Multi-Omics Characterization of E3 Regulatory Patterns in Different Cancer Types
Abstract
1. Introduction
2. Results
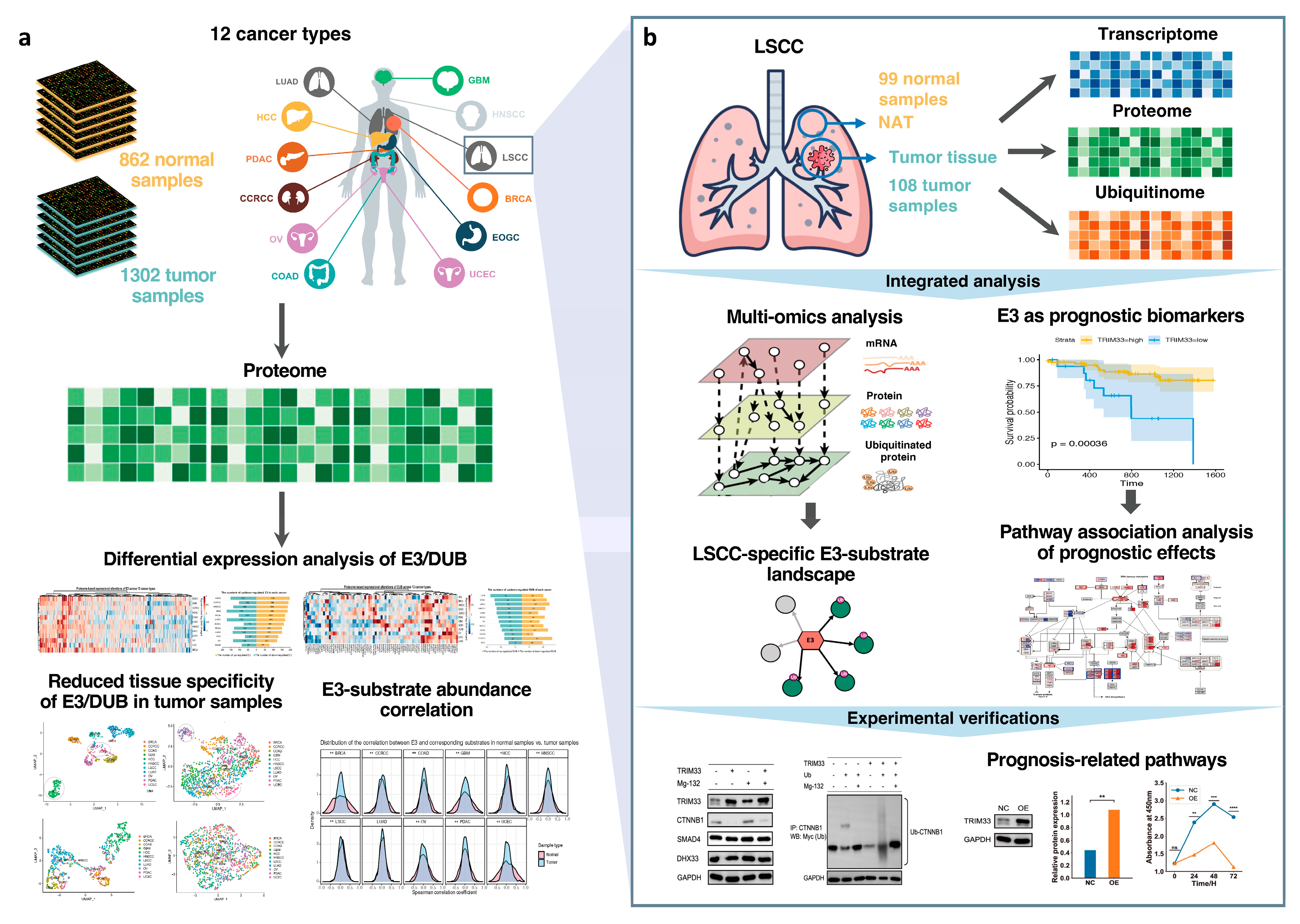
2.1. Assembly of Proteomic Data from Diverse Cancer Cohorts
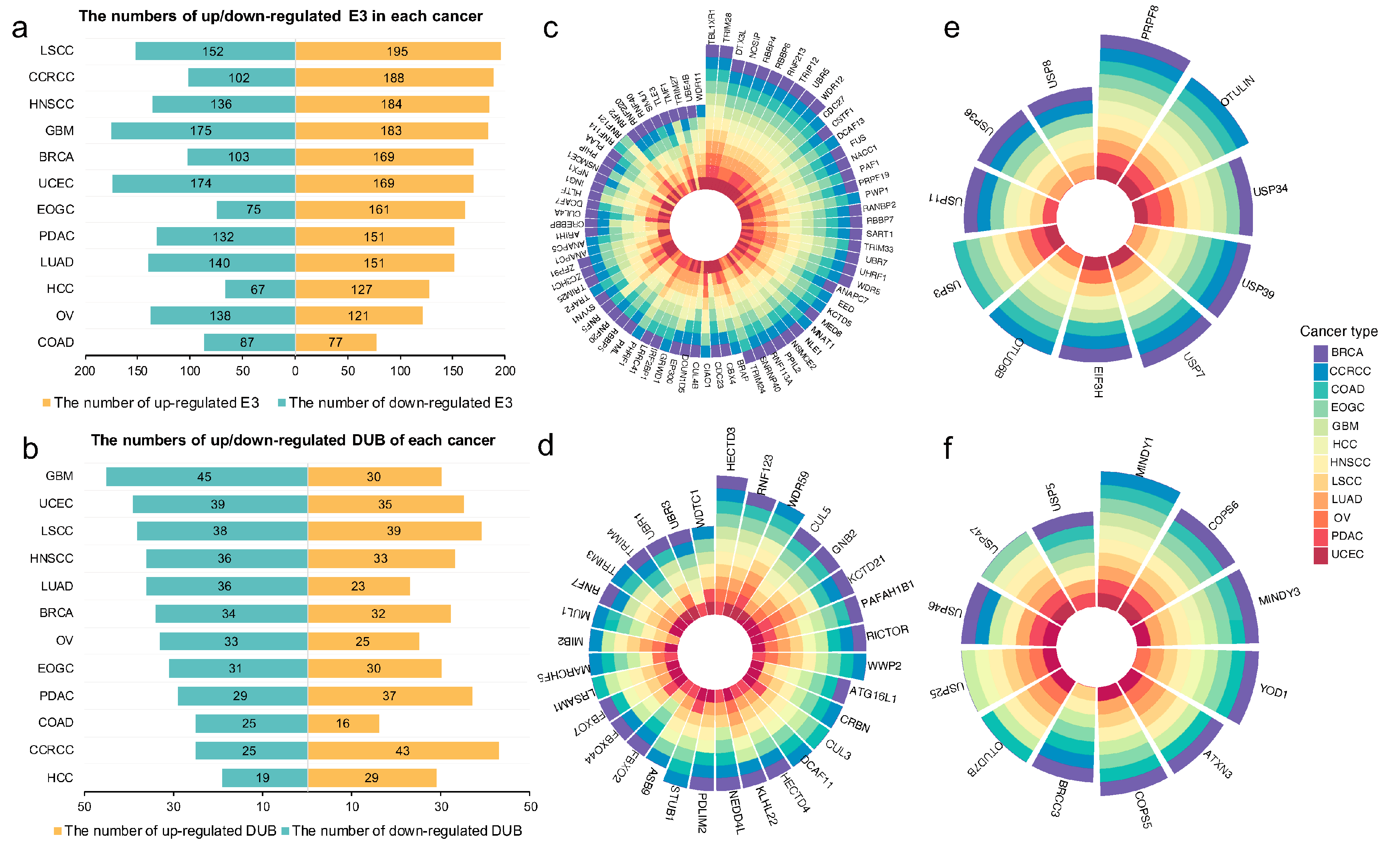
2.2. Proteome-Based Expressional Alterations of E3 Ligases and Deubiquitinases between Tumor and Normal Samples
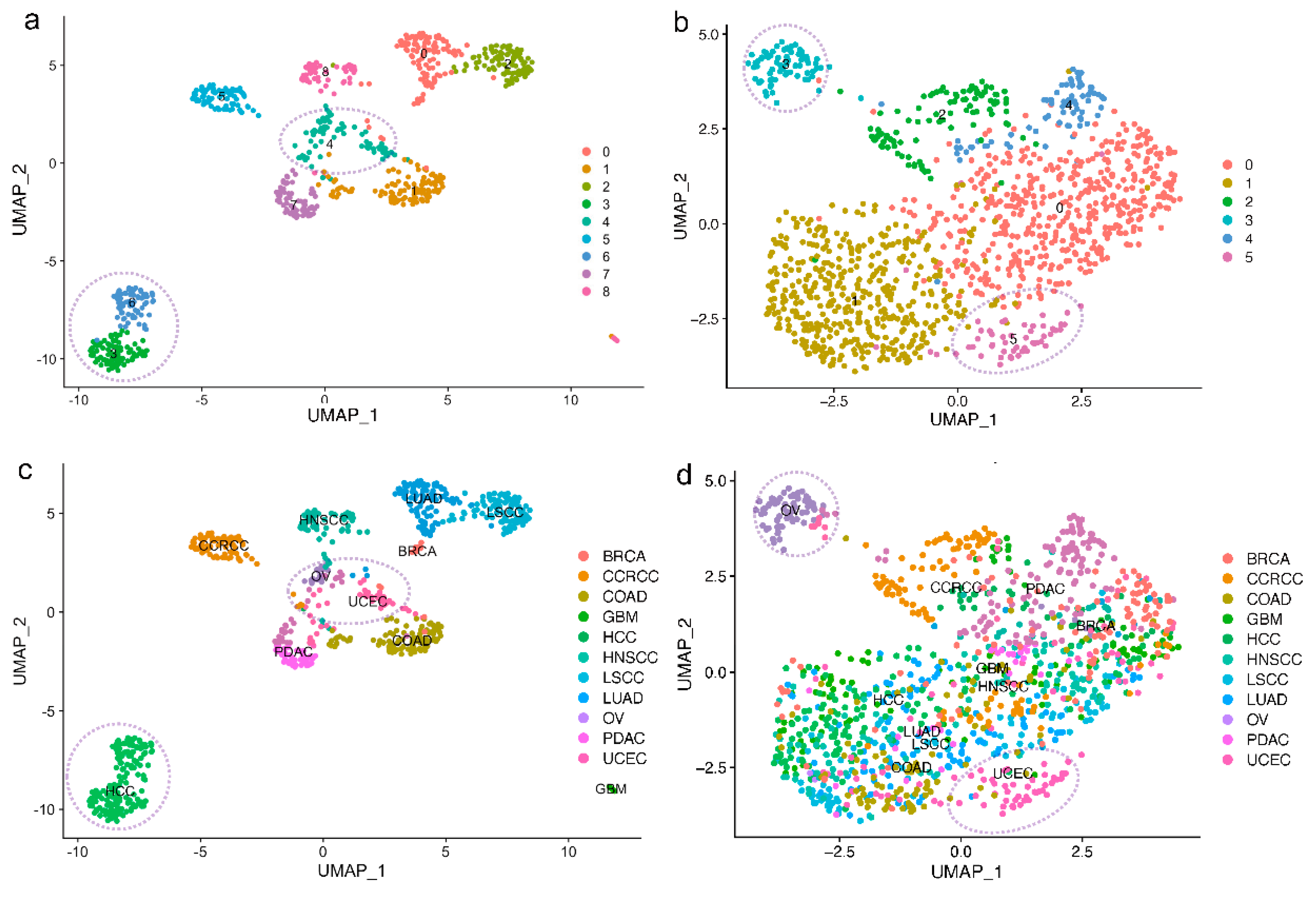
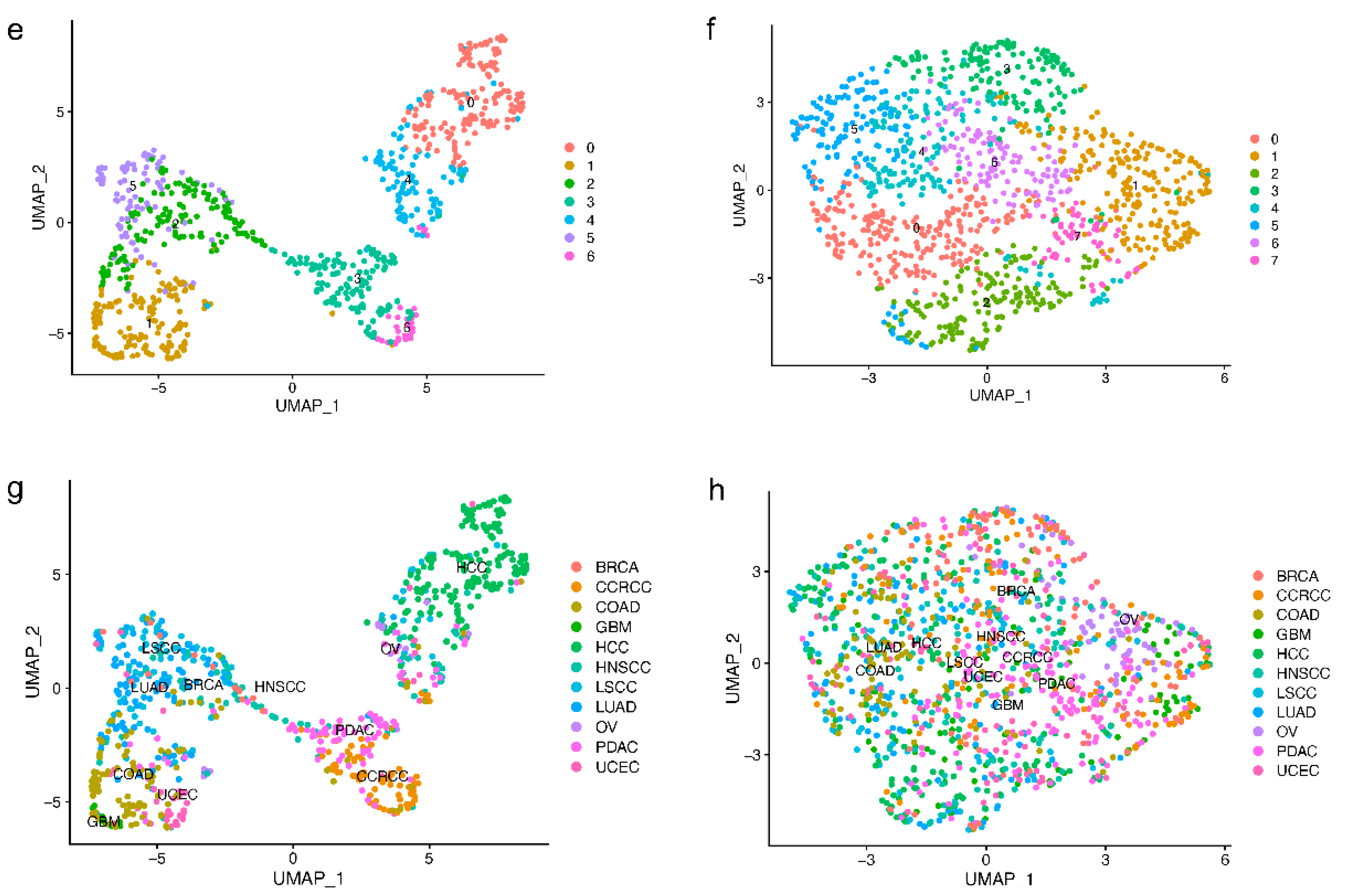
2.3. Reduced Tissue Specificity of E3 Expression Patterns in Tumor Samples Compared With Normal Samples
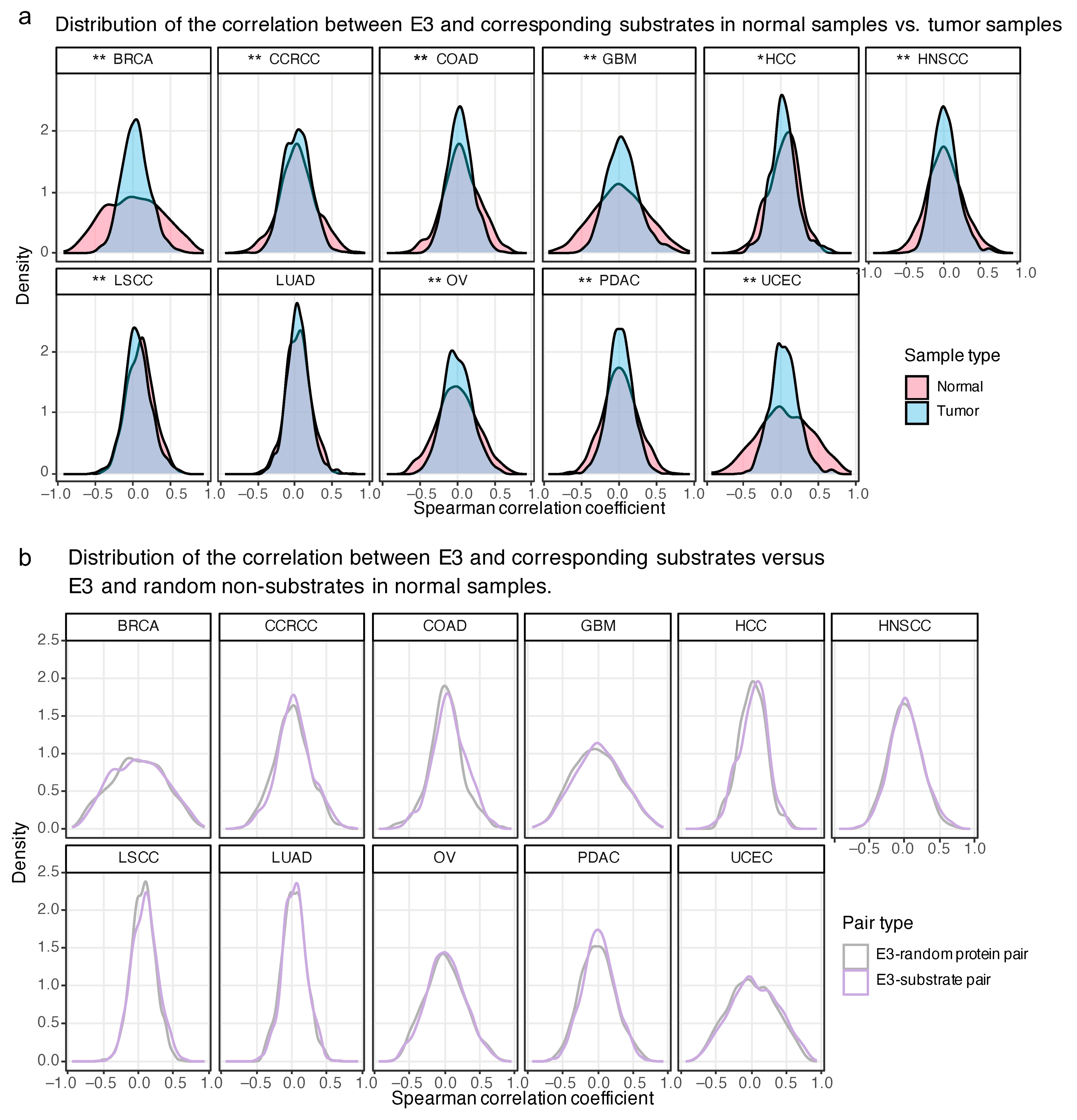
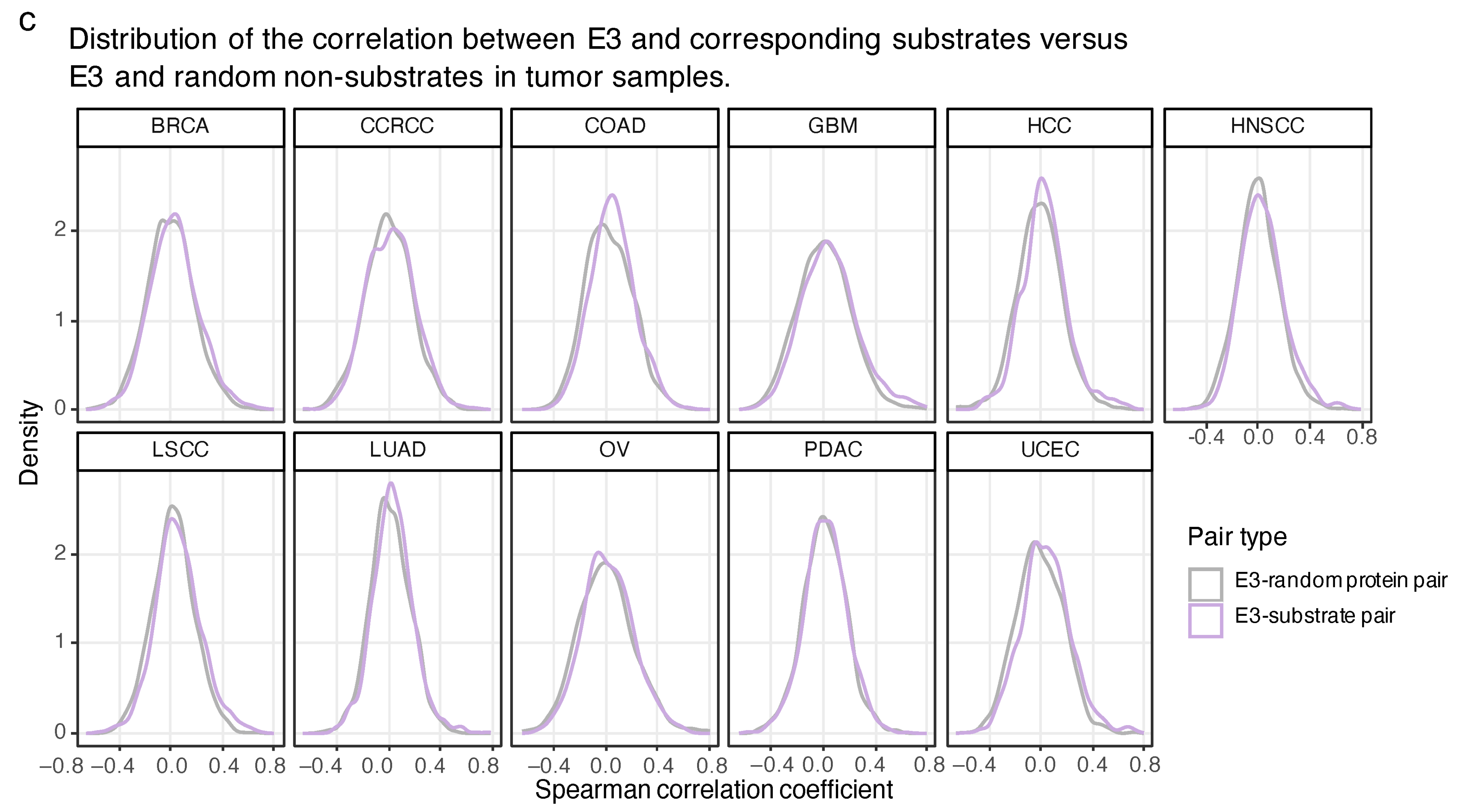
2.4. The Proteome-Based Correlation between E3 and Corresponding Substrates
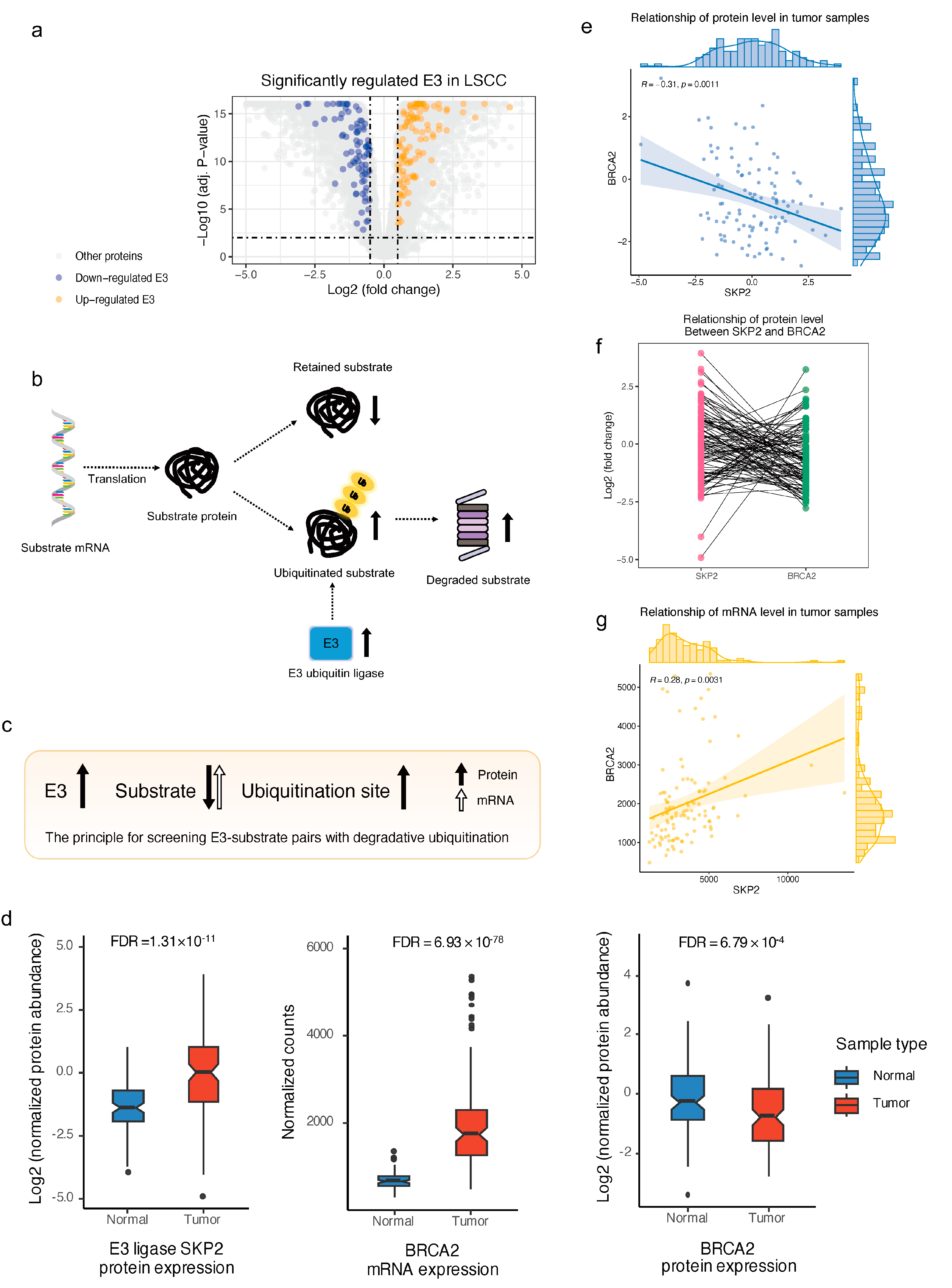
2.5. Landscape of E3–Substrate Regulatory Pattern in Lung Squamous Cell Carcinoma by Integrating Multi-Omics Data
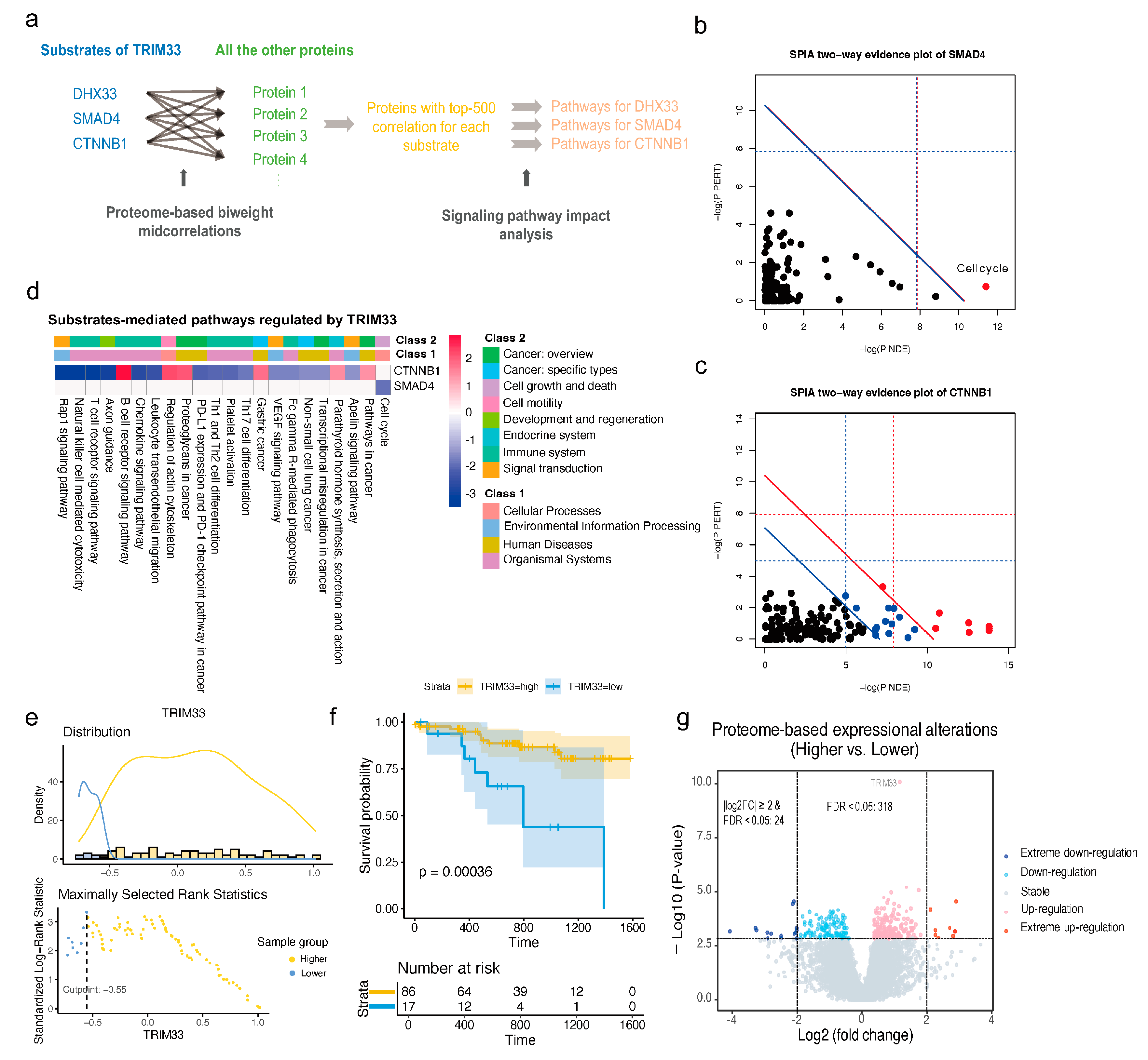
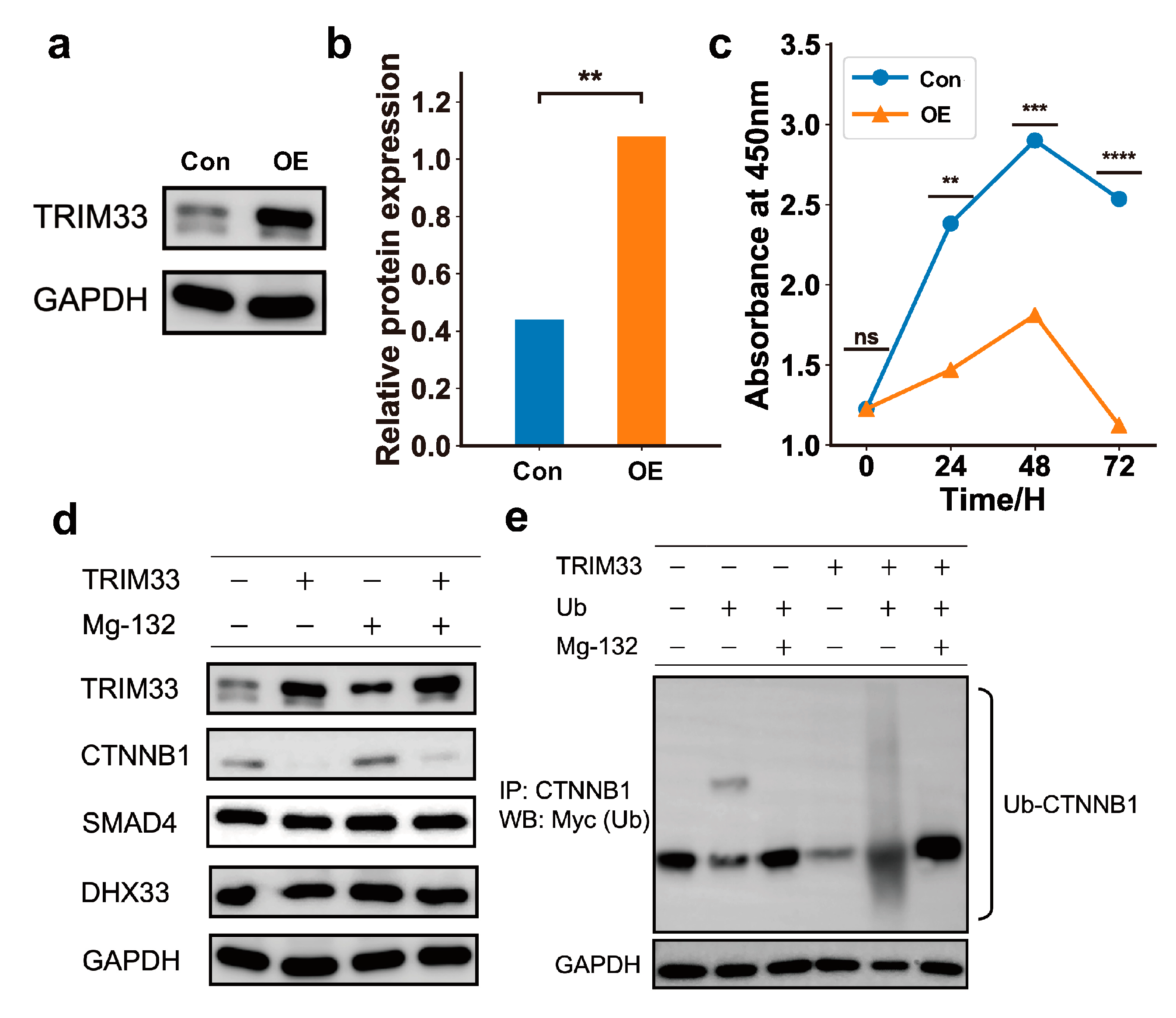
2.6. The Upregulation of TRIM33 Is a Biomarker of Favorable Prognosis by Inhibiting Cell Cycle
3. Discussion
4. Conclusions
5. Methods
5.1. The Proteome-Based Differential Expression Analysis
5.2. Heatmap of Proteome-Based Expressional Alterations
5.3. Cluster Determination of Cohorts across Cancer Types
5.4. The Proteome-Based Correlation between E3 and Substrates
5.5. Multi-Omics Analyses for the LSCC Cohort
5.6. TRIM33-Involved Pathway Analysis
5.7. Plasmid Preparation
5.8. Cell Culture
5.9. Transfection
5.10. Cell Counting Kit-8 (CCK-8) Assay
5.11. Protein Immuno-Co-Precipitation
5.12. Western Blot
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Komander, D.; Clague, M.J.; Urbe, S. Breaking the chains: Structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009, 10, 550–563. [Google Scholar] [CrossRef]
- French, M.E.; Koehler, C.F.; Hunter, T. Emerging functions of branched ubiquitin chains. Cell Discov. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Popovic, D.; Vucic, D.; Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 2014, 20, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Schwesinger, C. The ubiquitin–proteasome system in kidney physiology and disease. Nat. Rev. Nephrol. 2019, 15, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Burslem, G.M.; Crews, C.M. Proteolysis-Targeting Chimeras as Therapeutics and Tools for Biological Discovery. Cell 2020, 181, 102–114. [Google Scholar] [CrossRef]
- Mun, D.G.; Bhin, J.; Kim, S.; Kim, H.; Jung, J.H.; Jung, Y.; Jang, Y.E.; Park, J.M.; Kim, H.; Jung, Y.; et al. Proteogenomic characterization of human early-onset gastric cancer. Cancer Cell 2019, 35, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Tokheim, C.; Wang, X.; Timms, R.T.; Zhang, B.; Mena, E.L.; Wang, B.; Chen, C.; Ge, J.; Chu, J.; Zhang, W.; et al. Systematic characterization of mutations altering protein degradation in human cancers. Mol. Cell 2021, 81, 1292–1308.e11. [Google Scholar] [CrossRef]
- Thangudu, R.R.; Rudnick, P.A.; Holck, M.; Singhal, D.; MacCoss, M.J.; Edwards, N.J.; Ketchum, K.A.; Kinsinger, C.R.; Kim, E.; Basu, A. Abstract LB-242: Proteomic Data Commons: A resource for proteogenomic analysis. Cancer Res. 2020, 80 (Suppl. S16), LB-242. [Google Scholar] [CrossRef]
- Lu, M.; Chen, W.; Zhuang, W.; Zhan, X. Label-free quantitative identification of abnormally ubiquitinated proteins as useful biomarkers for human lung squamous cell carcinomas. EPMA J. 2020, 11, 73–94. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, M.; Yang, L.; Yang, J.; Zhan, X.; Zheng, S.; Guo, Y.; Li, B.; Wen, S.; Li, J.; et al. Ubiquitination-mediated molecular pathway alterations in human lung squamous cell carcinomas identified by quantitative ubiquitinomics. Front. Endocrinol. 2022, 13, 970843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, C.; Yu, Y.; Geng, J.; Meng, Q.; Xu, S.; Zhou, F.; Chen, Y.; Jin, S.; Shen, J.; et al. TBL1XR1 is involved in c-Met-mediated tumorigenesis of human nonsmall cell lung cancer. Cancer Gene Ther. 2020, 27, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Daniels, G.; Li, Y.; Gellert, L.L.; Zhou, A.; Melamed, J.; Wu, X.; Zhang, X.; Zhang, D.; Meruelo, D.; Logan, S.K.; et al. TBLR1 as an androgen receptor (AR) coactivator selectively ac-tivates AR target genes to inhibit prostate cancer growth. Endocr.-Relat. Cancer 2014, 21, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Venturutti, L.; Teater, M.; Zhai, A.; Chadburn, A.; Babiker, L.; Kim, D.; Béguelin, W.; Lee, T.C.; Kim, Y.; Chin, C.R.; et al. TBL1XR1 Mutations Drive Extranodal Lymphoma by Inducing a Pro-tumorigenic Memory Fate. Cell 2020, 182, 297–316.e27. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, Y.; Cao, Q.; Liu, N.; Zhang, W. TBL1XR1 Is Highly Expressed in Gastric Cancer and Predicts Poor Prognosis. Dis. Markers 2016, 2016, 2436518. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ning, Z.; Chen, H.; Lu, C.; Liu, X.; Xia, T.; Qi, H.; Wang, W.; Ling, T.; Guo, X.; et al. An integrative pan-cancer analysis of biological and clinical impacts un-derlying ubiquitin-specific-processing proteases. Oncogene 2020, 39, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, H.; Jiang, T.; Kuang, M.; Liu, B.; Guo, X.; He, D.; Chen, M.; Gu, J.; Gu, J.; et al. pVHL promotes lysosomal degradation of YAP in lung adenocarcinoma. Cell. Signal. 2021, 83, 110002. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; He, M.; Kong, X.; Jiang, P.; Liu, X.; Diao, L.; Zhang, X.; Li, H.; Ling, X.; et al. UbiBrowser 2.0: A comprehensive resource for proteome-wide known and predicted ubiquitin ligase/deubiquitinase–substrate interactions in eukaryotic species. Nucleic Acids Res. 2021, 50, D719–D728. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, S.; Jhong, J.H.; Pang, Y.; Huang, K.Y.; Li, S.; Lee, T.Y. UbiNet 2.0: A verified, classified, annotated and updated database of E3 ubiquitin ligase–substrate interactions. Database 2021, 2021, baab010. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, J.; Wang, C.; Jain, P.P.; Xiong, M.; Shi, X.; Lei, Y.; Chen, S.; Yin, Q.; Thistlethwaite, P.A.; et al. MDM2-Mediated Ubiquitination of Angiotensin-Converting Enzyme 2 Contributes to the Development of Pulmonary Arterial Hypertension. Circulation 2020, 142, 1190–1204. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, R.; Zeng, Y.; Li, Y.; Chen, Y.; Zhou, J.; Zhang, Y.; Wang, A.; Zhu, J.; Liu, Z.; et al. ALCAP2 inhibits lung adenocarcinoma cell proliferation, migration and invasion via the ubiquitination of β-catenin by upregulating the E3 ligase NEDD4L. Cell Death Dis. 2021, 12, 755. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hong, H.; Wang, Q.; Li, J.; Zhang, W.; Chen, T.; Li, P. NEDD4L-induced ubiquitination mediating UBE2T degradation in-hibits progression of lung adenocarcinoma via PI3K-AKT signaling. Cancer Cell Int. 2021, 21, 631. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, X.; Jiang, Y.; Deng, C.; Luo, X.; Han, J.; Hu, J.; Cao, X. Down-regulated NEDD4L facilitates tumor progression through activating Notch signaling in lung adenocarcinoma. PeerJ 2022, 10, e13402. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, L.; Tan, C.T.; Chapman, A.; Nonaka, D.; Mack, N.A.; Smith, D.; Booton, R.; Hurlstone, A.F.; Malliri, A. HUWE1 ubiquitylates and degrades the RAC activator TIAM1 promoting cell-cell adhesion disassembly, migration, and invasion. Cell Rep. 2015, 10, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, L.; Zeng, C.; Long, H.; Duan, G.; Yin, G.; Dai, X.; Lin, Z. E3 Ubiquitin Ligase HRD1 Promotes Lung Tumorigenesis by Promoting Sirtuin 2 Ubiquitination and Degradation. Mol. Cell. Biol. 2020, 40, e00257-19. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, X.; Xia, T.; Tekcham, D.S.; Wang, W.; Chen, H.; Li, T.; Lu, C.; Ning, Z.; Liu, X.; et al. A Multidimensional Characterization of E3 Ubiquitin Ligase and Substrate Interaction Network. iScience 2019, 16, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, S.; Krug, K.; Beltran, P.M.J.; Savage, S.R.; Petralia, F.; Kumar-Sinha, C.; Dou, Y.; Reva, B.; Kane, M.H.; Avanessian, S.C.; et al. A proteogenomic portrait of lung squamous cell carcinoma. Cell 2021, 184, 4348–4371.e40. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, J.; Wei, Q.; Wang, R.; Yang, W.; Ma, Y.; Chen, G.; Yu, Y. Proteomes and ubiquitylomes analysis reveals the involvement of ubiq-uitination in protein degradation in petunias. Plant Physiol. 2017, 173, 668–687. [Google Scholar] [CrossRef]
- Moro, L.; Arbini, A.A.; Marra, E.; Greco, M. Up-regulation of Skp2 after Prostate Cancer Cell Adhesion to Basement Membranes Results in BRCA2 Degradation and Cell Proliferation. J. Biol. Chem. 2006, 281, 22100–22107. [Google Scholar] [CrossRef]
- Xue, J.; Chen, Y.; Wu, Y.; Wang, Z.; Zhou, A.; Zhang, S.; Lin, K.; Aldape, K.; Majumder, S.; Lu, Z.; et al. Tumour suppressor TRIM33 targets nuclear β-catenin degradation. Nat. Commun. 2015, 6, 6156. [Google Scholar] [CrossRef]
- Weng, L.; Mitoma, H.; Tricot, C.; Bao, M.; Liu, Y.; Zhang, Z.; Liu, Y.J. The E3 ubiquitin ligase tripartite motif 33 is essential for cyto-solic RNA-induced NLRP3 inflammasome activation. J. Immunol. 2014, 193, 3676–3682. [Google Scholar] [CrossRef]
- Dupont, S.; Zacchigna, L.; Cordenonsi, M.; Soligo, S.; Adorno, M.; Rugge, M.; Piccolo, S. Germ-Layer Specification and Control of Cell Growth by Ectodermin, a Smad4 Ubiquitin Ligase. Cell 2005, 121, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, X.; Dong, C. Molecular mechanisms of T helper 17 cell differentiation: Emerging roles for transcription cofac-tors. Adv. Immunol. 2019, 144, 121–153. [Google Scholar] [PubMed]
- Xia, X.; Zuo, F.; Luo, M.; Sun, Y.; Bai, J.; Xi, Q. Role of TRIM33 in Wnt signaling during mesendoderm differentiation. Sci. China Life Sci. 2017, 60, 1142–1149. [Google Scholar] [CrossRef]
- Tarca, A.L.; Draghici, S.; Khatri, P.; Hassan, S.S.; Mittal, P.; Kim, J.-S.; Kim, C.J.; Kusanovic, J.P.; Romero, R. A novel signaling pathway impact analysis. Bioinformatics 2008, 25, 75–82. [Google Scholar] [CrossRef]
- Hothorn, T.; Lausen, B. Maximally selected rank statistics in R. R News 2002, 2, 3–5. [Google Scholar]
- Mo, X.; Niu, Q.; Ivanov, A.A.; Tsang, Y.H.; Tang, C.; Shu, C.; Li, Q.; Qian, K.; Wahafu, A.; Doyle, S.P.; et al. Systematic discovery of mutation-directed neo-protein-protein interactions in cancer. Cell 2022, 185, 1974–1985.e12. [Google Scholar] [CrossRef]
- Manohar, S.; Jacob, S.; Wang, J.; Wiechecki, K.A.; Koh, H.W.L.; Simões, V.; Choi, H.; Vogel, C.; Silva, G.M. Polyubiquitin chains linked by lysine residue 48 (K48) selectively target oxidized proteins in vivo. Antioxid. Redox Signal. 2019, 31, 1133–1149. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Ciechanover, A. The Ubiquitin Code in the Ubiquitin-Proteasome System and Autophagy. Trends Biochem. Sci. 2017, 42, 873–886. [Google Scholar] [CrossRef]
- Swatek, K.N.; Komander, D. Ubiquitin modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef] [PubMed]
- Vincent, D.F.; Yan, K.P.; Treilleux, I.; Gay, F.; Arfi, V.; Kaniewsky, B.; Marie, J.C.; Lepinasse, F.; Martel, S.; Goddard-Leon, S.; et al. Inactivation of TIF1gamma cooperates with Kras to in-duce cystic tumors of the pancreas. PLoS Genet. 2009, 5, e1000575. [Google Scholar] [CrossRef] [PubMed]
- Herquel, B.; Ouararhni, K.; Khetchoumian, K.; Ignat, M.; Teletin, M.; Mark, M.; Béchade, G.; Van Dorsselaer, A.; Sanglier-Cianférani, S.; Hamiche, A.; et al. Transcription cofactors TRIM24, TRIM28, and TRIM33 associate to form regulatory complexes that suppress murine hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 8212–8217. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, G.; Zhang, J.; Li, J.; Ruan, N.; Zhang, J.; Zhang, Z.; Chen, Y.; Zhang, Q.; Xia, Q. TRIM33 Overexpression Inhibits the Progression of Clear Cell Renal Cell Carcinoma In Vivo and In Vitro. BioMed Res. Int. 2020, 2020, 8409239. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Luo, R.X.; Dei Santi, A.; Postigo, A.A.; Dean, D.C. Cdk phosphorylation triggers sequential intramolecular interac-tions that progressively block Rb functions as cells move through G1. Cell 1999, 98, 859–869. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, R.S.; Brumbaugh, K.M.; Williams, J.M.; Sarkaria, J.N.; Cliby, W.A.; Shieh, S.Y.; Taya, Y.; Prives, C.; Abraham, R.T. A role for ATR in the DNA dam-age-induced phosphorylation of p53. Genes Dev. 1999, 13, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Kim, S.; Chowdhury, S.; Li, Z.; Yang, M.; Yoo, S.; Petralia, F.; Jacobsen, J.; Li, J.J.; Ge, X.; et al. DreamAI: Algorithm for the imputation of proteomics data. bioRxiv 2020. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M., 3rd; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef]
- Zhang, Z.; Hernandez, K.; Savage, J.; Li, S.; Miller, D.; Agrawal, S.; Ortuno, F.; Staudt, L.M.; Heath, A.; Grossman, R.L. Uniform genomic data analysis in the NCI Genomic Data Commons. Nat. Commun. 2021, 12, 1226. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Huang, C.; Zhou, D.C.; Hu, Y.; Lih, T.M.; Savage, S.R.; Krug, K.; Clark, D.J.; Schnaubelt, M.; Chen, L.; et al. Proteogenomic Characterization of Pancreatic Ductal Adenocarcinoma. Cell 2021, 184, 5031–5052. [Google Scholar] [CrossRef]
- Wang, L.-B.; Karpova, A.; Gritsenko, M.A.; Kyle, J.E.; Cao, S.; Li, Y.; Rykunov, D.; Colaprico, A.; Rothstein, J.H.; Hong, R.; et al. Proteogenomic and Metabolomic Characterization of Human Glioblastoma. Cancer Cell 2021, 39, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Pan, J.; Shah, P.; Ao, M.; Thomas, S.N.; Liu, Y.; Chen, L.; Schnaubelt, M.; Clark, D.J.; Rodriguez, H.; et al. Integrated Proteomic and Glycoproteomic Characterization of Human High-Grade Serous Ovarian Carcinoma. Cell Rep. 2020, 33, 108276. [Google Scholar] [CrossRef]
- Gillette, M.A.; Satpathy, S.; Cao, S.; Dhanasekaran, S.M.; Vasaikar, S.V.; Krug, K.; Petralia, F.; Li, Y.; Liang, W.-W.; Reva, B.; et al. Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell 2020, 182, 200–225. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, L.; Savage, S.R.; Eguez, R.V.; Dou, Y.; Li, Y.; da Veiga Leprevost, F.; Jaehnig, E.J.; Lei, J.T.; Wen, B.; et al. Proteogenomic Insights into the Biology and Treatment of HPV-Negative Head and Neck Squamous Cell Carcinoma. Cancer Cell 2021, 39, 361–379. [Google Scholar] [CrossRef]
- Dou, Y.; Kawaler, E.A.; Zhou, D.C.; Gritsenko, M.A.; Huang, C.; Blumenberg, L.; Karpova, A.; Petyuk, V.A.; Savage, S.R.; Satpathy, S.; et al. Proteogenomic Characterization of Endometrial Carcinoma. Cell 2020, 180, 729–748. [Google Scholar] [CrossRef]
- Krug, K.; Jaehnig, E.J.; Satpathy, S.; Blumenberg, L.; Karpova, A.; Anurag, M.; Miles, G.; Mertins, P.; Geffen, Y.; Tang, L.C.; et al. Proteogenomic Landscape of Breast Cancer Tumorigenesis and Targeted Therapy. Cell 2020, 183, 1436–1456. [Google Scholar] [CrossRef]
- Clark, D.J.; Dhanasekaran, S.M.; Petralia, F.; Pan, J.; Song, X.; Hu, Y.; da Veiga Leprevost, F.; Reva, B.; Lih, T.-S.M.; Chang, H.-Y.; et al. Integrated Proteogenomic Characterization of Clear Cell Renal Cell Carcinoma. Cell 2019, 179, 964–983. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.; Huang, C.; Wang, X.; Petyuk, V.A.; Savage, S.R.; Wen, B.; Dou, Y.; Zhang, Y.; Shi, Z.; Arshad, O.A.; et al. Proteogenomic Analysis of Human Colon Cancer Reveals New Therapeutic Opportunities. Cell 2019, 177, 1035–1049. [Google Scholar] [CrossRef]
- Gao, Q.; Zhu, H.; Dong, L.; Shi, W.; Chen, R.; Song, Z.; Huang, C.; Li, J.; Dong, X.; Zhou, Y.; et al. Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma. Cell 2019, 179, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.U. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2020, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Povey, S.; Lovering, R.; Bruford, E.; Wright, M.; Lush, M.; Wain, H. The HUGO Gene Nomenclature Committee (HGNC). Hum. Genet. 2001, 109, 678–680. [Google Scholar] [CrossRef]
- Ge, Z.; Leighton, J.S.; Wang, Y.; Peng, X.; Chen, Z.; Chen, H.; Sun, Y.; Yao, F.; Li, J.; Zhang, H.; et al. Integrated Genomic Analysis of the Ubiquitin Pathway across Cancer Types. Cell Rep. 2018, 23, 213–226. [Google Scholar] [CrossRef]
- Wu, X.-T.; Wang, Y.-H.; Cai, X.-Y.; Dong, Y.; Cui, Q.; Zhou, Y.-N.; Yang, X.-W.; Lu, W.-F.; Zhang, M. RNF115 Promotes Lung Adenocarcinoma through Wnt/β-Catenin Pathway Activation by Mediating APC Ubiquitination. Cancer Metab. 2021, 9, 7. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]

| Cancer Type | PDC Study Name | PDC Study ID | Tumor Sample Size | Normal Sample Size | Paired or Not | Ubiquitinome |
|---|---|---|---|---|---|---|
| Lung Squamous Cell Carcinoma (LSCC) | CPTAC LSCC Discovery Study | PDC000234 | 108 | 99 | Paired | √ |
| Pancreatic Ductal Adenocarcinoma (PDAC) | CPTAC PDA Discovery Study | PDC000270 | 141 | 77 | Paired | ✕ |
| Glioblastoma (GBM) | CPTAC GBM Discovery Study | PDC000204 | 99 | 10 | Unpaired | ✕ |
| Ovarian Serous Cystadenocarcinoma (OV) | Prospective Ovarian JHU Proteome | PDC000110 | 83 | 23 | Unpaired | ✕ |
| Lung Adenocarcinoma (LUAD) | CPTAC LUAD Discovery Study | PDC000153 | 110 | 101 | Paired | ✕ |
| Head and Neck Squamous Cell Carcinoma (HNSCC) | CPTAC HNSCC Discovery Study | PDC000221 | 108 | 67 | Paired | ✕ |
| Uterine Corpus Endometrial Carcinoma (UCEC) | CPTAC UCEC Discovery Study | PDC000125 | 104 | 49 | Paired | ✕ |
| Breast Invasive Carcinoma (BRCA) | Prospective Breast BI Proteome | PDC000120 | 96 | 14 | Paired | ✕ |
| Clear Cell Renal Cell Carcinoma (CCRCC) | CPTAC CCRCC Discovery Study | PDC000127 | 110 | 83 | Paired | ✕ |
| Hepatocellular carcinoma (HCC) | HBV-Related Hepatocellular Carcinoma | PDC000198 | 159 | 159 | Paired | ✕ |
| Colon Adenocarcinoma (COAD) | Prospective Colon VU Proteome | PDC000109 | 104 | 100 | Paired | ✕ |
| Early Onset Gastric Cancer (EOGC) | Proteogenomics of Gastric Cancer | PDC000214 | 80 | 80 | Paired | ✕ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wan, J.; Li, S.; Tang, Y.; Lin, Y.-C.-D.; Ni, J.; Cai, X.; Yu, J.; Huang, H.-D.; Lee, T.-Y. Multi-Omics Characterization of E3 Regulatory Patterns in Different Cancer Types. Int. J. Mol. Sci. 2024, 25, 7639. https://doi.org/10.3390/ijms25147639
Li Z, Wan J, Li S, Tang Y, Lin Y-C-D, Ni J, Cai X, Yu J, Huang H-D, Lee T-Y. Multi-Omics Characterization of E3 Regulatory Patterns in Different Cancer Types. International Journal of Molecular Sciences. 2024; 25(14):7639. https://doi.org/10.3390/ijms25147639
Chicago/Turabian StyleLi, Zhongyan, Jingting Wan, Shangfu Li, Yun Tang, Yang-Chi-Dung Lin, Jie Ni, Xiaoxuan Cai, Jinhan Yu, Hsien-Da Huang, and Tzong-Yi Lee. 2024. "Multi-Omics Characterization of E3 Regulatory Patterns in Different Cancer Types" International Journal of Molecular Sciences 25, no. 14: 7639. https://doi.org/10.3390/ijms25147639
APA StyleLi, Z., Wan, J., Li, S., Tang, Y., Lin, Y.-C.-D., Ni, J., Cai, X., Yu, J., Huang, H.-D., & Lee, T.-Y. (2024). Multi-Omics Characterization of E3 Regulatory Patterns in Different Cancer Types. International Journal of Molecular Sciences, 25(14), 7639. https://doi.org/10.3390/ijms25147639






