Analysis of the Actions of RARγ Agonists on Growing Osteochondromas in a Mouse Model
Abstract
1. Introduction
2. Results
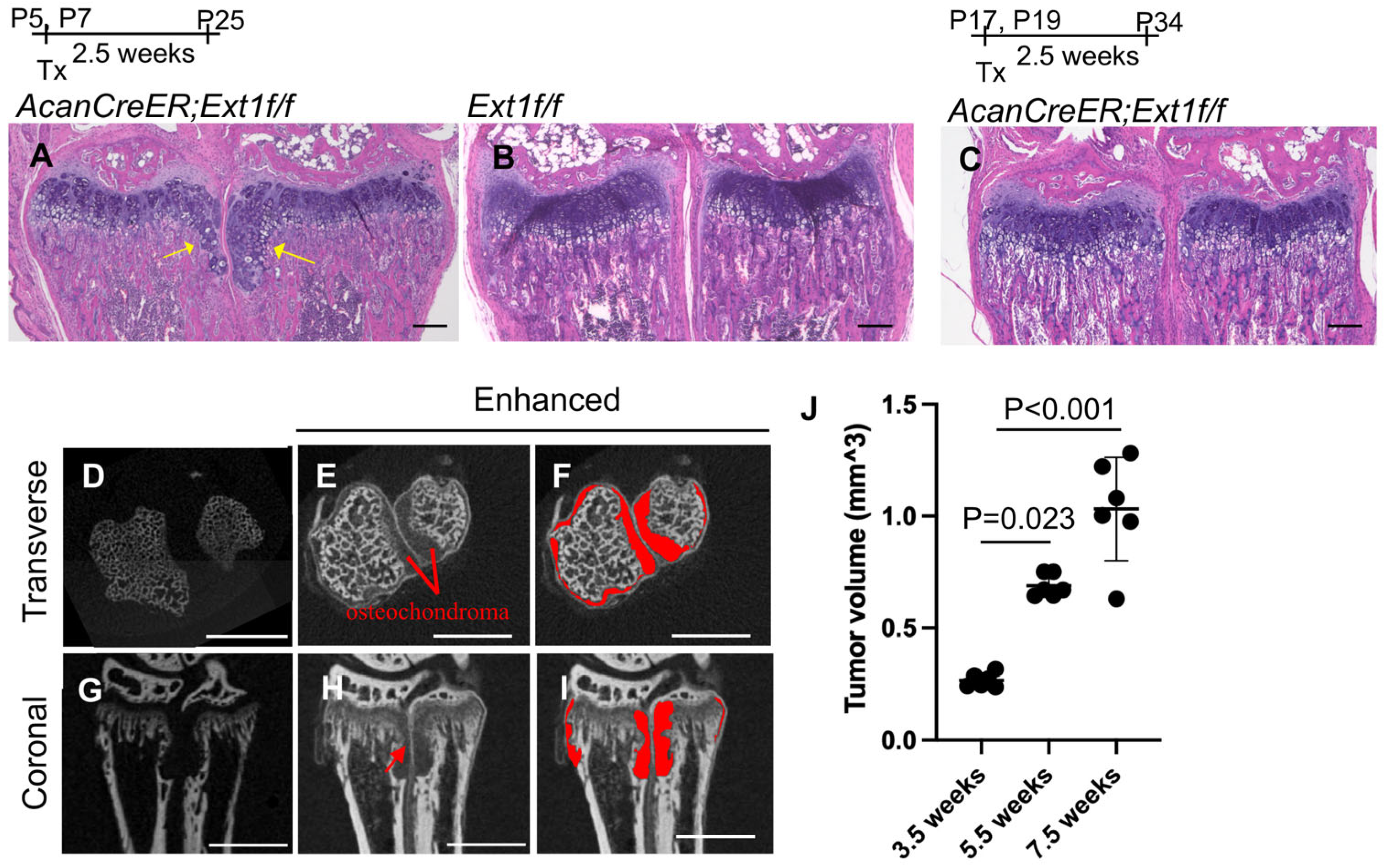
2.1. Measurement of Cartilage Tumor Volume by Enhanced microCT in an AcanCreER;Ext1f/f Mouse Osteochondroma Model
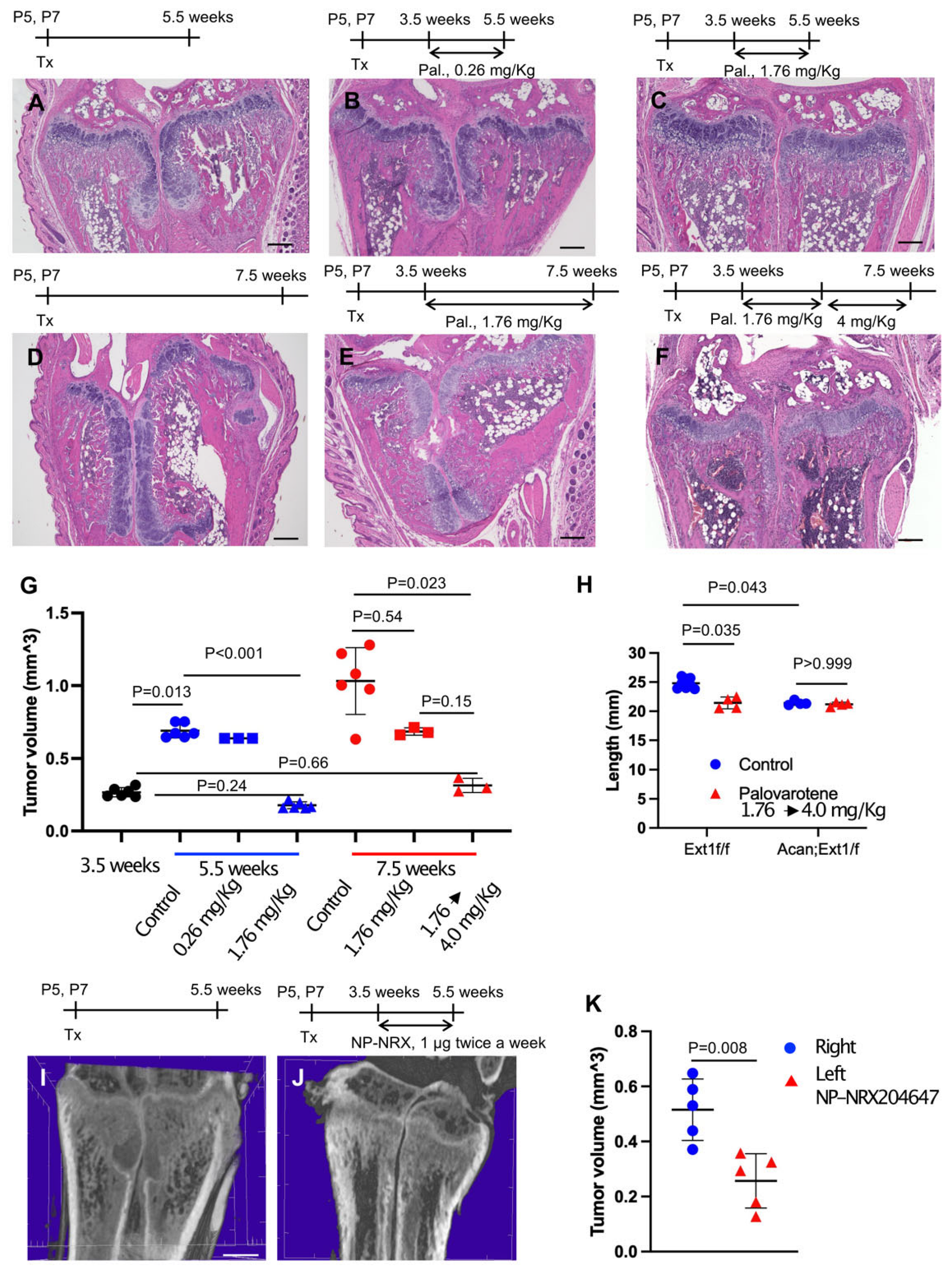
2.2. Inhibition of Pre-Existing Osteochondromas by Palovarotene
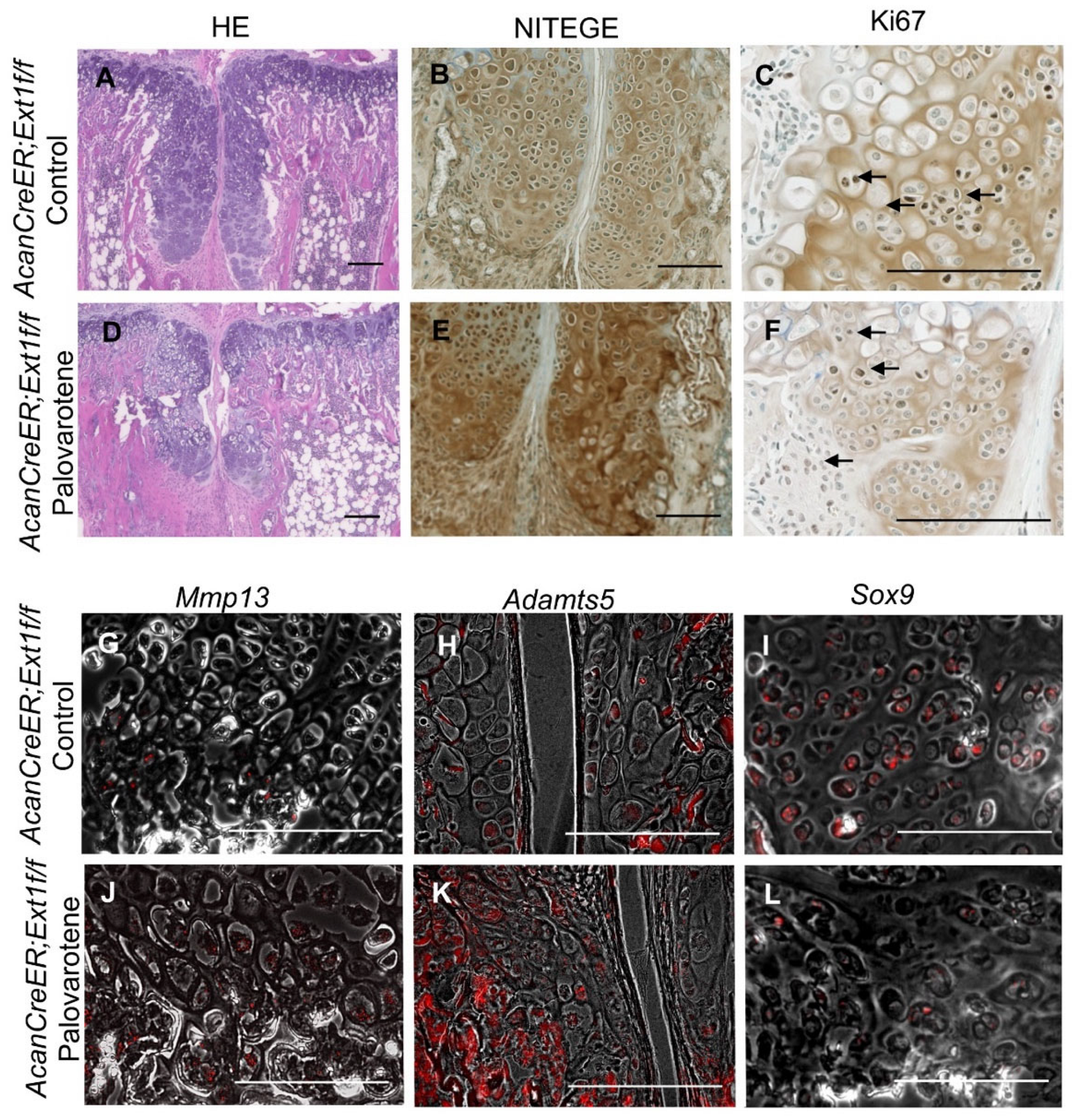
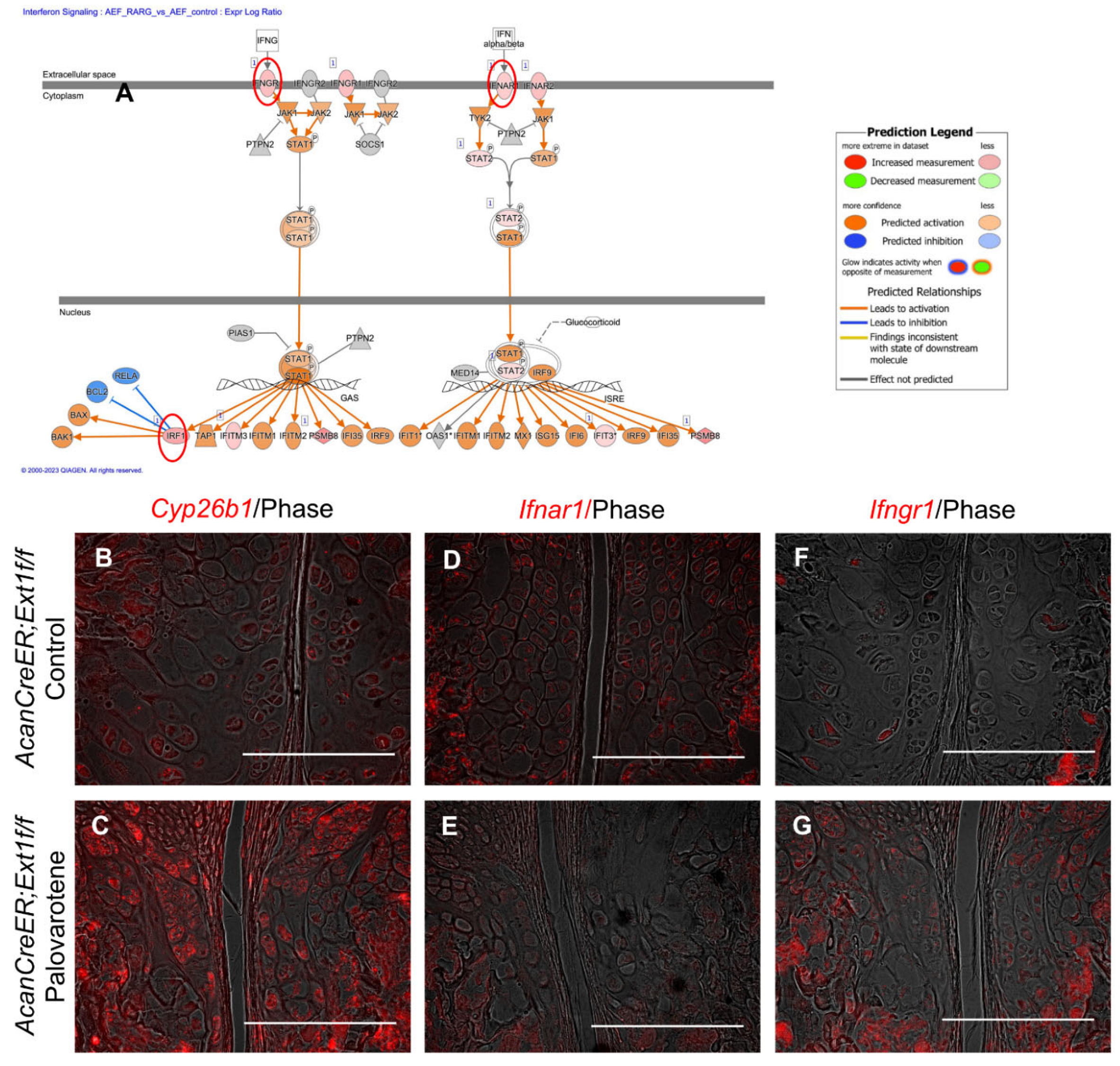
2.3. Actions of Palovarotene on Osteochondromas
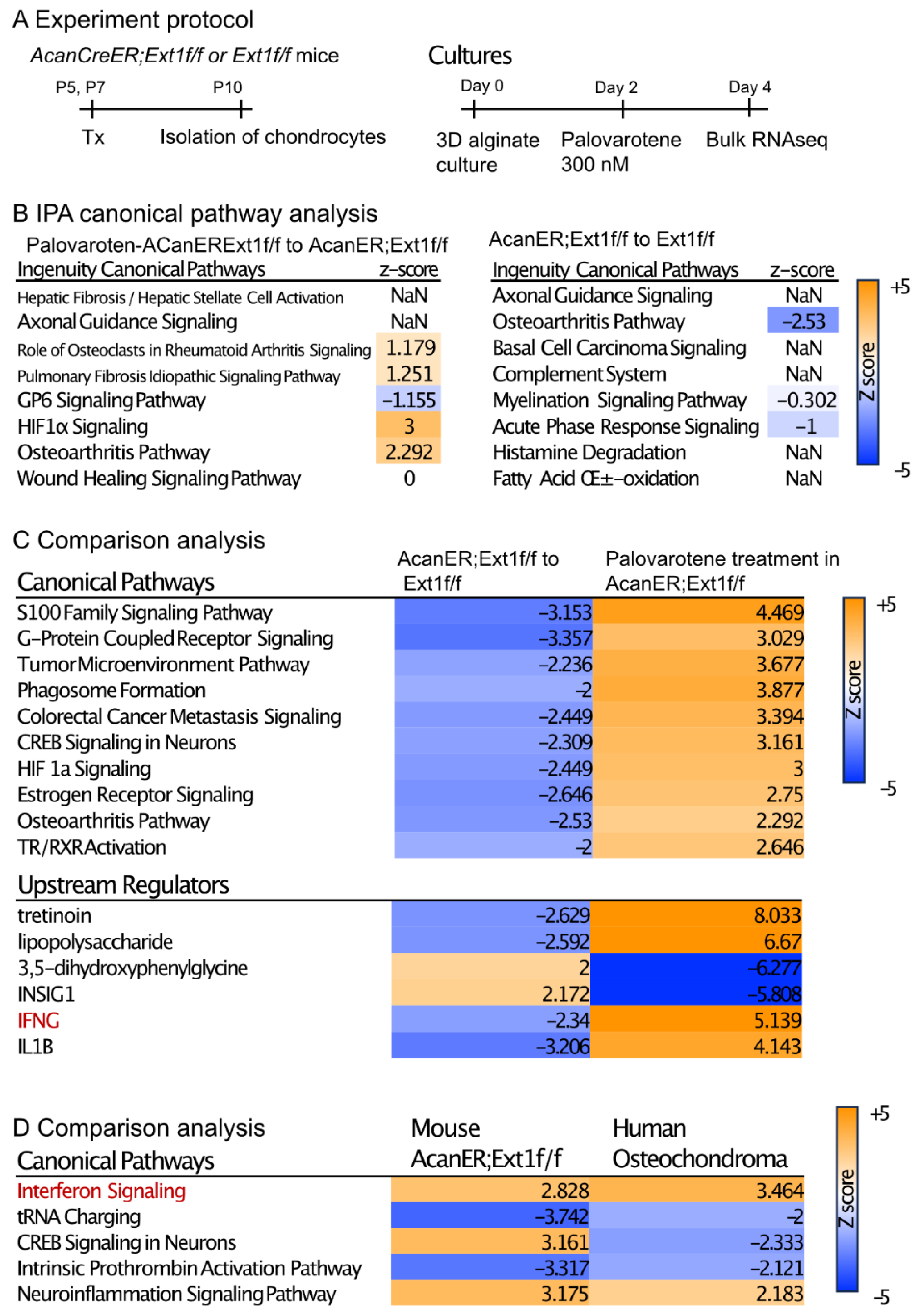
2.4. Actions of Palovarotene on Cultured Chondrocytes
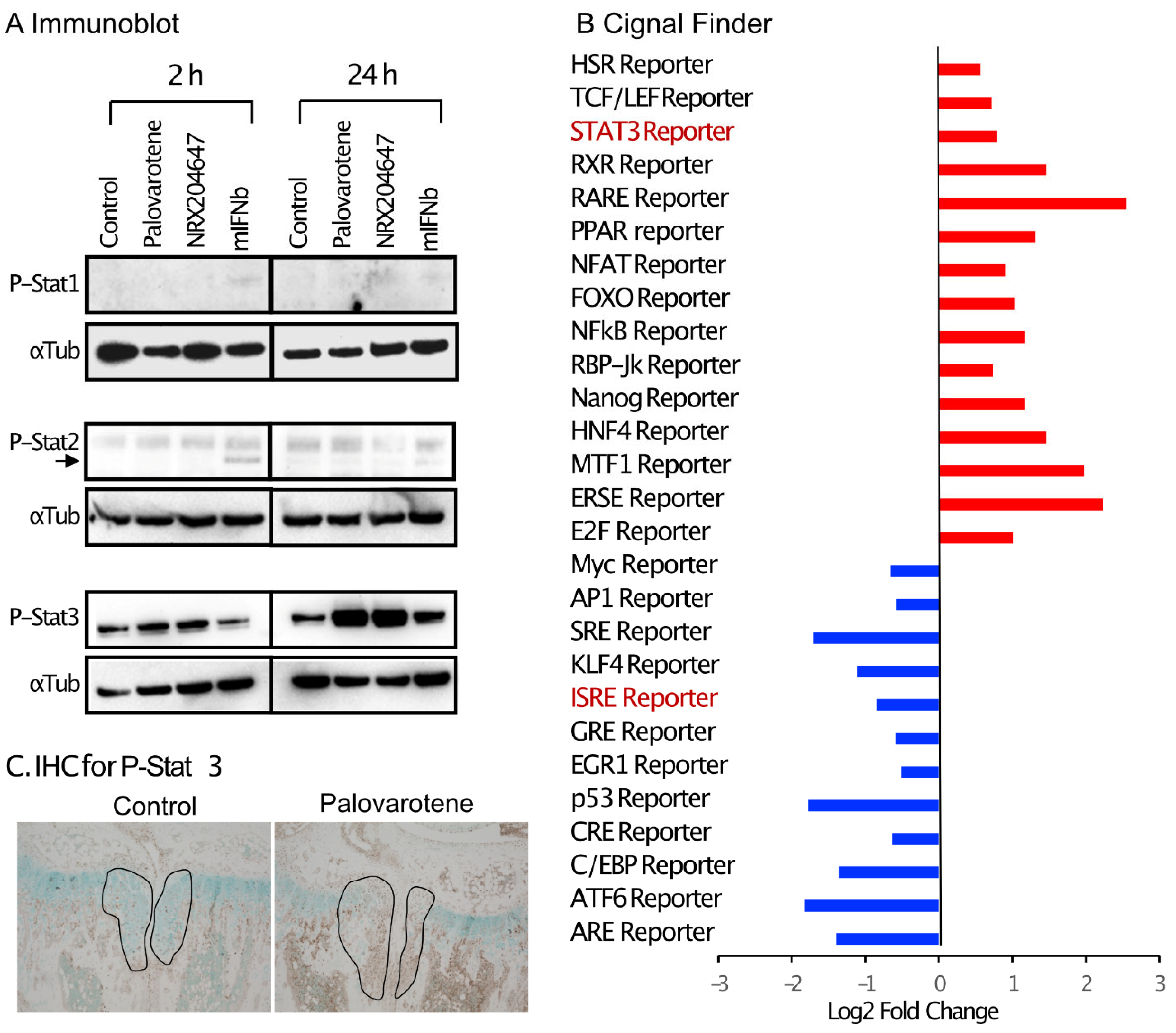
2.5. Stimulation of the Stat3 Pathway by Palovarotene
3. Discussion
3.1. Clinically Relevant Regimen of Drug Treatment for Osteochondromas
3.2. Actions of Palovarotene on Chondrocytes
4. Materials and Methods
4.1. Animals
4.2. Drug Treatment
4.3. Histology and Immunohistochemical Staining
4.4. Tumor Volumetric Analysis by PTA-Enhanced microCT
4.5. In Situ Hybridization by RNAscope
4.6. Chondrocyte Culture
4.7. Transcriptome Analysis
4.8. Immunoblot Protocol
4.9. Reporter Assay
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pannier, S.; Legeai-Mallet, L. Hereditary multiple exostoses and enchondromatosis. Best Pract. Res. Clin. Rheumatol. 2008, 22, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Ludecke, H.J.; Lindow, S.; Horton, W.A.; Lee, B.; Wagner, M.J.; Horsthemke, B.; Wells, D.E. Cloning of the putative tumour suppressor gene for hereditary multiple exostoses (EXT1). Nat. Genet. 1995, 11, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Stickens, D.; Clines, G.; Burbee, D.; Ramos, P.; Thomas, S.; Hogue, D.; Hecht, J.T.; Lovett, M.; Evans, G.A. The EXT2 multiple exostoses gene defines a family of putative tumour suppressor genes. Nat. Genet. 1996, 14, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chhina, H.; Davis, J.C.; Alvarez, C.M. Health-related quality of life in people with hereditary multiple exostoses. J. Pediatr. Orthop. 2012, 32, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Murphey, M.D.; Choi, J.J.; Kransdorf, M.J.; Flemming, D.J.; Gannon, F.H. Imaging of osteochondroma: Variants and complications with radiologic-pathologic correlation. Radiographics 2000, 20, 1407–1434. [Google Scholar] [CrossRef] [PubMed]
- Stieber, J.R.; Dormans, J.P. Manifestations of hereditary multiple exostoses. J. Am. Acad. Orthop. Surg. 2005, 13, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Mundy, C.; Bechtold, T.; Sgariglia, F.; Ibrahim, M.M.; Billings, P.C.; Carroll, K.; Koyama, E.; Jones, K.B.; Pacifici, M. Unsuspected osteochondroma-like outgrowths in the cranial base of Hereditary Multiple Exostoses patients and modeling and treatment with a BMP antagonist in mice. PLoS Genet. 2017, 13, e1006742. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, J.; Moore, D.C.; Liang, H.; Dooner, M.; Wu, Q.; Terek, R.; Chen, Q.; Ehrlich, M.G.; Quesenberry, P.J.; et al. Ptpn11 deletion in a novel progenitor causes metachondromatosis by inducing hedgehog signalling. Nature 2013, 499, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, T.; Lemire, I.; Irie, F.; Yamaguchi, Y. Palovarotene inhibits osteochondroma formation in a mouse model of multiple hereditary exostoses. J. Bone Miner. Res. 2018, 33, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Baujat, G.; Hsiao, E.C.; Keen, R.; Wilson, A.; Packman, J.; Strahs, A.L.; Grogan, D.R.; Kaplan, F.S. Palovarotene for Fibrodysplasia Ossificans Progressiva (FOP): Results of a Randomized, Placebo-Controlled, Double-Blind Phase 2 Trial. J. Bone Miner. Res. 2022, 37, 1891–1902. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Hsiao, E.C.; Al Mukaddam, M.; Baujat, G.; Berglund, S.K.; Brown, M.A.; Cheung, A.M.; De Cunto, C.; Delai, P.; Haga, N.; et al. Reduction of New Heterotopic Ossification (HO) in the Open-Label, Phase 3 MOVE Trial of Palovarotene for Fibrodysplasia Ossificans Progressiva (FOP). J. Bone Miner. Res. 2023, 38, 381–394. [Google Scholar] [CrossRef]
- Garcia, S.A.; Ng, V.Y.; Iwamoto, M.; Enomoto-Iwamoto, M. Osteochondroma Pathogenesis: Mouse Models and Mechanistic Insights into Interactions with Retinoid Signaling. Am. J. Pathol. 2021, 191, 2042–2051. [Google Scholar] [CrossRef]
- Piombo, V.; Jochmann, K.; Hoffmann, D.; Wuelling, M.; Vortkamp, A. Signaling systems affecting the severity of multiple osteochondromas. Bone 2018, 111, 71–81. [Google Scholar] [CrossRef]
- Garcia, S.A.; Tian, H.; Imamura-Kawasawa, Y.; Fisher, A.; Cellini, A.; Codd, C.; Herzenberg, J.E.; Abzug, J.M.; Ng, V.; Iwamoto, M.; et al. Understanding the Action of RARgamma Agonists on Human Osteochondroma Explants. Int. J. Mol. Sci. 2020, 21, 2686. [Google Scholar] [CrossRef]
- Jones, K.B.; Piombo, V.; Searby, C.; Kurriger, G.; Yang, B.; Grabellus, F.; Roughley, P.J.; Morcuende, J.A.; Buckwalter, J.A.; Capecchi, M.R.; et al. A mouse model of osteochondromagenesis from clonal inactivation of Ext1 in chondrocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 2054–2059. [Google Scholar] [CrossRef]
- Walter, M.R. The Role of Structure in the Biology of Interferon Signaling. Front. Immunol. 2020, 11, 606489. [Google Scholar] [CrossRef]
- Ma, J.H.; Qin, L.; Li, X. Role of STAT3 signaling pathway in breast cancer. Cell Commun. Signal 2020, 18, 33. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Shield, W.P., 3rd; Cellini, A.; Tian, H.; Wilson, K.; Dan, Y.; Abzug, J.M.; Garcia, S.; Moritani, N.; Alferiev, I.; Chorny, M.; et al. Selective Agonists of Nuclear Retinoic Acid Receptor Gamma Inhibit Growth of HCS-2/8 Chondrosarcoma Cells. J. Orthop. Res. 2020, 38, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Kondo, N.; Okabe, T.; Takeshita, N.; Pilchak, D.M.; Koyama, E.; Ochiai, T.; Jensen, D.; Chu, M.L.; Kane, M.A.; et al. Retinoic acid receptors are required for skeletal growth, matrix homeostasis and growth plate function in postnatal mouse. Dev. Biol. 2009, 328, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Kane, M.; Okabe, T.; Enomoto-Iwamoto, M.; Napoli, J.L.; Pacifici, M.; Iwamoto, M. Endogenous retinoids in mammalian growth plate cartilage: Analysis and roles in matrix homeostasis and turnover. J. Biol. Chem. 2010, 285, 36674–36681. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, M.; Takano, T.; Suzuki, F. Restoration by cyclic AMP of the differentiated phenotype of chondrocytes from de-differentiated cells pretreated with retinoids. Mol. Cell. Biochem. 1982, 42, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Biddulph, D.M.; Dozier, M.M.; Julian, N.C.; Sawyer, L.M. Inhibition of chondrogenesis by retinoic acid in limb mesenchymal cells in vitro: Effects on PGE2 and cyclic AMP concentrations. Cell Differ. Dev. 1988, 25, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Horton, W.; Hassell, J.R. Independence of cell shape and loss of cartilage matrix production during retinoic acid treatment of cultured chondrocytes. Dev. Biol. 1986, 115, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, T.; Nozawa, S.; Matsumoto, K.; Irie, F.; Yamaguchi, Y. Aberrant perichondrial BMP signaling mediates multiple osteochondromagenesis in mice. JCI Insight 2017, 2, e90049. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, Y.; Sakai, Y.; Yahara, Y.; Akiyama, H.; Yoshikawa, H.; Hosokawa, K.; Tsumaki, N. Cyp26b1 within the growth plate regulates bone growth in juvenile mice. Biochem. Biophys. Res. Commun. 2014, 454, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E.; Mangiavini, L.; Merceron, C. HIF-1alpha and growth plate development: What we really know. Bonekey Rep. 2015, 4, 730. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E.; Ryan, H.E.; Didrickson, S.; Kobayashi, T.; Knight, M.; Johnson, R.S. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes. Dev. 2001, 15, 2865–2876. [Google Scholar] [CrossRef] [PubMed]
- Provot, S.; Zinyk, D.; Gunes, Y.; Kathri, R.; Le, Q.; Kronenberg, H.M.; Johnson, R.S.; Longaker, M.T.; Giaccia, A.J.; Schipani, E. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J. Cell Biol. 2007, 177, 451–464. [Google Scholar] [CrossRef]
- Elzakra, N.; Kim, Y. HIF-1alpha Metabolic Pathways in Human Cancer. Adv. Exp. Med. Biol. 2021, 1280, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Kalvakolanu, D.V. The GRIMs: A new interface between cell death regulation and interferon/retinoid induced growth suppression. Cytokine Growth Factor. Rev. 2004, 15, 169–194. [Google Scholar] [CrossRef] [PubMed]
- Nallar, S.C.; Kalvakolanu, D.V. GRIM-19: A master regulator of cytokine induced tumor suppression, metastasis and energy metabolism. Cytokine Growth Factor. Rev. 2017, 33, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, Z.; Huang, B.; Xu, K.; Su, J. Stat3 Signaling Pathway: A Future Therapeutic Target for Bone-Related Diseases. Front. Pharmacol. 2022, 13, 897539. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Chen, T.; Qiu, J.; Gao, W.; Qiu, X.; Zhu, Y.; Wang, X.; Chen, Y.; Zhou, H.; Deng, Z.; et al. Inhibition of nuclear receptor RORalpha attenuates cartilage damage in osteoarthritis by modulating IL-6/STAT3 pathway. Cell Death Dis. 2021, 12, 886. [Google Scholar] [CrossRef]
- Chen, B.; Ning, K.; Sun, M.L.; Zhang, X.A. Regulation and therapy, the role of JAK2/STAT3 signaling pathway in OA: A systematic review. Cell Commun. Signal 2023, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Cherifi, C.; Maillet, J.; Ea, H.K.; Bouaziz, W.; Funck-Brentano, T.; Cohen-Solal, M.; Hay, E.; Richette, P. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 2017, 76, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.Z.; Hu, A.X.; Liu, X.S. DUSP19 regulates IL-1beta-induced apoptosis and MMPs expression in rat chondrocytes through JAK2/STAT3 signaling pathway. Biomed. Pharmacother. 2017, 96, 1209–1215. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef]
- Chapellier, B.; Mark, M.; Garnier, J.M.; Dierich, A.; Chambon, P.; Ghyselinck, N.B. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor gamma (RARgamma) gene. Genesis 2002, 32, 95–98. [Google Scholar] [CrossRef]
- Chorny, M.; Fishbein, I.; Adamo, R.F.; Forbes, S.P.; Folchman-Wagner, Z.; Alferiev, I.S. Magnetically targeted delivery of therapeutic agents to injured blood vessels for prevention of in-stent restenosis. Methodist. Debakey Cardiovasc. J. 2012, 8, 23–27. [Google Scholar] [CrossRef]
- Lesciotto, K.M.; Motch Perrine, S.M.; Kawasaki, M.; Stecko, T.; Ryan, T.M.; Kawasaki, K.; Richtsmeier, J.T. Phosphotungstic acid-enhanced microCT: Optimized protocols for embryonic and early postnatal mice. Dev. Dyn. 2020, 249, 573–585. [Google Scholar] [CrossRef]
- Yasuhara, R.; Yuasa, T.; Williams, J.A.; Byers, S.W.; Shah, S.; Pacifici, M.; Iwamoto, M.; Enomoto-Iwamoto, M. Wnt/beta-catenin and retinoic acid receptor signaling pathways interact to regulate chondrocyte function and matrix turnover. J. Biol. Chem. 2010, 285, 317–327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, S.A.; Wilson, K.; Tang, N.; Tian, H.; Oichi, T.; Gunawardena, A.T.; Chorny, M.; Alferiev, I.S.; Herzenberg, J.E.; Ng, V.Y.; et al. Analysis of the Actions of RARγ Agonists on Growing Osteochondromas in a Mouse Model. Int. J. Mol. Sci. 2024, 25, 7610. https://doi.org/10.3390/ijms25147610
Garcia SA, Wilson K, Tang N, Tian H, Oichi T, Gunawardena AT, Chorny M, Alferiev IS, Herzenberg JE, Ng VY, et al. Analysis of the Actions of RARγ Agonists on Growing Osteochondromas in a Mouse Model. International Journal of Molecular Sciences. 2024; 25(14):7610. https://doi.org/10.3390/ijms25147610
Chicago/Turabian StyleGarcia, Sonia A., Kimberly Wilson, Ningfeng Tang, Hongying Tian, Takeshi Oichi, Aruni T. Gunawardena, Michael Chorny, Ivan S. Alferiev, John E. Herzenberg, Vincent Y. Ng, and et al. 2024. "Analysis of the Actions of RARγ Agonists on Growing Osteochondromas in a Mouse Model" International Journal of Molecular Sciences 25, no. 14: 7610. https://doi.org/10.3390/ijms25147610
APA StyleGarcia, S. A., Wilson, K., Tang, N., Tian, H., Oichi, T., Gunawardena, A. T., Chorny, M., Alferiev, I. S., Herzenberg, J. E., Ng, V. Y., Iwamoto, M., & Enomoto-Iwamoto, M. (2024). Analysis of the Actions of RARγ Agonists on Growing Osteochondromas in a Mouse Model. International Journal of Molecular Sciences, 25(14), 7610. https://doi.org/10.3390/ijms25147610





