A Combined Metabolome and Transcriptome Reveals the Lignin Metabolic Pathway during the Developmental Stages of Peel Coloration in the ‘Xinyu’ Pear
Abstract
:1. Introduction
2. Results
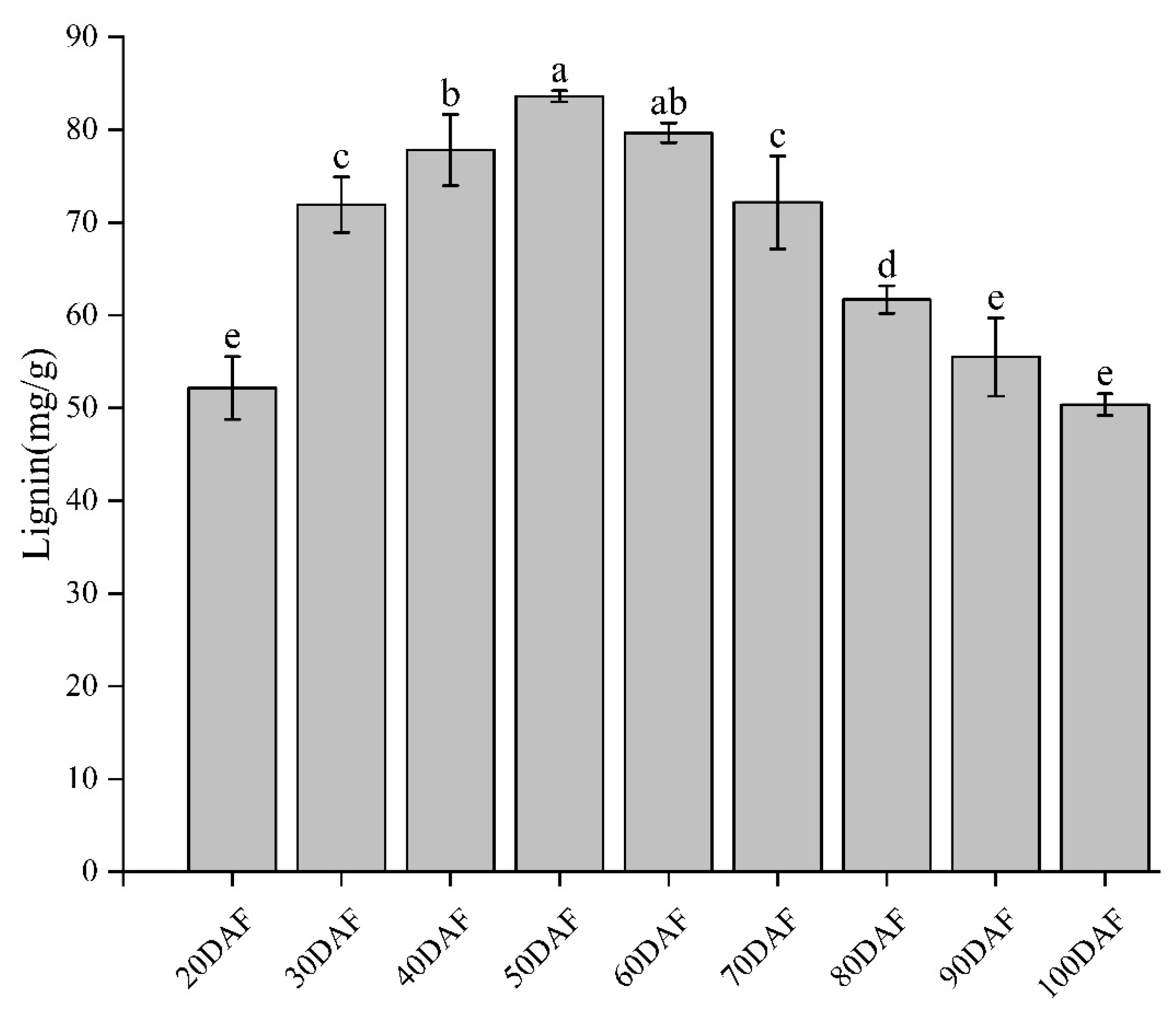
2.1. Changes in Lignin Contents during Fruit Development of ‘Xinyu’ Pear
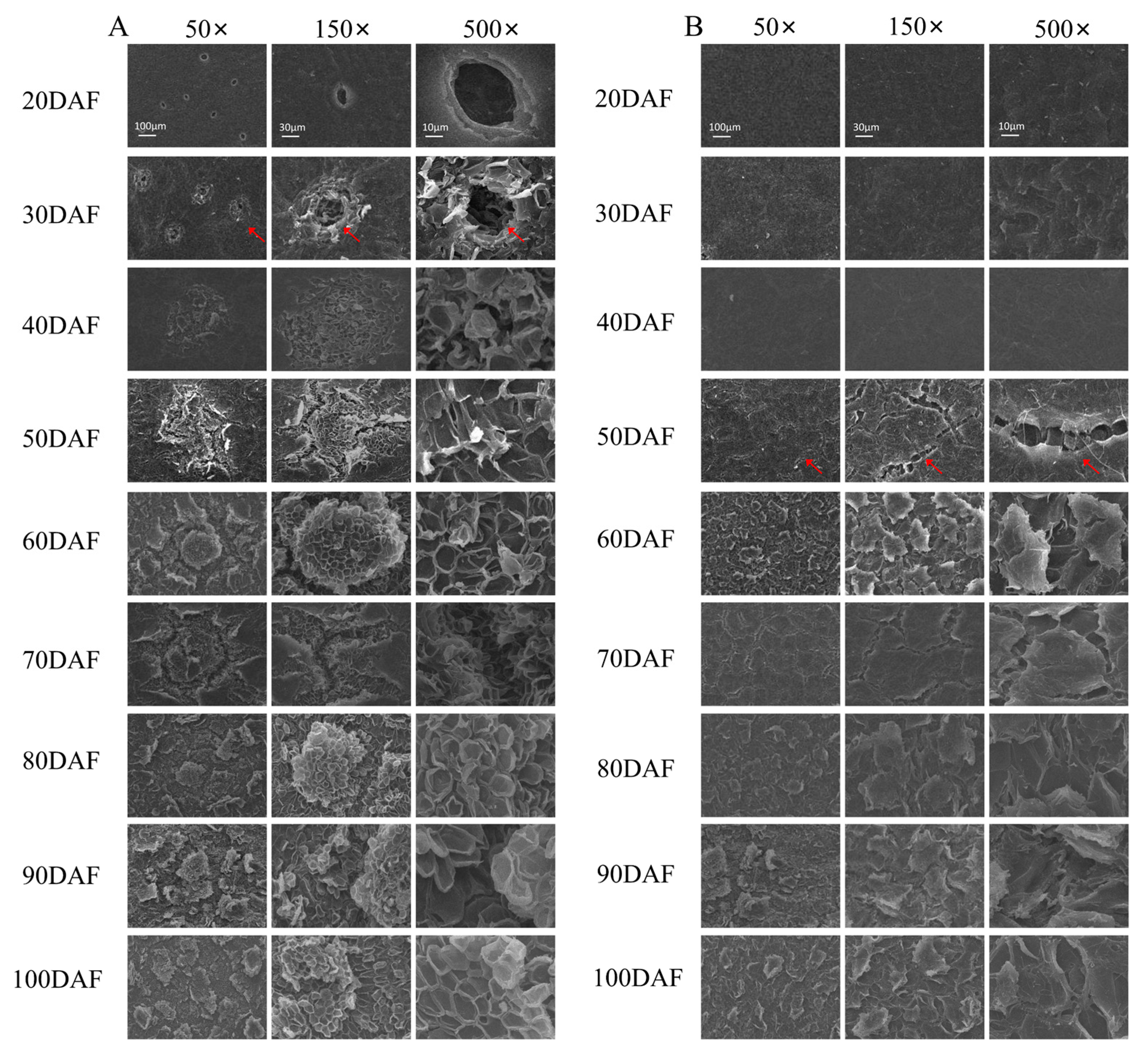
2.2. Observation of Fruit Peel by Scanning Electron Microscope (SEM) in ‘Xinyu’ Pear
2.3. Metabolomic Analysis of the ‘Xinyu’ Pear during the Development of Fruit
2.4. Transcriptome Analysis of the ‘Xinyu’ Pear during the Development of Fruit
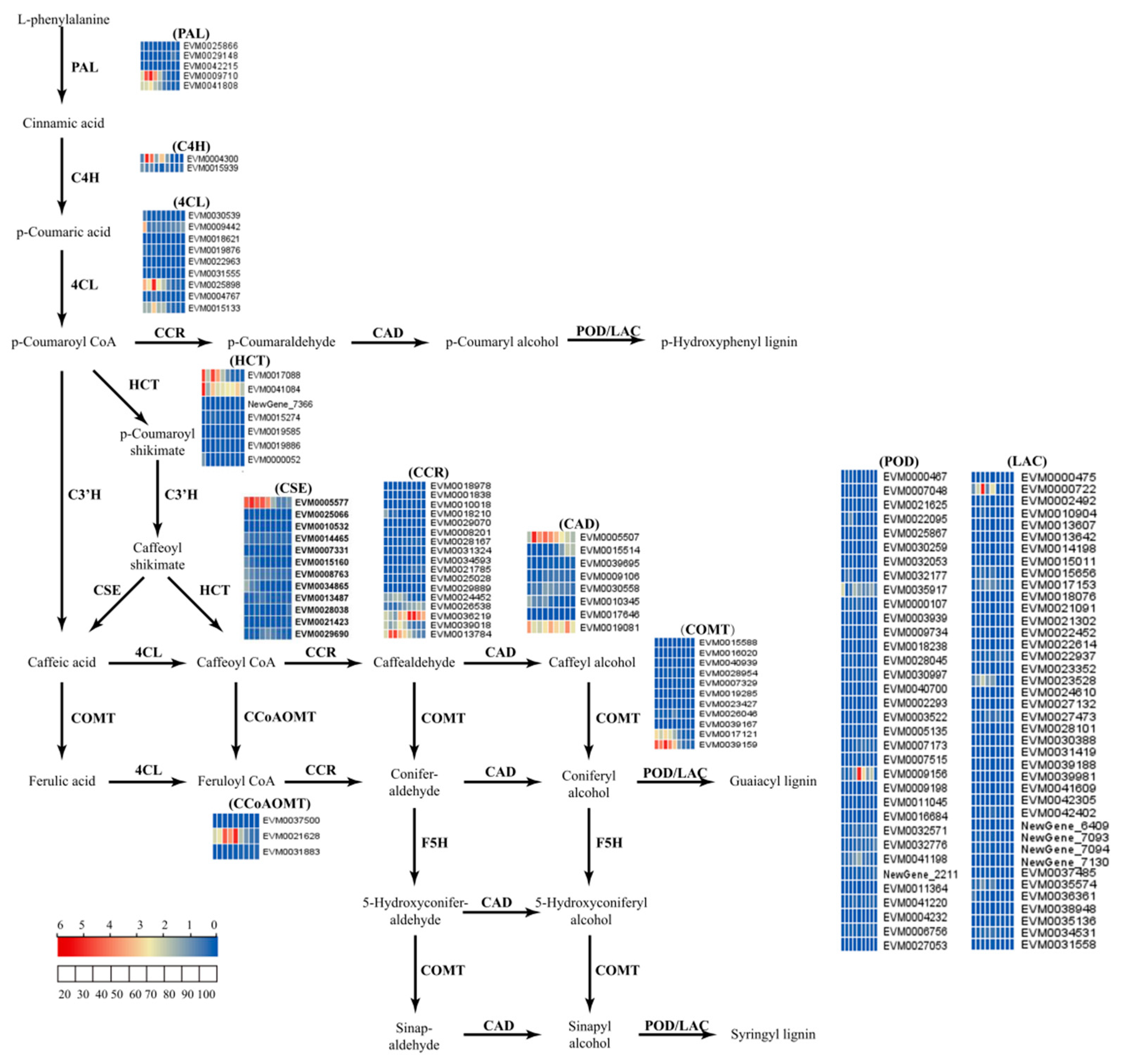
2.5. Analysis of Unigenes Related to Lignin Biosynthesis Pathways in Fruit Peel
2.6. Co-Expression Analysis of Genes Related to Lignin Synthesis Pathway
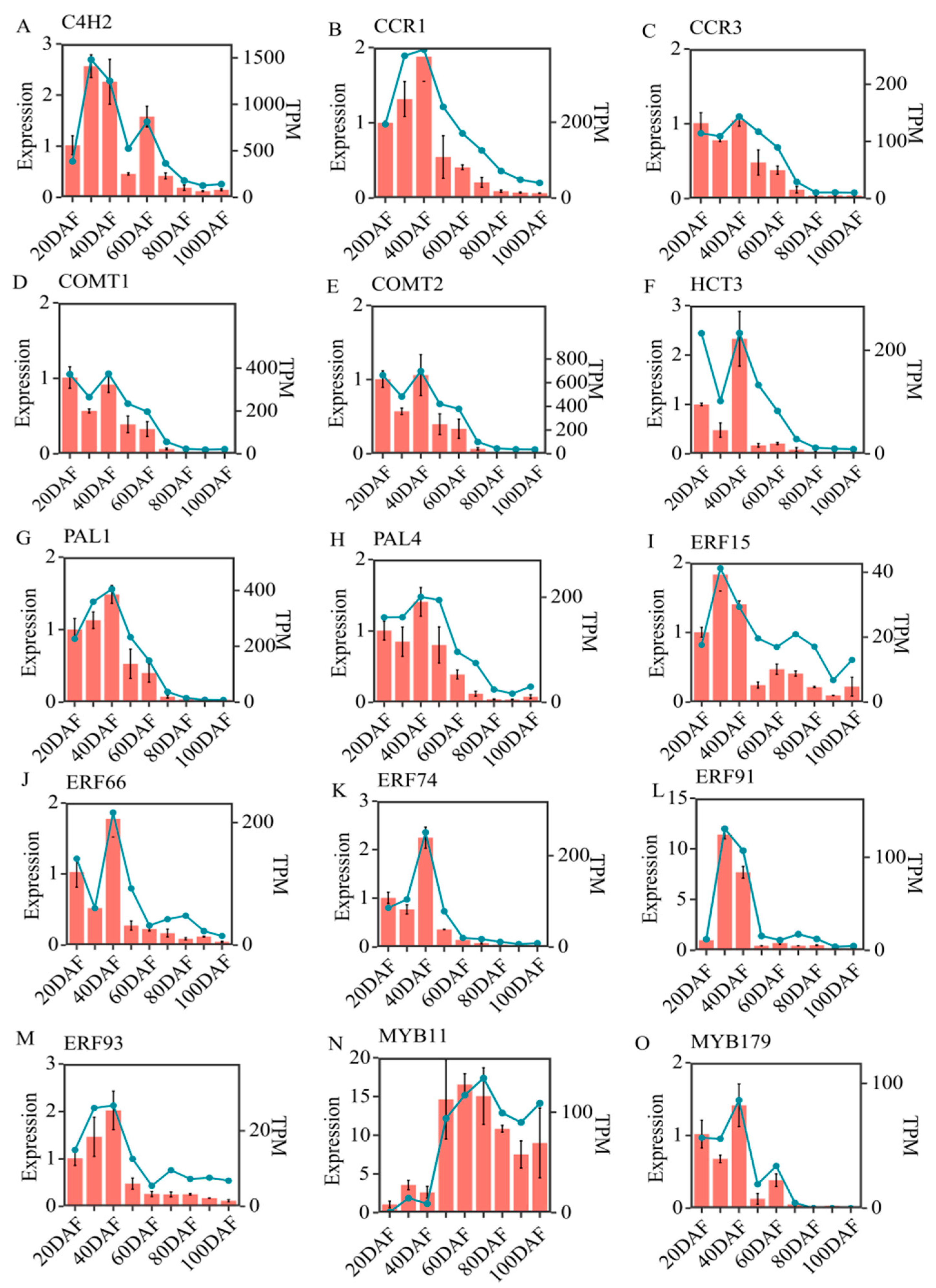
2.7. RT-qPCR Validation of the Results of Transcriptomic Data in ‘Xinyu’ Pear Peel
3. Discussion
4. Material and Methods
4.1. Plant Materials
4.2. Lignin Extractions and Analysis by Spectrophotometer
4.3. ‘Xinyu’ Pear Peel at Different Periods of Fruit Growth and Development Were Observed by Scanning Electron Microscope (SEM)
4.4. Metabolomic Profiling Analysis
4.5. Transcriptome Analysis
4.6. Co-Expression Network Construction of Metabolome and Transcriptome
4.7. RT-qPCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heng, W.W.; Yang, M.D.; Yang, J.Y.; Wang, Z.T.; Jiang, X.H.; Zhu, L.W. Relationship between H2O2 in polyamine metabolism and lignin in the exocarp of a russet mutant of ‘Dangshansuli’ pear (Pyrus bretschneideri Rehd.). Plant Mol. Biol. Rep. 2016, 34, 1056–1063. [Google Scholar] [CrossRef]
- Sun, J. Discussion on the current situation and countermeasures of pear industry development in Zhejiang province. China Fruit News 2005, 21, 11–13. (In Chinese) [Google Scholar]
- Pereira, H. Chemical composition and variability of cork from Quercus suber. Wood Sci. Technol. 1988, 22, 211–218. [Google Scholar] [CrossRef]
- Lopes, M.H.; Barros, A.S.; Pascoal Neto, C.; Rutledge, D.; Delgadillo, I.; Gil, A.M. Variability of cork from Portuguese Quercus suber studied by solid-state 13C-NMR and FTIR spectroscopie. Biopolymers 2001, 62, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.B. The Mechanisms of Fruit Appearance Characters Formation in Sand Pear. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2011. (In Chinese). [Google Scholar]
- Liu, Y.; Yang, J.; Wang, L.; Wang, S.K.; Su, Y.L.; Li, X.G.; Xue, H.B. Advances in studies on lignin synthesis pathway and its regulation of pear brown peel characteristic. J. Fruit Sci. 2018, 35, 17–25. (In Chinese) [Google Scholar]
- Xie, M.; Zhang, J.; Tschaplinski, T.J.; Tuskan, G.A.; Chen, J.G.; Muchero, W. Regulation of lignin biosynthesis and its role in growth-defense trade offs. Front. Plant Sci. 2018, 9, 1427. [Google Scholar] [CrossRef]
- Liang, X.; Dron, M.; Cramer, C.L.; Dixon, R.A.; Lamb, C.J. Differential regulation of phenylalanine ammonia-lyase genes during plant development and by environmental cues. J. Biol. Chem. 1989, 264, 14486–14492. [Google Scholar] [CrossRef]
- Yoon, J.; Heebak, C.; An, G. Roles of lignin biosynthesis and regulatory genes in plant development. J. Integr. Plant Biol. 2015, 57, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, H.; Scully, E.D.; Gries, T.; Palmer, N.A.; Funnell-Harris, D.L.; Baird, L.; Seravalli, J.; Dien, B.S.; Sarath, G.; Clemente, T.E.; et al. Overexpression of the Sorghum bicolor SbCCoAOMT alters cell wall associated hydroxycinnamoyl groups. PLoS ONE 2018, 13, e0204153. [Google Scholar] [CrossRef]
- Cheng, X.; Li, G.; Ma, C.H.; Abdullah, M.; Zhang, J.Y.; Zhao, H.; Jin, Q.; Cai, Y.P.; Lin, Y. Comprehensive genome-wide analysis of the pear (Pyrus bretschneideri) laccase gene (PbLAC) family and functional identification of PbLAC1 involved in lignin biosynthesis. PLoS ONE 2019, 14, e0210892. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Xu, C.; Movahedi, A.; Sun, W.; Qiang, Z.G. Characterization, expression profiling, and functional analyses of a 4CL-like gene of Populus trichocarpa. Processes 2019, 7, 45. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, N.F.; Hisano, H.; Cao, Y.P.; Wu, F.Y.; Liu, W.W.; Bao, Y.; Wang, Z.Y.; Fu, C.X. Simultaneous regulation of F5H in COMT-RNAi transgenic switchgrass alters effects of COMT suppression on syringyl lignin biosynthesis. Plant Biotechnol. J. 2019, 17, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Liu, J.; Kim, H.; Liu, B.G.; Huang, X.; Yang, Z.C.; Lin, Y.C.J.; Chen, H.; Yang, C.M.; Wang, J.P.; et al. CAD1 and CCR2 protein complex formation in monolignol biosynthesis in Populus trichocarpa. New Phytol. 2019, 222, 244–260. [Google Scholar] [CrossRef]
- Kim, W.C.; Ko, J.H.; Kim, J.Y.; Kim, J.M.; Bae, H.J.; Han, K.H. MYB46 directly regulates the gene expression of secondary wall associated cellulose synthases in Arabidopsis. Plant J. 2013, 73, 26–36. [Google Scholar] [CrossRef]
- Xu, Q.; Yin, X.R.; Zeng, J.K.; Ge, H.; Song, M.; Xu, C.J.; Li, X.; Ferguson, I.B.; Chen, K.S. Activator- and repressor-type MYB transcription factors are involved in chilling injury induced flesh lignification in loquat via their interactions with the phenylpropanoid pathway. J. Exp. Bot. 2014, 65, 4349–4359. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yao, J.L.; Xue, Y.S.; Su, G.Q.; Wang, L.; Lin, L.K.; Allan, A.C.; Zhang, S.L.; Wu, J. PbrMYB169 positively regulates lignification of stone cells in pear fruit. J. Exp. Bot. 2019, 70, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Xue, C.; Wang, R.Z.; Zhang, M.Y.; Qi, K.J.; Fan, J.; Hu, H.G.; Zhang, S.L.; Wu, J. The variation of stone cell content in 236 germplasms of sand pear (Pyrus pyrifolia) and identification of related candidate genes. Hortic. Plant J. 2020, 7, 108–116. [Google Scholar] [CrossRef]
- Zhong, R.Q.; Ye, Z.H. Transcriptional regulation of lignin biosynthesis. Plant Signal. Behav. 2009, 4, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Zhang, S.; Liu, J.; Zhao, C.; Wu, J.; Cao, Y.; Fu, C.; Han, X.; He, H.; Zhao, Q. MYB20, MYB42, MYB43, and MYB85 regulate phenylalanine and lignin biosynthesis during secondary cell wall formation. Plant Physiol. 2019, 182, 1272–1283. [Google Scholar] [CrossRef]
- Kim, M.H.; Cho, J.S.; Jeon, H.W.; Sangsawang, K.; Shim, D.; Choi, Y.I.; Park, E.J.; Lee, H.; Ko, J.H. Wood transcriptome profiling identifies critical pathway genes of secondary wall biosynthesis and novel regulators for vascular cambium development in Populus. Genes 2019, 10, 690. [Google Scholar] [CrossRef]
- Song, L.Y. Identification and Characterization of the SG4-R2R3-MYB Genes That Related with Anthocyanin and Lignin Biosynthesis and Regulation in Pear. Ph.D. Thesis, Northwest Agriculture and Forestry University, Xi’an, China, 2021. (In Chinese). [Google Scholar]
- Xue, Y.; Shan, Y.; Yao, J.; Wang, T.; Xu, S.; Liu, D.; Ye, Z.; Lin, J.; Li, X.; Xue, C.; et al. PbrMYB24 simultaneously regulates lignin and cellulose biosynthesis in stone cells of pear fruits by binding to different cis-elements. Plant Physiol. 2023, 192, 1997–2014. [Google Scholar] [CrossRef] [PubMed]
- Ambavaram, M.M.R.; Krishnan, A.; Trijatmiko, K.R.; Pereira, A. Coordinated activation of cellulose and repression of lignin biosynthesis pathways in rice. Plant Physiol. 2011, 155, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Vahala, J.; Felten, J.; Love, J.; Gorzsas, A.; Gerber, L.; Lamminmaki, A.; Kangasjarvi, J.; Sundberg, B. A genome-wide screen for ethylene-induced Ethylene Response Factors (ERFs) in hybrid aspen stem identifies ERF genes that modify stem growth and wood properties. New Phytol. 2013, 200, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Chikagawa, Y.; Kato, M.; Maeda, K.; Ozeki, Y. Up regulation of the promoter activity of the carrot (Daucus carota) phenylalanine ammonia-lyase gene (DcPAL3) is caused by new members of the transcriptional regulatory proteins, DcERF1 and DcERF2, which bind to the GCC-box homolog and act as an activator to the DcPAL3 promoter. J. Plant Res. 2008, 121, 499–508. [Google Scholar]
- Jin, X.; Cheng, C.; Qi, Q.; Zhou, S.; Wang, C.; Zhang, Y.; Sun, C.; Wang, Y.; Dang, R.; Yang, S. PpERF1b-like enhances lignin synthesis in pear (Pyrus pyrifolia) ‘hard-end’ fruit. Front. Plant Sci. 2022, 13, 1087388. [Google Scholar] [CrossRef]
- Cai, Y.; Li, G.; Nie, J.; Lin, Y.; Nie, F.; Zhang, J.; Xu, Y. Study of the structure and biosynthetic pathway of lignin in stone cells of pear. Sci. Hortic. 2010, 125, 374–379. [Google Scholar] [CrossRef]
- Jin, Q.; Yan, C.C.; Qiu, J.X.; Zhang, N.; Lin, Y.; Cai, Y.P. Structural characterization and deposition of stone cell lignin in Dangshan su pear. Sci. Hortic. 2013, 155, 123–130. [Google Scholar] [CrossRef]
- Zhao, S.G.; Zhang, J.G.; Zhao, Y.P.; Zhang, Y.X. New discoveries of stone cell differentiation in fruitlets of ‘Yali’ pears (Pyrus bretschneideri Rehd). J. Food Agric. Environ. 2017, 11, 937–942. [Google Scholar]
- Li, X.F.; Li, X.; Jia, B.; Liu, L.; Ye, Z.F.; Heng, W.; Zhu, L.W. Analysis of enzyme activity and lignin content and expression of CCoAOMT gene in the pericarp of ‘Dangshan Suli’ and its russet mutant. Acta Hortic. Sin. 2012, 39, 828–836. [Google Scholar]
- Liu, L.; Sun, H.L.; Cheng, Z.Y.; Jia, B.; Liu, P.; Ye, Z.F.; Zhu, L.W.; Heng, W. Cloning and expression of enzyme genes related to lignin biosynthesis in the pericarp of russet mutant of Dangshansuli. Acta Agric Boreali Sin. 2013, 28, 88–92. [Google Scholar]
- Lv, Z.Q.; Ren, D.D.; Zhou, H.; Qiao, Y.S. Cloning an expression of HHT gene in ‘Huanghua’ pear and its bud mutant ‘Luhuanghua’ pear (Pyrus pyrifolia Nakai). Acta Bot. Boreali-Occident. Sin. 2016, 36, 1105–1109. [Google Scholar]
- Yang, W.L.; Li, X.G.; Yang, Q.S.; Lin, J.; Sheng, B.L.; Chang, Y.H.; Wang, H. Comparative metabolic and transcriptomic analysis of the pericarp of Sucui 1, Cuiguan and Huasu pears. J. Fruit Sci. 2022, 39, 1989–2006. [Google Scholar]
- Wang, H.A.; An, Y.Y.; Wang, L.J. Morphological and anatomic observation on fruit russet formation of pear (Pyrus pyrifolia Nakai ‘Cuiguan’). J. Fruit Sci. 2017, 34, 1415–1425. (In Chinese) [Google Scholar]
- Shi, C.H.; Wang, X.Q.; Zhang, X.Y.; Hang, X.Y.; Shen, L.Y.; Luom, J.; Zhang, Y.X. Response of fruit bagging to lignin biosynthesis and expression of related genes in fruit peel of sand pear (Pyrus pyrifolia Nakai) cv. Cuiguan. Hortscience 2019, 54, 1989–1997. [Google Scholar] [CrossRef]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin biosynthesis and its integration into metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kamitakahara, H.; Murayama, H.; Ohsako, T.; Itai, A. Analysis of fruit lignin content, composition, and linkage types in pear cultivars and related species. J. Agric. Food Chem. 2020, 68, 2493–2505. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.-F.; Zhang, P.-F.; Bian, Y.-H.; Liu, Z.-Y.; Zhang, C.; Liu, X.; Wang, C.-L.; Street, N. An integrated metabolic and transcriptomic analysis reveals the mechanism through which fruit bagging alleviates exocarp semi-russeting in pear fruit. Tree Physiol. 2021, 41, 1306–1318. [Google Scholar] [CrossRef]
- Zhang, Z.T. Study on the Cork Layer Formation of the Brown Mutant of Suisho Pear (Pyrus pyrifolia) and Its Related Enzymes. Master’s Thesis, Hebei Normal University of Science and Technology, Shijiazhuang, China, 2011. (In Chinese). [Google Scholar]
- Cheng, S.; Yan, J.; Meng, X.; Zhang, W.; Liao, Y.; Ye, J.; Xu, F. Characterization and expression patterns of a cinnamate-4-hydroxylase gene involved in lignin biosynthesis and in response to various stresses and hormonal treatments in Ginkgo biloba. Acta Physiol. Plant. 2018, 40, 7. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, J.Y.; Wang, H.; Chen, T.Z.; Li, G.H.; Yan, C.C.; Jin, Q.; Lin, Y.; Cai, Y.P. Effects of metaxenia on stone cell formation in Pear (Pyrus bretschneideri) based on transcriptomic analysis and functional characterization of the lignin-related gene PbC4H2. Forests 2020, 11, 53. [Google Scholar] [CrossRef]
- Xue, J.; Luo, D.; Xu, D.; Zeng, M.; Cui, X.; Li, L.; Huang, H. CCR1, an enzyme required for lignin biosynthesis in Arabidopsis, mediates cell proliferation exit for leaf development. Plant J. 2015, 83, 375–387. [Google Scholar] [CrossRef]
- Besseau, S.; Hoffmann, L.; Geoffroy, P.; Lapierre, C.; Pollet, B.; Legrand, M. Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 2007, 19, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, B.; Cesarino, I.; Goeminne, G.; Kim, H.; Marroni, F.; Van Acker, R.; Vanholme, R.; Morreel, K.; Ivens, B.; Pinosio, S.; et al. Breeding with rare defective alleles (BRDA): A natural Populus nigra HCT mutant with modified lignin as a case study. New Phytol. 2013, 198, 765–776. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Whetten, R.W.; Bao, W.; Chen, C.L.; Sederoff, R.R. The role of laccase in lignification. Plant J. 1993, 4, 751–757. [Google Scholar] [CrossRef]
- Vanholme, R.; Ralph, J.; Akiyama, T.; Lu, F.; Pazo, J.R.; Kim, H.; Christensen, J.H.; Brecht, V.R.; Storme, V.; Rycke, R.D.; et al. Engineering traditional monolignols out of lignin by concomitant up-regulation of F5H1 and down-regulation of COMT in Arabidopsis. Plant J. 2010, 64, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, C.; Li, J.; Qiao, X.; Li, L.; Yu, L.; Huang, Y.; Wu, J. Genome-Wide Identification, Evolution and Functional Divergence of MYB Transcription Factors in Chinese White Pear (Pyrus bretschneideri). Plant Cell Physiol. 2016, 57, 824–847. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Jiang, H.; Liu, W.; Zhang, S.; Hou, X.; Zhang, S.; Wang, N.; Zhang, R.; Zhang, Z.; et al. Transcriptome analysis reveals that PbMYB61 and PbMYB308 are involved in the regulation of lignin biosynthesis in pear fruit stone cells. Plant J. 2023, 116, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, H.; Rao, X.; Li, L.; Dixon, R.A. Abscisic acid regulates secondary cell-wall formation and lignin deposition in Arabidopsis thaliana through phosphorylation of NST1. Proc. Natl. Acad. Sci. USA 2021, 118, e2106367118. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, C.; Jiang, R.; Wang, F.; Zhang, Z.; Zeng, J. A novel NAC transcription factor from Eucalyptus, EgNAC141, positively regulates lignin biosynthesis and increases lignin deposition. Front. Plant Sci. 2021, 12, 642090. [Google Scholar] [CrossRef]
- Li, M.T.; Cheng, C.X.; Zhang, X.F.; Zhou, S.P.; Wang, C.H.; Ma, C.H.; Yang, S.L. PpNAC187 enhances lignin synthesis in ‘Whangkeumbae’ pear (Pyrus pyrifolia) ‘Hard-End’ Fruit. Molecules 2019, 24, 4338. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, X.R.; Li, H.; Xu, M.; Zhang, M.X.; Li, S.J.; Liu, X.F.; Shi, Y.N.; Grierson, D.; Chen, K.S. ETHYLENE RESPONSE FACTOR39-MYB8 complex regulates low-temperature-induced lignification of loquat fruit. J. Exp. Bot. 2020, 71, 3172–3184. [Google Scholar] [CrossRef]
- Li, X.; Yang, R.; Liang, Y.; Gao, B.; Li, S.; Bai, W.; Oliver, M.J.; Zhang, D. The ScAPD1-like gene from the desert moss Syntrichia caninervis enhances resistance to Verticillium dahliae via phenylpropanoid gene regulation. Plant J. 2023, 113, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, X.; Li, Z.; Song, Q.; Xu, C.; Luo, K. Jasmonic acid regulates lignin deposition in poplar through JAZ5-MYB/NAC interaction. Front. Plant Sci. 2023, 14, 1232880. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. 2012, 131, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.E.; Bailey, T.L.; Noble, W.S. FIMO: Scanning for occurrences of a given motif. Bioinformatics 2011, 27, 1017–1018. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol. Biol. 2011, 696, 291–303. [Google Scholar]




| Substance | Different Stages of Fruit Development | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20DAF | 30DAF | 40DAF | 50DAF | 60DAF | 70DAF | 80DAF | 90DAF | 100DAF | |
| Coumaric aldehyde | 0.09 ± 0.01 | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.08 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.10 ± 0.00 | 0.09 ± 0.02 |
| Ferulic acid | 0.03 ± 0.01 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.00 |
| Sinapyl alcohol | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.05 ± 0.01 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Sinapaldehyde | 0.49 ± 0.16 | 0.98 ± 0.02 | 1.18 ± 0.17 | 1.16 ± 0.13 | 1.40 ± 0.02 | 1.05 ± 0.11 | 0.675 ± 0.09 | 0.41 ± 0.06 | 0.26 ± 0.06 |
| L-phenylalanine | 143.33 ± 3.40 | 53.40 ± 1.31 | 21.40 ± 2.16 | 39.30 ± 2.57 | 43.27 ± 1.54 | 89.83 ± 2.31 | 80.77 ± 14.47 | 96.27 ± 19.07 | 51.77 ± 12.19 |
| p-coumaric acid | 1.20 ± 0.03 | 0.77 ± 0.07 | 0.78 ± 0.09 | 0.70 ± 0.05 | 0.58 ± 0.026 | 0.44 ± 0.04 | 0.35 ± 0.04 | 0.22 ± 0.03 | 0.16 ± 0.05 |
| Caffeic aldehyde | 0.05 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 |
| Caffeyl alcohol | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.03 ± 0.01 |
| Conifer aldehyde | 0.98 ± 0.19 | 1.04 ± 0.10 | 1.12 ± 0.15 | 1.00 ± 0.07 | 0.83 ± 0.11 | 0.69 ± 0.15 | 0.53 ± 0.01 | 0.38 ± 0.09 | 0.25 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Lyu, K.; Zeng, S.; Wang, X.; Chen, X. A Combined Metabolome and Transcriptome Reveals the Lignin Metabolic Pathway during the Developmental Stages of Peel Coloration in the ‘Xinyu’ Pear. Int. J. Mol. Sci. 2024, 25, 7481. https://doi.org/10.3390/ijms25137481
Jiang C, Lyu K, Zeng S, Wang X, Chen X. A Combined Metabolome and Transcriptome Reveals the Lignin Metabolic Pathway during the Developmental Stages of Peel Coloration in the ‘Xinyu’ Pear. International Journal of Molecular Sciences. 2024; 25(13):7481. https://doi.org/10.3390/ijms25137481
Chicago/Turabian StyleJiang, Cuicui, Keliang Lyu, Shaomin Zeng, Xiao’an Wang, and Xiaoming Chen. 2024. "A Combined Metabolome and Transcriptome Reveals the Lignin Metabolic Pathway during the Developmental Stages of Peel Coloration in the ‘Xinyu’ Pear" International Journal of Molecular Sciences 25, no. 13: 7481. https://doi.org/10.3390/ijms25137481






