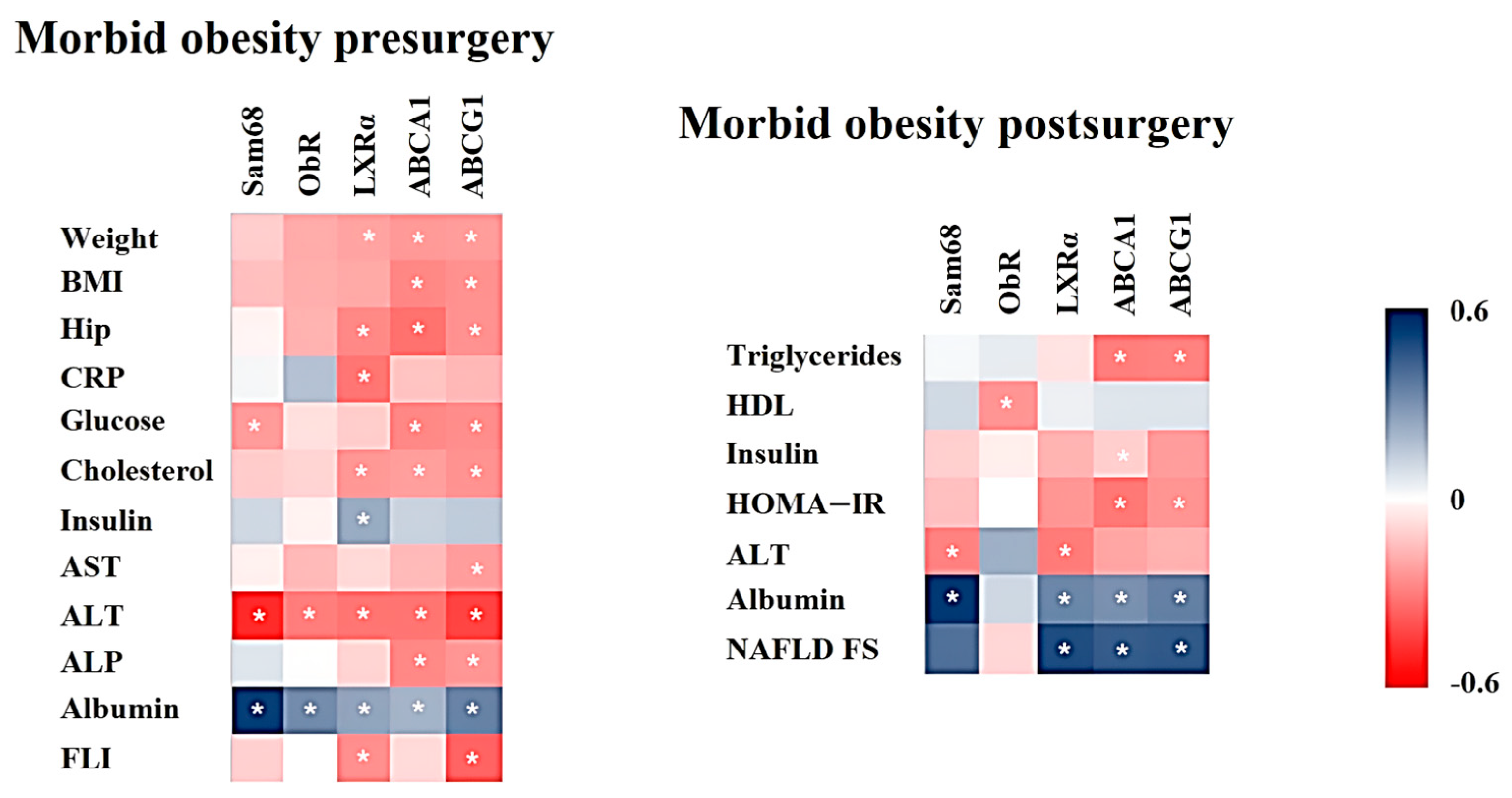
2.4. Association between mRNA Expression and Anthropometric/Biochemical Variables in Patients with MO Pre- and Post-Surgery
3. Discussion
4. Methods
4.1. Subjects
4.2. Sample Collection
4.3. Laboratory Measurements
4.4. Real-Time Quantitative PCR of mRNA Levels
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murri, M.; García-Fuentes, E.; García-Almeida, J.M.; Garrido-Sánchez, L.; Mayas, M.D.; Bernal, R.; Tinahones, F.J. Changes in Oxidative Stress and Insulin Resistance in Morbidly Obese Patients After Bariatric Surgery. Obes. Surg. 2010, 20, 363–368. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Vilariño-García, T.; Fernández-Riejos, P.; Martín-González, J.; Segura-Egea, J.J.; Sánchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor. Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Jiménez, F.; Pérez-Pérez, A.; González-Yanes, C.; Najib, S.; Varone, C.L.; Sánchez-Margalet, V. Leptin receptor activation increases Sam68 tyrosine phosphorylation and expression in human trophoblastic cells. Mol. Cell Endocrinol. 2011, 332, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; de la Cruz, L.; Virizuela, J.A.; Sánchez-Margalet, V. Sam68 Mediates the Activation of Insulin and Leptin Signalling in Breast Cancer Cells. PLoS ONE 2016, 11, e0158218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vilariño-García, T.; Pérez-Pérez, A.; Santamaría-López, E.; Prados, N.; Fernández-Sánchez, M.; Sánchez-Margalet, V. Sam68 mediates leptin signaling and action in human granulosa cells: Possible role in leptin resistance in PCOS. Endocr. Connect. 2020, 9, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Qiao, A.; Zhou, J.; Xu, S.; Ma, W.; Boriboun, C.; Kim, T.; Yan, B.; Deng, J.; Yang, L.; Zhang, E.; et al. Sam68 promotes hepatic gluconeogenesis via CRTC2. Nat. Commun. 2021, 12, 3340. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Petersen, K.F.; Shulman, G.I. Pleotropic effects of leptin to reverse insulin resistance and diabetic ketoacidosis. Diabetologia 2016, 59, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, J.; Alba, G.; Reyes-Quiroz, M.E.; Geniz, I.; Jiménez, J.; Sobrino, F.; Alba, G. Grapefruit Flavonoid Naringenin Regulates the Expression of LXRα in THP-1 Macrophages by Modulating AMP-Activated Protein Kinase. Mol. Pharm. 2018, 15, 1735–1745. [Google Scholar] [CrossRef]
- Moreno-Santos, I.; Garcia-Serrano, S.; Boughanem, H.; Garrido-Sanchez, L.; Tinahones, F.J.; Garcia-Fuentes, E.; Macias-Gonzalez, M. The Antagonist Effect of Arachidonic Acid on GLUT4 Gene Expression by Nuclear Receptor Type II Regulation. Int. J. Mol. Sci. 2019, 20, 963. [Google Scholar] [CrossRef]
- Zelcer, N.; Tontonoz, P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Investig. 2006, 116, 607–614. [Google Scholar] [CrossRef]
- Sáenz, J.; Alba, G.; Reyes-Quiroz, M.E.; Geniz, I.; Jiménez, J.; Sobrino, F.; Santa-María, C. Curcumin enhances LXRα in an AMP-activated protein kinase-dependent manner in human macrophages. J. Nutr. Biochem. 2018, 54, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Quiroz, M.E.; Alba, G.; Saenz, J.; Santa-María, C.; Geniz, I.; Jiménez, J.; Ramírez, R.; Martín-Nieto, J.; Pintado, E.; Sobrino, F. Oleic acid modulates mRNA expression of liver X receptor (LXR) and its target genes ABCA1 and SREBP1c in human neutrophils. Eur. J. Nutr. 2014, 53, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Alba, G.; Reyes, M.E.; Santa-María, C.; Ramírez, R.; Geniz, I.; Jiménez, J.; Martín-Nieto, J.; Pintado, E.; Sobrino, F. Transcription of liver X receptor is down-regulated by 15-deoxy-Δ(12,14)-prostaglandin J(2) through oxidative stress in human neutrophils. PLoS ONE 2012, 7, e42195. [Google Scholar] [CrossRef]
- Wong, R.; Yuan, L.Y. Sarcopenia and metabolic dysfunction associated steatotic liver disease: Time to address both. World J. Hepatol. 2024, 16, 871–877. [Google Scholar] [CrossRef] [PubMed]
- García-Suárez, C.; Crespo, J.; Fernández-Gil, P.L.; Amado, J.A.; García-Unzueta, M.T.; Pons Romero, F. Concentraciones plasmáticas de leptina en los pacientes con cirrosis biliar primaria y su relación con el grado de fibrosis [Plasma leptin levels in patients with primary biliary cirrhosis and their relationship with degree of fibrosis]. Gastroenterol. Hepatol. 2004, 27, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.K.; Ikeda, K.; Rockey, D.C.; Friedman, S.L.; Anania, F.A. Leptin in hepatic fibrosis: Evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002, 35, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Dixon, E.D.; Nardo, A.D.; Claudel, T.; Trauner, M. The Role of Lipid Sensing Nuclear Receptors (PPARs and LXR) and Metabolic Lipases in Obesity, Diabetes and NAFLD. Genes 2021, 12, 645. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.; Zhou, E.; Müller, C.; Mohammed, Y.; Herceg, S.; Bracher, F.; Rensen, P.C.N.; Wang, Y.; Mirakaj, V.; Giera, M. Inhibition of Δ24-dehydrocholesterol reductase activates pro-resolving lipid mediator biosynthesis and inflammation resolution. Proc. Natl. Acad. Sci. USA 2019, 116, 20623–20634. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.N.; Wang, C.C.N.; Chang, H.Y.; Chu, F.Y.; Hsu, Y.A.; Cheng, W.K.; Ma, W.C.; Chen, C.J.; Wan, L.; Lim, Y.P. Ursolic Acid, a Novel Liver X Receptor α (LXRα) Antagonist Inhibiting Ligand-Induced Nonalcoholic Fatty Liver and Drug-Induced Lipogenesis. J. Agric. Food Chem. 2018, 66, 11647–11662. [Google Scholar] [CrossRef]
- Liu, Y.; Han, X.; Bian, Z.; Peng, Y.; You, Z.; Wang, Q.; Chen, X.; Qiu, D.; Ma, X. Activation of liver X receptors attenuates endotoxin-induced liver injury in mice with nonalcoholic fatty liver disease. Dig. Dis. Sci. 2012, 57, 390–398. [Google Scholar] [CrossRef]
- García-Fuentes, E.; García-Almeida, J.M.; García-Arnés, J.; Rivas-Marín, J.; Gallego-Perales, J.L.; González-Jiménez, B.; Cardona, I.; García-Serrano, S.; Garriga, M.J.; Gonzalo, M.; et al. Morbidly obese individuals with impaired fasting glucose have a specific pattern of insulin secretion and sensitivity: Effect of weight loss after bariatric surgery. Obes. Surg. 2006, 16, 1179–1188. [Google Scholar] [CrossRef]
- Genua, I.; Puig, N.; Miñambres, I.; Benítez, S.; Gil, P.; Grau-Agramunt, M.; Rivas-Urbina, A.; Balagué, C.; Fernández-Alanin, S.; García-Osuna, Á.; et al. Changes in the composition and function of lipoproteins after bariatric surgery in patients with severe obesity. J. Clin. Med. 2021, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Choromanska, B.; Mysliwiec, P.; Hady, H.R.; Dadan, J.; Mysliwiec, H.; Bonda, T.; Chabowski, A.; Miklosz, A. The implication of adipocyte atp-binding cassette A1 and G1 transporters in metabolic complications of obesity. J. Physiol. Pharmacol. 2019, 70, 143–152. [Google Scholar]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldán, A.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Smith, J.D. ABCA1 and nascent HDL biogenesis. Biofactors 2014, 40, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Julia, Z.; Poitou, C.; Bouillot, J.L.; Basdevant, A.; Chapman, M.J.; Clement, K.; Guerin, M. Effect of bariatric surgery-induced weight loss on SR-BI-, ABCG1-, and ABCA1-mediated cellular cholesterol efflux in obese women. J. Clin. Endocrinol. Metab. 2011, 96, 1151–1159. [Google Scholar] [CrossRef]
- Alaminos-Castillo, M.Á.; Ho-Plagaro, A.; García-Serrano, S.; Santiago-Fernandez, C.; Rodríguez-Pacheco, F.; Garrido-Sanchez, L.; Rodriguez, C.; Valdes, S.; Gonzalo, M.; Moreno-Ruiz, F.J.; et al. Increased PON lactonase activity in morbidly obese patients is associated with impaired lipid profile. Int. J. Clin. Pract. 2019, 73, e13315. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2023, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, A.; Joseph, S.B.; Vaidya, S.A.; Haberland, M.; Fogelman, A.M.; Cheng, G.; Tontonoz, P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell. 2003, 12, 805–816. [Google Scholar] [CrossRef]
- Tanaka, N.; Aoyama, T.; Kimura, S.; Gonzalez, F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Ther. 2017, 179, 142–157. [Google Scholar] [CrossRef]
- Vassilev, G.; Galata, C.; Finze, A.; Weiss, C.; Otto, M.; Reissfelder, C.; Blank, S. Sarcopenia after Roux-en-Y Gastric Bypass: Detection by Skeletal Muscle Mass Index vs. Bioelectrical Impedance Analysis. J. Clin. Med. 2022, 11, 1468. [Google Scholar] [CrossRef] [PubMed]
- Armandi, A.; Rosso, C.; Caviglia, G.P.; Bugianesi, E. Insulin Resistance across the Spectrum of Nonalcoholic Fatty Liver Disease. Metabolites 2021, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Boyle, C.A.; Kola, P.K.; Oraegbuna, C.S.; Lei, S. Leptin excites basolateral amygdala principal neurons and reduces food intake by LepRb-JAK2-PI3K-dependent depression of GIRK channels. J. Cell Physiol. 2024, 239, e31117. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Kratzsch, J.; Kiess, W.; Andler, W. Circulating soluble leptin receptor, leptin, and insulin resistance before and after weight loss in obese children. Int. J. Obes. 2005, 29, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, W.; Zhang, D.; Lin, C.; He, H.; Xie, F.; Gan, L.; Fu, W.; Wu, L.; Wu, Y. TNF-α Antagonizes the Effect of Leptin on Insulin Secretion through FOXO1-Dependent Transcriptional Suppression of LepRb in INS-1 Cells. Oxid. Med. Cell Longev. 2022, 2022, 9142798. [Google Scholar] [CrossRef] [PubMed]
- Nozari, Y.; Park, C.; Brietzke, E.; Iacobucci, M.; Gill, H.; McIntyre, R. Correlation between improved leptin signaling and cognitive function post bariatric surgery. J. Affect. Disord. 2023, 326, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Mazahreh, T.S.; Alfaqih, M.; Saadeh, R.; Al-Zoubi, N.A.; Hatamleh, M.; Alqudah, A.; Aleshawi, A.J.; Alzoubi, A. The effects of laparoscopic sleeve gastrectomy on the parameters of leptin resistance in obesity. Biomolecules 2019, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Laimer, M.; Ebenbichler, C.F.; Kaser, S.; Sandhofer, A.; Weiss, H.; Nehoda, H.; Aigner, F.; Patsch, J.R. Weight loss increases soluble leptin receptor levels and the soluble receptor bound fraction of leptin. Obes. Res. 2002, 10, 597–601. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, W.; Ma, C.; Du, C.; Huang, Y.; Xu, H.; Li, C.; Cheng, X.; Hao, R.; Xu, Y. RNA-Binding Proteins in the Regulation of Adipogenesis and Adipose Function. Cells 2022, 11, 2357. [Google Scholar] [CrossRef]
- Loomba, R.; Hwang, S.J.; O’Donnell, C.J.; Ellison, R.C.; Vasan, R.S.; D’Agostino, R.B., Sr.; Liang, T.J.; Fox, C.S. Parental obesity and offspring serum alanine and aspartate aminotransferase levels: The Framingham heart study. Gastroenterology 2008, 134, 953–959. [Google Scholar] [CrossRef]
- Ruhl, C.E.; Everhart, J.E. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology 2003, 124, 71–79. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Sakai, Y.; Terashima, T.; Shimode, T.; Seki, A.; Orita, N.; Takeshita, Y.; Shimakami, T.; Takatori, H.; Arai, K.; et al. Decline in serum albumin concentration is a predictor of serious events in nonalcoholic fatty liver disease. Medicine 2021, 100, e26835. [Google Scholar] [CrossRef]
- Ahn, S.B.; Jang, K.; Jun, D.W.; Lee, B.H.; Shin, K.J. Expression of liver X receptor correlates with intrahepatic inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Dig. Dis. Sci. 2014, 59, 2975–2982. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Chen, W.L. Examining the Association Between Serum Leptin and Sarcopenic Obesity. J. Inflamm. Res. 2021, 14, 3481–3487. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Ciminnisi, S.; Di Marco, V.; Cabibi, D.; Cammà, C.; Licata, A.; Marchesini, G.; Craxì, A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017, 45, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, L.; Wang, J.; Wang, Y.; Hao, H.; Huang, L. Characterization of serum protein expression profiles in the early sarcopenia older adults with low grip strength: A cross-sectional study. BMC Musculoskelet. Disord. 2022, 23, 894. [Google Scholar] [CrossRef]
- Casado, M.E.; Collado-Pérez, R.; Frago, L.M.; Barrios, V. Recent Advances in the Knowledge of the Mechanisms of Leptin Physiology and Actions in Neurological and Metabolic Pathologies. Int. J. Mol. Sci. 2023, 24, 1422. [Google Scholar] [CrossRef]
- Nason, S.R.; Kim, T.; Antipenko, J.P.; Finan, B.; DiMarchi, R.; Hunter, C.S.; Habegger, K.M. Glucagon-Receptor Signaling Reverses Hepatic Steatosis Independent of Leptin Receptor Expression. Endocrinology 2020, 161, bqz013. [Google Scholar] [CrossRef]
- Jung, U.J.; Seo, Y.R.; Ryu, R.; Choi, M.S. Differences in metabolic biomarkers in the blood and gene expression profiles of peripheral blood mononuclear cells among normal weight, mildly obese and moderately obese subjects. Br. J. Nutr. 2016, 116, 1022–1032. [Google Scholar] [CrossRef]
- DiBlasio-Smith, E.A.; Arai, M.; Quinet, E.M.; Evans, M.J.; Kornaga, T.; Basso, M.D.; Chen, L.; Feingold, I.; Halpern, A.R.; Liu, Q.Y.; et al. Discovery and implementation of transcriptional biomarkers of synthetic LXR agonists in peripheral blood cells. J. Transl. Med. 2008, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [PubMed]
- López, S.; García-Serrano, S.; Gutierrez-Repiso, C.; Rodríguez-Pacheco, F.; Ho-Plagaro, A.; Santiago-Fernandez, C.; Alba, G.; Cejudo-Guillen, M.; Rodríguez-Cañete, A.; Valdes, S.; et al. Tissue-Specific Phenotype and Activation of iNKT Cells in Morbidly Obese Subjects: Interaction with Adipocytes and Effect of Bariatric Surgery. Obes. Surg. 2018, 28, 2774–2782. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
| Non-Obese Subjects | MO Pre-Surgery | MO Post-Surgery | |
|---|---|---|---|
| Sex (Men/Women) | 35 (12/23) | 87 (22/65) | - |
| Age (years) | 43.4 ± 13.9 | 42.5 ± 10.0 | - |
| %EWL | - | - | 36 ± 31.5 |
| %TWL | - | - | 17.3 ± 14.6 |
| Weight (kg) | 70.3 ± 13.0 | 135.3 ± 25.4 3 | 96.5 ± 18.4 3,b |
| BMI (kg/m2) | 26.1 ± 3.9 | 50.4 ± 7.6 3 | 36.1 ± 6.6 3,b |
| Waist (cm) | 92.5 ± 9.2 | 138.3 ± 16.2 3 | 110.6 ± 13.1 3,b |
| Hip (cm) | 99.7 ± 8.0 | 147.5 ± 18.5 3 | 121.6 ± 16.9 3,b |
| Glucose (mg/dL) | 85.7 ± 8.8 | 108.8 ± 49.6 3 | 81.1 ± 13.5 b |
| Cholesterol (mg/dL) | 204.9 ± 30.6 | 197.3 ± 35.7 | 183.6 ± 33.5 2,b |
| Triglycerides (mg/dL) | 109.2 ± 61.3 | 149.7 ± 80.2 2 | 109.9 ± 47.4 b |
| HDL (mg/dL) | 54.8 ± 12.1 | 47.2 ± 12.7 2 | 47.0 ± 8.9 2,a |
| LDL (mg/dL) | 128.2 ± 27.0 | 120.0 ± 31.1 | 114.2 ± 30.3 1,b |
| Insulin (µU/mL) | 10.1 ± 4.5 | 22.0 ± 13.8 3 | 8.8 ± 4.1 b |
| HOMA-IR | 2.17 ± 1.05 | 5.95 ± 4.5 3 | 1.81 ± 0.88 b |
| CRP (mg/dL) | 3.3 ± 3.3 | 11.4 ± 7.0 3 | 4.6 ± 4.1 b |
| Leptin (ng/mL) | 17.5 ± 12.7 | 71.1 ± 38.4 3 | 19.28 ± 13.9 b |
| Adiponectin (µg/mL) | 10.7 ± 6.8 | 6.5 ± 3.3 2 | 10.5 ± 5.5 c |
| AST (IU/L) | 22.0 ± 13.5 | 27.6 ± 18.6 | 20.9 ± 8.8 c |
| ALT (IU/L) | 25.5 ± 17.0 | 44.3 ± 33.1 3 | 27.5 ± 14.5 c |
| GGT (IU/L) | 31.8 ± 32.2 | 38.4 ± 31.4 | 24.6 ± 26.3 2,c |
| ALP (IU/L) | 64.4 ± 30.1 | 79.5 ± 24.1 1 | 75.1 ± 39.5 1 |
| Albumin (g/dL) | 4.7 ± 0.3 | 4.0 ± 0.4 3 | 4.0 ± 0.3 3,c |
| FLI | 53.5 ± 32.9 | 98.6 ± 2.9 3 | 72.0 ± 25.7 2 |
| NAFLD FS | −1.17 ± 1.47 | −0.69 ± 1.48 | −1.53 ± 1.31 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Cortegana, C.; López-Enríquez, S.; Alba, G.; Santa-María, C.; Martín-Núñez, G.M.; Moreno-Ruiz, F.J.; Valdés, S.; García-Serrano, S.; Rodríguez-Díaz, C.; Ho-Plágaro, A.; et al. The Expression of Genes Related to Reverse Cholesterol Transport and Leptin Receptor Pathways in Peripheral Blood Mononuclear Cells Are Decreased in Morbid Obesity and Related to Liver Function. Int. J. Mol. Sci. 2024, 25, 7549. https://doi.org/10.3390/ijms25147549
Jiménez-Cortegana C, López-Enríquez S, Alba G, Santa-María C, Martín-Núñez GM, Moreno-Ruiz FJ, Valdés S, García-Serrano S, Rodríguez-Díaz C, Ho-Plágaro A, et al. The Expression of Genes Related to Reverse Cholesterol Transport and Leptin Receptor Pathways in Peripheral Blood Mononuclear Cells Are Decreased in Morbid Obesity and Related to Liver Function. International Journal of Molecular Sciences. 2024; 25(14):7549. https://doi.org/10.3390/ijms25147549
Chicago/Turabian StyleJiménez-Cortegana, Carlos, Soledad López-Enríquez, Gonzalo Alba, Consuelo Santa-María, Gracia M. Martín-Núñez, Francisco J. Moreno-Ruiz, Sergio Valdés, Sara García-Serrano, Cristina Rodríguez-Díaz, Ailec Ho-Plágaro, and et al. 2024. "The Expression of Genes Related to Reverse Cholesterol Transport and Leptin Receptor Pathways in Peripheral Blood Mononuclear Cells Are Decreased in Morbid Obesity and Related to Liver Function" International Journal of Molecular Sciences 25, no. 14: 7549. https://doi.org/10.3390/ijms25147549
APA StyleJiménez-Cortegana, C., López-Enríquez, S., Alba, G., Santa-María, C., Martín-Núñez, G. M., Moreno-Ruiz, F. J., Valdés, S., García-Serrano, S., Rodríguez-Díaz, C., Ho-Plágaro, A., Fontalba-Romero, M. I., García-Fuentes, E., Garrido-Sánchez, L., & Sánchez-Margalet, V. (2024). The Expression of Genes Related to Reverse Cholesterol Transport and Leptin Receptor Pathways in Peripheral Blood Mononuclear Cells Are Decreased in Morbid Obesity and Related to Liver Function. International Journal of Molecular Sciences, 25(14), 7549. https://doi.org/10.3390/ijms25147549






