Microvascular Dysfunction across the Spectrum of Heart Failure Pathology: Pathophysiology, Clinical Features and Therapeutic Implications
Abstract
1. Introduction
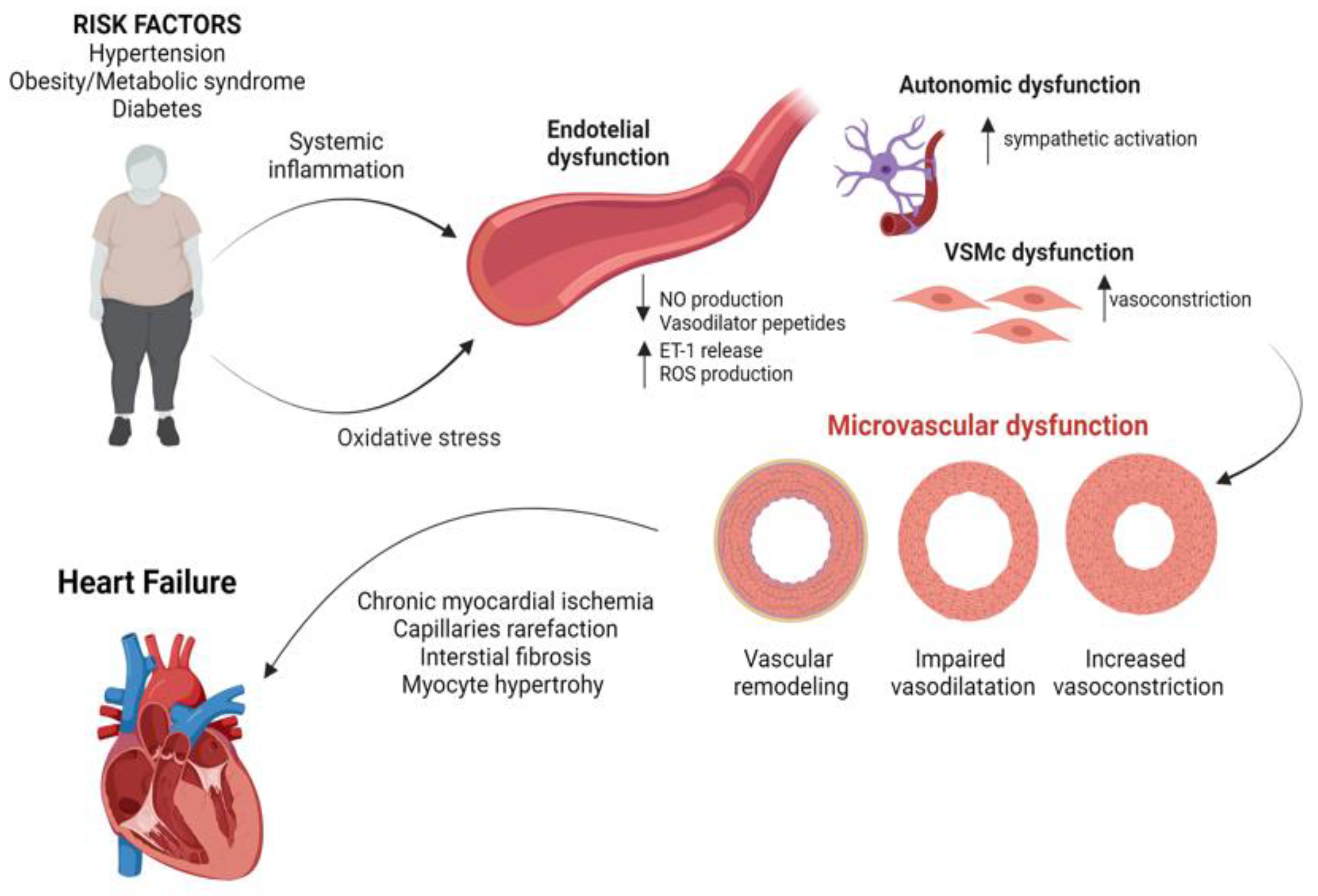
2. Pathophysiology of CMD
3. CMD across the Spectrum of Heart Failure Pathology
4. Chronic Inflammation, Endothelial Dysfunction and HF
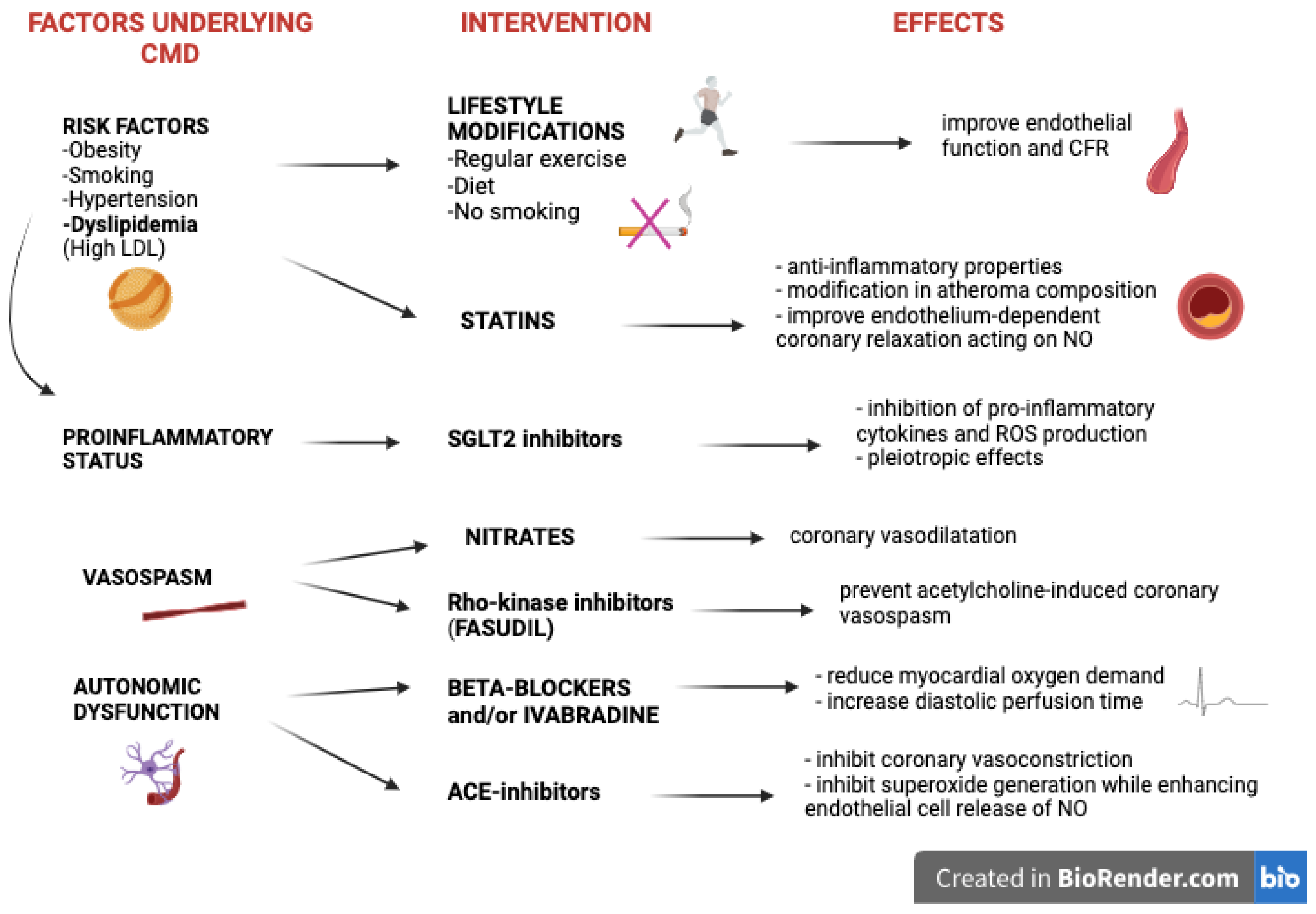
5. Targeting CMD in Patients with HF
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef] [PubMed]
- Rush, C.J.; Berry, C.; Oldroyd, K.G.; Rocchiccioli, J.P.; Lindsay, M.M.; Touyz, R.M.; Murphy, C.L.; Ford, T.J.; Sidik, N.; McEntegart, M.B.; et al. Prevalence of Coronary Artery Disease and Coronary Microvascular Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction. JAMA Cardiol. 2021, 6, 1130–1143. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef] [PubMed]
- D’amario, D.; Migliaro, S.; Borovac, J.A.; Restivo, A.; Vergallo, R.; Galli, M.; Leone, A.M.; Montone, R.A.; Niccoli, G.; Aspromonte, N.; et al. Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction. Front. Physiol. 2019, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Montone, R.A.; Rinaldi, R. Pathophysiology of Coronary Microvascular Dysfunction. Circ J. 2022, 86, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Laborante, R.; Bianchini, E.; Ciliberti, G.; Paglianiti, D.A.; Galli, M.; Restivo, A.; Stolfo, D.; Vergallo, R.; Rosano, G.M.; et al. Impact of coronary microvascular dysfunction in heart failure with preserved ejection fraction: A meta-analysis. ESC Heart Fail. 2024. [Google Scholar] [CrossRef]
- Paulus, W.J.; Zile, M.R. From Systemic Inflammation to Myocardial Fibrosis: The Heart Failure with Preserved Ejection Fraction Paradigm Revisited. Circ. Res. 2021, 128, 1451–1467. [Google Scholar] [CrossRef]
- Crea, F.; Montone, R.A. Pathophysiology of coronary microvascular dysfunction. Vasc. Pharmacol. 2023, 153, 107239. [Google Scholar] [CrossRef]
- Shimokawa, H.; Suda, A.; Takahashi, J.; Berry, C.; Camici, P.G.; Crea, F.; Escaned, J.; Ford, T.; Yii, E.; Kaski, J.C.; et al. Clinical characteristics and prognosis of patients with microvascular angina: An international and prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) Group. Eur. Heart J. 2021, 42, 4592–4600. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Meucci, M.C.; De Vita, A.; Lanza, G.A.; Niccoli, G. Coronary provocative tests in the catheterization laboratory: Pathophysiological bases, methodological considerations and clinical implications. Atherosclerosis 2021, 318, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Greco, S.; Capogrossi, M.C.; Gaetano, C.; Martelli, F. Nitric Oxide, Oxidative Stress, andp66ShcInterplay in Diabetic Endothelial Dysfunction. BioMed Res. Int. 2014, 2014, 193095. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-H.; Lu, G.; Xu, X.; Ren, Y.; Hein, T.W.; Kuo, L. Enhanced endothelin-1/Rho-kinase signalling and coronary microvascular dysfunction in hypertensive myocardial hypertrophy. Cardiovasc. Res. 2017, 113, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Cecchi, F.; Olivotto, I.; Camici, P.G. Microvascular Dysfunction in Hypertrophic Cardiomyopathy. J. Clin. Med. 2022, 11, 6560. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Lillo, R.; Biagini, E.; Limongelli, G.; Autore, C.; Pieroni, M.; Lanzillo, C.; Calò, L.; Musumeci, M.B.; Ingrasciotta, G.; et al. Myocardial infarction with non-obstructive coronary arteries in hypertrophic cardiomyopathy vs. Fabry disease. Int. J. Cardiol. 2022, 369, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Aspromonte, N.; Zaninotto, M.; Aimo, A.; Fumarulo, I.; Plebani, M.; Clerico, A. Measurement of Cardiac-Specific Biomarkers in the Emergency Department: New Insight in Risk Evaluation. Int. J. Mol. Sci. 2023, 24, 15998. [Google Scholar] [CrossRef]
- Ronco, C.; McCullough, P.A.; Anker, S.D.; Anand, I.; Aspromonte, N.; Bagshaw, S.M.; Bellomo, R.; Berl, T.; Bobek, I.; Cruz, D.N.; et al. Cardiorenal syndromes: An executive summary from the consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib. Nephrol. 2010, 165, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Aspromonte, N.; Fumarulo, I.; Petrucci, L.; Biferali, B.; Liguori, A.; Gasbarrini, A.; Massetti, M.; Miele, L. The Liver in Heart Failure: From Biomarkers to Clinical Risk. Int. J. Mol. Sci. 2023, 24, 15665. [Google Scholar] [CrossRef]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet 2018, 392, 1789–1858, Erratum in Lancet 2019, 393, e44. [CrossRef]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Rahman, H.; Perera, D. Coronary microvascular dysfunction and heart failure with preserved ejection fraction: What are the mechanistic links? Curr. Opin. Cardiol. 2023, 38, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Ljung Faxén, U.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450, Erratum in Eur. Heart J. 2019, 40, 541. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, G.; Wang, S.; Huang, J. The prevalence of coronary microvascular dysfunction (CMD) in heart failure with preserved ejection fraction (HFpEF): A systematic review and meta-analysis. Heart Fail. Rev. 2023, 29, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Obokata, M.; Reddy, Y.N.; Redfield, M.M.; Lerman, A.; Borlaug, B.A. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2019, 22, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.R.; Kanagala, P.; Budgeon, C.A.; Jerosch-Herold, M.; Gulsin, G.S.; Singh, A.; Khan, J.N.; Chan, D.C.; Squire, I.B.; Ng, L.L.; et al. Prevalence and Prognostic Significance of Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2022, 15, 1001–1011. [Google Scholar] [CrossRef]
- Paolisso, P.; Gallinoro, E.; Belmonte, M.; Bertolone, D.T.; Bermpeis, K.; De Colle, C.; Shumkova, M.; Leone, A.; Caglioni, S.; Esposito, G.; et al. Coronary Microvascular Dysfunction in Patients with Heart Failure: Characterization of Patterns in HFrEF Versus HFpEF. Circ. Heart Fail. 2024, 17, e010805. [Google Scholar] [CrossRef]
- Srivaratharajah, K.; Coutinho, T.; Dekemp, R.; Liu, P.; Haddad, H.; Stadnick, E.; Davies, R.A.; Chih, S.; Dwivedi, G.; Guo, A.; et al. Reduced Myocardial Flow in Heart Failure Patients with Preserved Ejection Fraction. Circ. Heart Fail. 2016, 9, e002562. [Google Scholar] [CrossRef]
- Dryer, K.; Gajjar, M.; Narang, N.; Lee, M.; Paul, J.; Shah, A.P.; Nathan, S.; Butler, J.; Davidson, C.J.; Fearon, W.F.; et al. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H1033–H1042. [Google Scholar] [CrossRef]
- Kato, S.; Fukui, K.; Kodama, S.; Azuma, M.; Nakayama, N.; Iwasawa, T.; Kimura, K.; Tamura, K.; Utsunomiya, D. Cardiovascular magnetic resonance assessment of coronary flow reserve improves risk stratification in heart failure with preserved ejection fraction. J. Cardiovasc. Magn. Reson. 2021, 23, 112. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Corban, M.T.; Toya, T.; Verbrugge, F.H.; Sara, J.D.; Lerman, L.O.; Borlaug, B.A.; Lerman, A. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.-Q.; Abdu, F.A.; Su, Y.; Liu, L.; Yin, G.; Feng, Y.; Zhang, W.; Xu, Y.; Xu, D.; Che, W. Prognostic Significance of Coronary Microvascular Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction. Can. J. Cardiol. 2023, 39, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477, Erratum in Eur. Heart J. 2020, 41, 4242. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Role of Inflammation in Coronary Epicardial and Microvascular Dysfunction. Eur. Cardiol. Rev. 2021, 16, e13. [Google Scholar] [CrossRef] [PubMed]
- Teragawa, H.; Fukuda, Y.; Matsuda, K.; Ueda, K.; Higashi, Y.; Oshima, T.; Yoshizumi, M.; Chayama, K. Relation between C reactive protein concentrations and coronary microvascular endothelial function. Heart 2004, 90, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; West, N.E.J.; Black, E.; McDonald, D.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Vascular Superoxide Production by NAD(P)H Oxidase: Association with endothelial dysfunction and clinical risk factors. Circ. Res. 2000, 86, E85–E90. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Rizzoni, D.; Taddei, S.; Widmer, R.J.; Montezano, A.C.; Lüscher, T.F.; Schiffrin, E.L.; Touyz, R.M.; Paneni, F.; Lerman, A.; et al. Assessment and pathophysiology of microvascular disease: Recent progress and clinical implications. Eur. Heart J. 2020, 42, 2590–2604. [Google Scholar] [CrossRef]
- Godo, S.; Suda, A.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Coronary Microvascular Dysfunction. Arter. Thromb. Vasc. Biol. 2021, 41, 1625–1637. [Google Scholar] [CrossRef]
- Miura, H.; Bosnjak, J.J.; Ning, G.; Saito, T.; Miura, M.; Gutterman, D.D. Role for Hydrogen Peroxide in Flow-Induced Dilation of Human Coronary Arterioles. Circ. Res. 2003, 92, e31–e40. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Camilli, M.; Russo, M.; Rinaldi, R.; Caffè, A.; La Vecchia, G.; Bonanni, A.; Iannaccone, G.; Basile, M.; Vergallo, R.; Aurigemma, C.; et al. Air Pollution and Coronary Vasomotor Disorders in Patients with Myocardial Ischemia and Unobstructed Coronary Arteries. J. Am. Coll. Cardiol. 2022, 80, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Camilli, M.; Calvieri, C.; Magnani, G.; Bonanni, A.; Bhatt, D.L.; Rajagopalan, S.; Crea, F.; Niccoli, G. Exposome in ischaemic heart disease: Beyond traditional risk factors. Eur. Heart J. 2024, 45, 419–438. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Rinaldi, R.; Bonanni, A.; Severino, A.; Pedicino, D.; Crea, F.; Liuzzo, G. Impact of air pollution on ischemic heart disease: Evidence, mechanisms, clinical perspectives. Atherosclerosis 2023, 366, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Iannaccone, G.; Meucci, M.C.; Gurgoglione, F.; Niccoli, G. Myocardial and Microvascular Injury Due to Coronavirus Disease. Eur. Cardiol. Rev. 2020, 15, e52. [Google Scholar] [CrossRef] [PubMed]
- Carris, N.W.; Mhaskar, R.; Coughlin, E.; Bracey, E.; Tipparaju, S.M.; Halade, G.V. Novel biomarkers of inflammation in heart failure with preserved ejection fraction: Analysis from a large prospective cohort study. BMC Cardiovasc. Disord. 2022, 22, 221. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.; Michaëlsson, E.; Kull, B.; Miliotis, T.; Svedlund, S.; Linde, C.; Donal, E.; Daubert, J.; Gan, L.; Lund, L.H. Myeloperoxidase and related biomarkers are suggestive footprints of endothelial microvascular inflammation in HFpEF patients. ESC Heart Fail. 2020, 7, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac Inflammation Contributes to Changes in the Extracellular Matrix in Patients with Heart Failure and Normal Ejection Fraction. Circ. Heart Fail. 2011, 4, 44–52. [Google Scholar] [CrossRef]
- Tromp, J.; Khan, M.A.F.; Klip, I.T.; Meyer, S.; de Boer, R.A.; Jaarsma, T.; Hillege, H.; van Veldhuisen, D.J.; van der Meer, P.; Voors, A.A. Biomarker Profiles in Heart Failure Patients with Preserved and Reduced Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e003989. [Google Scholar] [CrossRef]
- Simmonds, S.J.; Cuijpers, I.; Heymans, S.; Jones, E.A.V. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells 2020, 9, 242. [Google Scholar] [CrossRef]
- Lommi, J.; Pulkki, K.; Koskinen, P.; Naveri, H.; Leinonen, H.; Harkonen, M.; Kupari, M. Haemodynamic, neuroendocrine and metabolic correlates of circulating cytokine concentrations in congestive heart failure. Eur. Heart J. 1997, 18, 1620–1625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alem, M.M. Endothelial Dysfunction in Chronic Heart Failure: Assessment, Findings, Significance, and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 3198. [Google Scholar] [CrossRef]
- Olsen, R.H.; Pedersen, L.R.; Jürs, A.; Snoer, M.; Haugaard, S.B.; Prescott, E. A randomised trial comparing the effect of exercise training and weight loss on microvascular function in coronary artery disease. Int. J. Cardiol. 2015, 185, 229–235. [Google Scholar] [CrossRef]
- Rooks, C.; Faber, T.; Votaw, J.; Veledar, E.; Goldberg, J.; Raggi, P.; Quyyumi, A.A.; Bremner, J.D.; Vaccarino, V. Effects of smoking on coronary microcirculatory function: A twin study. Atherosclerosis 2011, 215, 500–506. [Google Scholar] [CrossRef]
- Hambrecht, R.; Gielen, S.; Linke, A.; Fiehn, E.; Yu, J.; Walther, C.; Schoene, N.; Schuler, G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA 2000, 283, 3095–3101. [Google Scholar] [CrossRef]
- Fukuta, H.; Goto, T.; Wakami, K.; Ohte, N. The effect of statins on mortality in heart failure with preserved ejection fraction: A meta-analysis of propensity score analyses. Int. J. Cardiol. 2016, 214, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Eshtehardi, P.; McDaniel, M.C.; Dhawan, S.S.; Binongo, J.N.G.; Krishnan, S.K.; Golub, L.; Corban, M.T.; Raggi, P.; Quyyumi, A.A.; Samady, H. Effect of intensive atorvastatin therapy on coronary atherosclerosis progression, composition, arterial remodeling, and microvascular function. J. Invasive Cardiol. 2012, 24, 522–529. [Google Scholar] [PubMed]
- Marume, K.; Takashio, S.; Nagai, T.; Tsujita, K.; Saito, Y.; Yoshikawa, T.; Anzai, T. Effect of Statins on Mortality in Heart Failure with Preserved Ejection Fraction Without Coronary Artery Disease—Report From the JASPER Study. Circ. J. 2019, 83, 357–367. [Google Scholar] [CrossRef]
- Padro, T.; Manfrini, O.; Bugiardini, R.; Canty, J.; Cenko, E.; De Luca, G.; Duncker, D.J.; Eringa, E.C.; Koller, A.; Tousoulis, D.; et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on ‘coronary microvascular dysfunction in cardiovascular disease’. Cardiovasc. Res. 2020, 116, 741–755. [Google Scholar] [CrossRef]
- Lanza, G.A.; Crea, F. Response to Letter Regarding Article, “Primary Coronary Microvascular Dysfunction: Clinical Presentation, Pathophysiology, and Management”. Circulation 2010, 121, 2317–2325. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Iannaccone, G.; Scacciavillani, R.; Carbone, S.; Camilli, M.; Niccoli, G.; Borlaug, B.A.; Lavie, C.J.; Arena, R.; Crea, F.; et al. Heart failure with preserved ejection fraction diagnosis and treatment: An updated review of the evidence. Prog. Cardiovasc. Dis. 2020, 63, 570–584. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Assmus, B.; Bekfani, T.; Dworatzek, E.; Edelmann, F.; Hashemi, D.; Hellenkamp, K.; Kempf, T.; Raake, P.; Schütt, K.A.; et al. Heart failure with preserved ejection fraction: Diagnosis, risk assessment, and treatment. Clin. Res. Cardiol. 2024, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wernhart, S.; Papathanasiou, M.; Rassaf, T.; Luedike, P. The controversial role of beta-blockers in heart failure with preserved ejection fraction. Pharmacol. Ther. 2023, 243, 108356. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.B.; Henry, G.C.; Macaya, C.; O’Neill, B.J.; Pucillo, A.L.; Carere, R.G.; Wargovich, T.J.; Mudra, H.; Lüscher, T.F.; Klibaner, M.I.; et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation 1996, 94, 258–265, Erratum in Circulation 1996, 94, 1490. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: Rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur. J. Heart Fail. 2013, 15, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Zile, M.; Pieske, B.; Voors, A.; Shah, A.; Kraigher-Krainer, E.; Shi, V.; Bransford, T.; Takeuchi, M.; Gong, J.; et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: A phase 2 double-blind randomised controlled trial. Lancet 2012, 380, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Mentz, R.J.; Claggett, B.L.; Miao, Z.M.; Kulac, I.J.; Ward, J.H.; Hernandez, A.F.; Morrow, D.A.; Starling, R.C.; Velazquez, E.J.; et al. Sacubitril/valsartan in heart failure with mildly reduced or preserved ejection fraction: A pre-specified participant-level pooled analysis of PARAGLIDE-HF and PARAGON-HF. Eur. Heart J. 2023, 44, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; DeMets, D.L.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; Bengtsson, O.; Ponikowski, P.; Sabatine, M.S.; et al. A trial to evaluate the effect of the sodium–glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur. J. Heart Fail. 2019, 21, 665–675. [Google Scholar] [CrossRef]
- Adingupu, D.D.; Göpel, S.O.; Grönros, J.; Behrendt, M.; Sotak, M.; Miliotis, T.; Dahlqvist, U.; Gan, L.-M.; Jönsson-Rylander, A.-C. SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob−/− mice. Cardiovasc. Diabetol. 2019, 18, 16. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Packer, M.; Butler, J.; Zannad, F.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients with Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation 2021, 144, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639, Erratum in Eur. Heart J. 2024, 45, 53. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.; Lund, L.H. Inflammation and myeloperoxidase—The next treatment targets in heart failure? Int. J. Cardiol. 2024, 401, 131834. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Botros, F.T.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and renal outcomes in type 2 diabetes: An exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 2019, 394, 131–138. [Google Scholar] [CrossRef]
- Alharby, H.; Abdelati, T.; Rizk, M.; Youssef, E.; Gaber, N.; Moghazy, K.; Yafei, S. Association of fasting glucagon-like peptide-1 with oxidative stress and subclinical atherosclerosis in type 2 diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Vinué, Á.; Navarro, J.; Herrero-Cervera, A.; García-Cubas, M.; Andrés-Blasco, I.; Martínez-Hervás, S.; Real, J.T.; Ascaso, J.F.; González-Navarro, H. The GLP-1 analogue lixisenatide decreases atherosclerosis in insulin-resistant mice by modulating macrophage phenotype. Diabetologia 2017, 60, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Bruen, R.; Curley, S.; Kajani, S.; Lynch, G.; O’reilly, M.E.; Dillon, E.T.; Brennan, E.P.; Barry, M.; Sheehan, S.; McGillicuddy, F.C.; et al. Liraglutide Attenuates Preestablished Atherosclerosis in Apolipoprotein E–Deficient Mice via Regulation of Immune Cell Phenotypes and Proinflammatory Mediators. J. Pharmacol. Exp. Ther. 2019, 370, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.M.; Yu, H.M.; Lei, X.M.; Wen, P.M. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of double-blind, randomized, placebo-controlled trials. Medicine 2024, 103, e37432. [Google Scholar] [CrossRef] [PubMed]
- del Olmo-Garcia, M.I.; Merino-Torres, J.F. GLP-1 Receptor Agonists and Cardiovascular Disease in Patients with Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 4020492. [Google Scholar] [CrossRef]
- Tadic, M.; Sala, C.; Saeed, S.; Grassi, G.; Mancia, G.; Rottbauer, W.; Cuspidi, C. New antidiabetic therapy and HFpEF: Light at the end of tunnel? Heart Fail. Rev. 2021, 27, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Rinaldi, R.; Niccoli, G.; Andò, G.; Gragnano, F.; Piccolo, R.; Pelliccia, F.; Moscarella, E.; Zimarino, M.; Fabris, E.; et al. Optimizing Management of Stable Angina: A Patient-Centered Approach Integrating Revascularization, Medical Therapy and Lifestyle Interventions. J. Am. Coll. Cardiol. 2024; in press. [Google Scholar]
- Villano, A.; Di Franco, A.; Nerla, R.; Sestito, A.; Tarzia, P.; Lamendola, P.; Di Monaco, A.; Sarullo, F.M.; Lanza, G.A.; Crea, F. Effects of Ivabradine and Ranolazine in Patients with Microvascular Angina Pectoris. Am. J. Cardiol. 2013, 112, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef]
- Mohri, M.; Shimokawa, H.; Hirakawa, Y.; Masumoto, A.; Takeshita, A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J. Am. Coll. Cardiol. 2002, 41, 15–19. [Google Scholar] [CrossRef]
- Masumoto, A.; Mohri, M.; Shimokawa, H.; Urakami, L.; Usui, M.; Takeshita, A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 2002, 105, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Sample Size (n) | Study Design | Age (Years) | Objective | Definition of CMD | Prevalence of CMD | Results | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Rush et al. | 2021 | 106 | nROS | 72 ± 9 | To assess the prevalence of CAD and CMD in hospitalized patients with HFpEF | CMD defined both invasively (as CFR < 2.0 and/or iMR > 25 and/or positive Ach provocative test) and non-invasively (as MPRi < 1.84 at CMR perfusion imaging) | 85% | CMD is highly prevalent in HFpEF with and without CAD | [4] |
| Mohammed et al. | 2015 | 228 | nROS | 75 (66–83) | To evaluate structural changes associated with HFpEF | Microvascular rarefaction defined as vessels/mm2 at myocardial biopsy | 23% | HFpEF showed a high prevalence of myocardial fibrosis and coronary microvascular rarefaction | [5] |
| Shah et al. | 2018 | 202 | nROS | 74.7 ± 8.7 in CMD pts vs. 72.4 ± 9 in those without | To investigate the prevalence of CMD and its association with endothelial dysfunction and HF severity in HFpEF | CMD defined as CFR < 2.5 assessed by stress transthoracic echocardiography | 75% | CMD is highly prevalent in HFpEF and is associated with clinical biomarkers of HF severity | [24] |
| Lin et al. | 2023 | 1267 | Systematic review and meta-analysis | N/A | To assess the prevalence of CMD in HFpEF | CMD assessed both invasively (including both cut-offs of CFR < 2.0 and CFR < 2.5) and non-invasively | 71% | CMD is highly prevalent in HFpEF and is associated with worse clinical outcomes | [25] |
| Yang et al. | 2020 | 162 | nROS | 56 ± 11 in CMD pts vs. 54 ± 11 in those without | To assess the prevalence of endothelium-dependent vs. -independent CMD in HFpEF | CMD defined invasively by Ach provocative test | 72% | Endothelium-dependent and -independent CMD are equally prevalent in HFpEF | [26] |
| Arnold et al. | 2021 | 144 | nROS | 73 ± 5 | To examine the prevalence of CMD, the relationship between perfusion and fibrosis, and the impact of CMD on clinical outcomes in HFpEF | CMD defined as MPR < 2.0 assessed by stress perfusion CMR study | 70% | CMD is highly prevalent in HFpEF (up to 70% of cases) and is independently associated with worse clinical outcomes | [27] |
| Paolisso et al. | 2024 | 56 | nROS | N/A | To characterize coronary CMD in HFpEF vs. HFrEF | CMD defined as CFR < 2.5 assessed invasively by intracoronary thermodilution | 52% | In HFrEF, CMD was mainly functional while in HFpEF, it was mainly characterized by structural changes | [28] |
| Srivaratharajah et al. | 2016 | 376 | nROS | 63 ± 11 | To assess myocardial flow reserve (MFR) in HFpEF | MFR > 2.0 assessed by cardiac positron emission tomography | 40% | HFpEF was associated with a significant reduction in global MFR | [29] |
| Dryer et al. | 2018 | 44 | nROS | 65.4 ± 9.6 in HF pts vs. 55.1 ± 3.1 in controls | To assess the prevalence of CMD in HFpEF | CMD defined invasively as CFR < 2.0 and/or iMR > 23 | 37% with overt CMD and 37% with either abnormal iMR or CFR | Distinctive coronary physiology groups are present in HFpE | [30] |
| Kato et al. | 2021 | 163 | nROS | 73 ± 9 | To assess the prognostic value of CMD in HFpEF | CMD defined as CFR < 2 assessed by stress perfusion CMR | 9% | CFR is a valuable prognostic marker in HFpEF | [31] |
| Ahmad et al. | 2021 | 51 | nROS | 59.6 ± 10.1 in pts with diagnosis of HFpEF vs. 54.3 ± 10.4 in pts without | To assess the relationship between microvascular function and exercise hemodynamics | CMD defined invasively as CFR ≤ 2.5 and/or abnormal Ach provocative test | 86% | CMD is associated with higher left ventricular filling pressures at peak exercise level | [32] |
| Mohammed et al. | 2023 | 137 | nROS | N/A | To assess the prognostic significance of CMD in HFpEF | CMD defined as coronary angiography-derived index of microcirculatory resistance ≥ 25 | 64% | CMD is an independent prognostic predictor of HFpEF | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Vecchia, G.; Fumarulo, I.; Caffè, A.; Chiatto, M.; Montone, R.A.; Aspromonte, N. Microvascular Dysfunction across the Spectrum of Heart Failure Pathology: Pathophysiology, Clinical Features and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 7628. https://doi.org/10.3390/ijms25147628
La Vecchia G, Fumarulo I, Caffè A, Chiatto M, Montone RA, Aspromonte N. Microvascular Dysfunction across the Spectrum of Heart Failure Pathology: Pathophysiology, Clinical Features and Therapeutic Implications. International Journal of Molecular Sciences. 2024; 25(14):7628. https://doi.org/10.3390/ijms25147628
Chicago/Turabian StyleLa Vecchia, Giulia, Isabella Fumarulo, Andrea Caffè, Mario Chiatto, Rocco A. Montone, and Nadia Aspromonte. 2024. "Microvascular Dysfunction across the Spectrum of Heart Failure Pathology: Pathophysiology, Clinical Features and Therapeutic Implications" International Journal of Molecular Sciences 25, no. 14: 7628. https://doi.org/10.3390/ijms25147628
APA StyleLa Vecchia, G., Fumarulo, I., Caffè, A., Chiatto, M., Montone, R. A., & Aspromonte, N. (2024). Microvascular Dysfunction across the Spectrum of Heart Failure Pathology: Pathophysiology, Clinical Features and Therapeutic Implications. International Journal of Molecular Sciences, 25(14), 7628. https://doi.org/10.3390/ijms25147628






