The Effect of Alternative Splicing Sites on Mirtron Formation and Arm Selection of Precursor microRNAs
Abstract
1. Introduction
2. Results
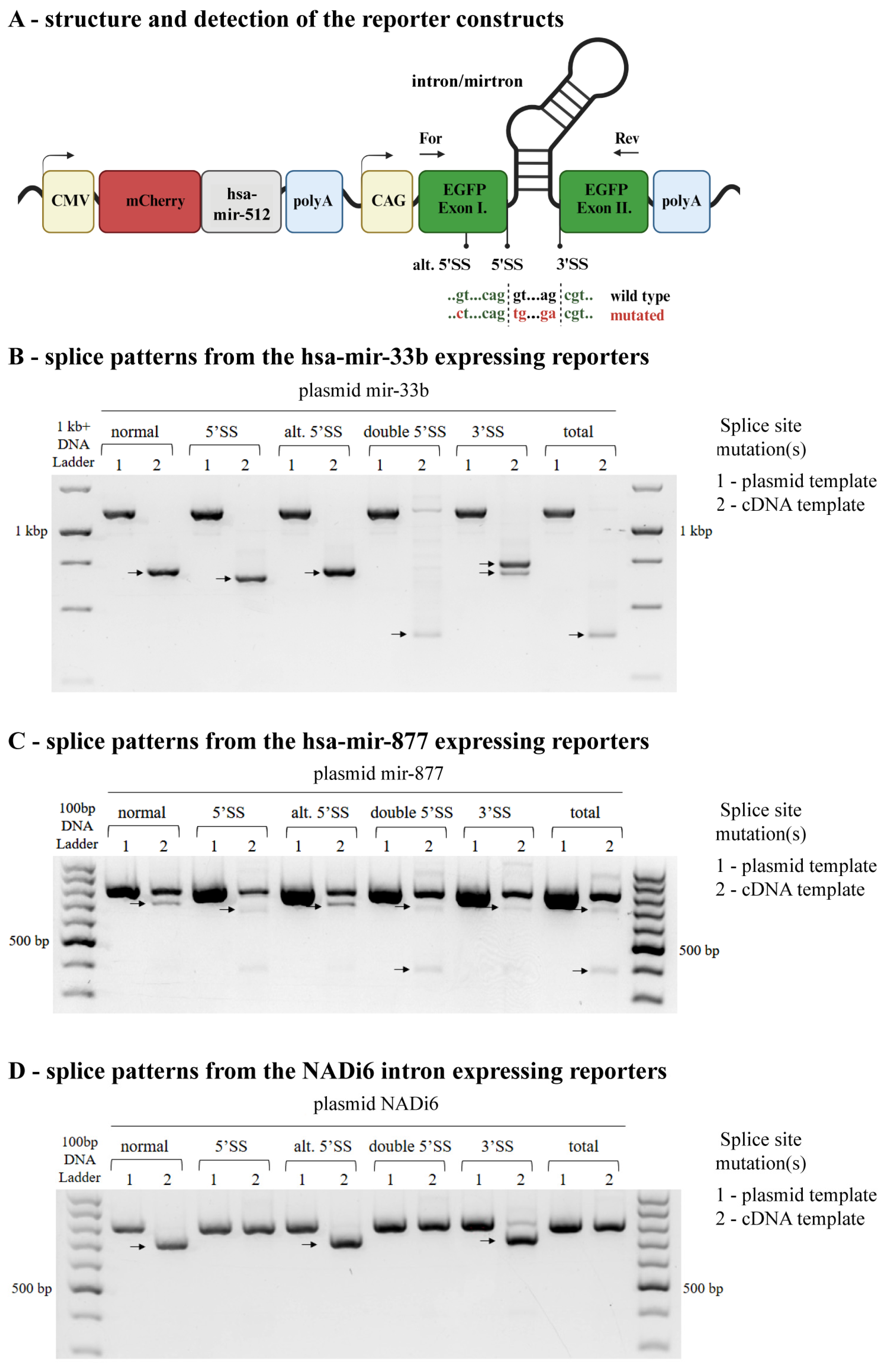
2.1. Mutations in the 5′ or 3′ Splice Sites Initiate the Usage of Alternative Donor or Acceptor Sites
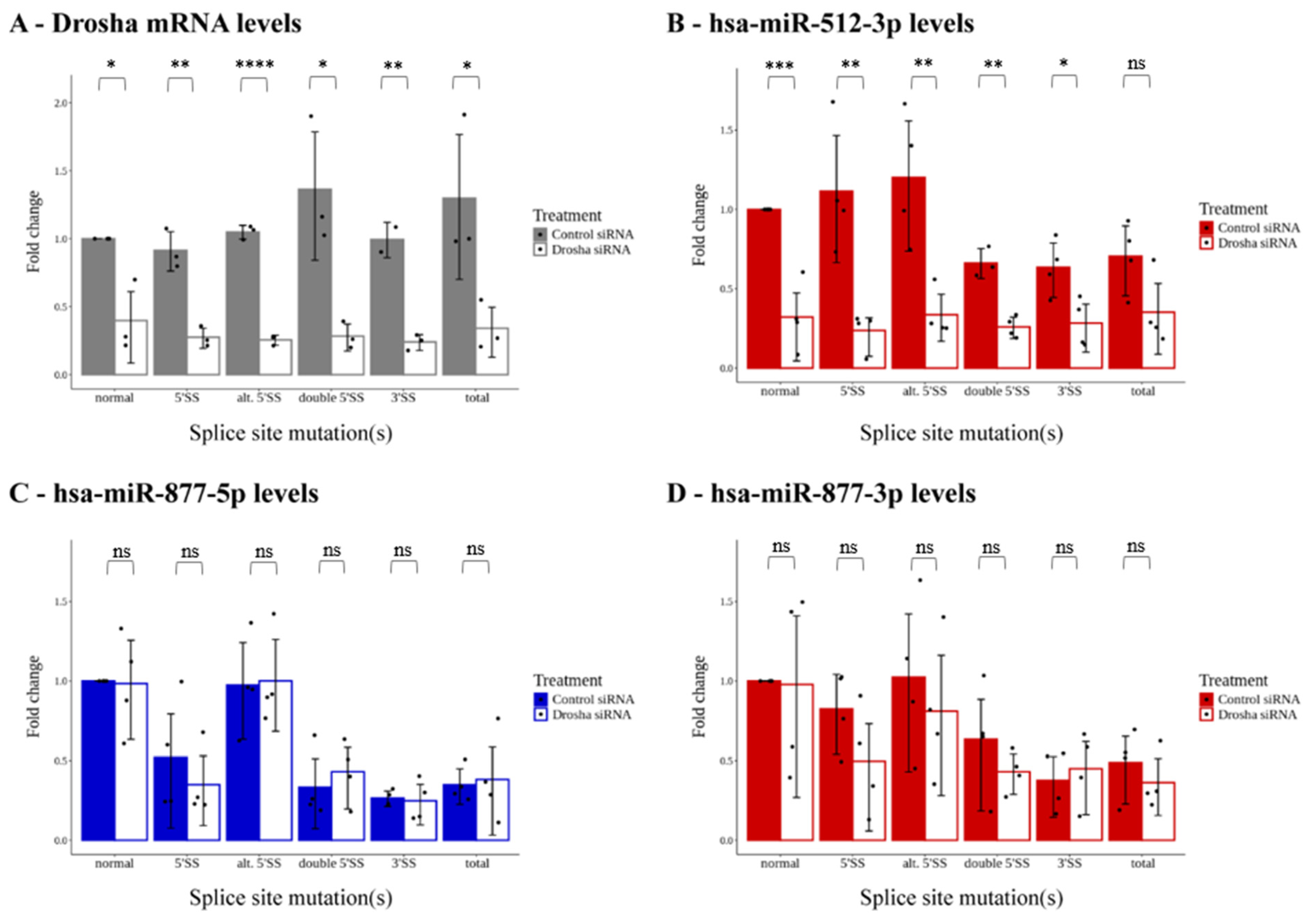
2.2. Interactions between the Splicing Apparatus and the miRNA Maturation Pathway
2.3. Arm Selection of Mirtron-Originated miRNAs Is Determined via Drosha-Independent Mechanisms
3. Discussion
4. Materials and Methods
4.1. Plasmid Constructs
4.2. Cell Culturing and Treatments
4.3. RNA Extraction
4.4. Reverse Transcription
4.5. Splicing Detection Assay
4.6. Quantitative Real-Time PCR (qPCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Trabucchi, M.; Mategot, R. Subcellular Heterogeneity of the microRNA Machinery. Trends Genet. 2019, 35, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009, 10, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Rana, R.; Chhabra, A.; Jaiswal, A.; Rani, V. miRNA-transcription factor interactions: A combinatorial regulation of gene expression. Mol. Genet. Genom. 2013, 288, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Leitao, A.L.; Enguita, F.J. A Structural View of miRNA Biogenesis and Function. Non-Coding RNA 2022, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Keller, A.; Meese, E. The miRNA-target interactions: An underestimated intricacy. Nucleic Acids Res. 2024, 52, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef]
- Berezikov, E. Evolution of microRNA diversity and regulation in animals. Nat. Rev. Genet. 2011, 12, 846–860. [Google Scholar] [CrossRef]
- Pasquinelli, A.E. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012, 13, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Farberov, L.; Ionescu, A.; Zoabi, Y.; Shapira, G.; Ibraheem, A.; Azan, Y.; Perlson, E.; Shomron, N. Multiple Copies of microRNA Binding Sites in Long 3’UTR Variants Regulate Axonal Translation. Cells 2023, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef]
- Miyoshi, K.; Miyoshi, T.; Siomi, H. Many ways to generate microRNA-like small RNAs: Non-canonical pathways for microRNA production. Mol. Genet. Genom. 2010, 284, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.G.; Jan, C.H.; Bartel, D.P. Intronic microRNA precursors that bypass Drosha processing. Nature 2007, 448, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Hagen, J.W.; Duan, H.; Tyler, D.M.; Lai, E.C. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 2007, 130, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.J.; Agius, P.; Westholm, J.O.; Chen, M.; Okamura, K.; Robine, N.; Leslie, C.S.; Lai, E.C. Computational and experimental identification of mirtrons in Drosophila melanogaster and Caenorhabditis elegans. Genome Res. 2011, 21, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Berezikov, E.; Chung, W.J.; Willis, J.; Cuppen, E.; Lai, E.C. Mammalian mirtron genes. Mol. Cell 2007, 28, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Babiarz, J.E.; Ruby, J.G.; Wang, Y.; Bartel, D.P.; Blelloch, R. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes. Dev. 2008, 22, 2773–2785. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Shao, C. Large-scale identification of mirtrons in Arabidopsis and rice. PLoS ONE 2012, 7, e31163. [Google Scholar] [CrossRef]
- Zhu, Q.H.; Spriggs, A.; Matthew, L.; Fan, L.; Kennedy, G.; Gubler, F.; Helliwell, C. A diverse set of microRNAs and microRNA-like small RNAs in developing rice grains. Genome Res. 2008, 18, 1456–1465. [Google Scholar] [CrossRef]
- Glazov, E.A.; Cottee, P.A.; Barris, W.C.; Moore, R.J.; Dalrymple, B.P.; Tizard, M.L. A microRNA catalog of the developing chicken embryo identified by a deep sequencing approach. Genome Res. 2008, 18, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Glazov, E.A.; Kongsuwan, K.; Assavalapsakul, W.; Horwood, P.F.; Mitter, N.; Mahony, T.J. Repertoire of bovine miRNA and miRNA-like small regulatory RNAs expressed upon viral infection. PLoS ONE 2009, 4, e6349. [Google Scholar] [CrossRef] [PubMed]
- Schamberger, A.; Orban, T.I. Experimental validation of predicted mammalian microRNAs of mirtron origin. Methods Mol. Biol. 2014, 1182, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Schamberger, A.; Sarkadi, B.; Orban, T.I. Human mirtrons can express functional microRNAs simultaneously from both arms in a flanking exon-independent manner. RNA Biol. 2012, 9, 1177–1185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sibley, C.R.; Seow, Y.; Saayman, S.; Dijkstra, K.K.; El Andaloussi, S.; Weinberg, M.S.; Wood, M.J. The biogenesis and characterization of mammalian microRNAs of mirtron origin. Nucleic Acids Res. 2012, 40, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Havens, M.A.; Reich, A.A.; Duelli, D.M.; Hastings, M.L. Biogenesis of mammalian microRNAs by a non-canonical processing pathway. Nucleic Acids Res. 2012, 40, 4626–4640. [Google Scholar] [CrossRef] [PubMed]
- Curtis, H.J.; Sibley, C.R.; Wood, M.J. Mirtrons, an emerging class of atypical miRNA. Wiley Interdiscip. Rev. RNA 2012, 3, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Ladewig, E.; Okamura, K.; Flynt, A.S.; Westholm, J.O.; Lai, E.C. Discovery of hundreds of mirtrons in mouse and human small RNA data. Genome Res. 2012, 22, 1634–1645. [Google Scholar] [CrossRef]
- Kock, K.H.; Kong, K.W.; Hoon, S.; Seow, Y. Functional VEGFA knockdown with artificial 3’-tailed mirtrons defined by 5’ splice site and branch point. Nucleic Acids Res. 2015, 43, 6568–6578. [Google Scholar] [CrossRef]
- Wen, J.; Ladewig, E.; Shenker, S.; Mohammed, J.; Lai, E.C. Analysis of Nearly One Thousand Mammalian Mirtrons Reveals Novel Features of Dicer Substrates. PLoS Comput. Biol. 2015, 11, e1004441. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; You, X.; Chen, T.; Mackowiak, S.D.; Friedlander, M.R.; Weigt, M.; Du, H.; Gogol-Doring, A.; Chang, Z.; Dieterich, C.; et al. Global profiling of miRNAs and the hairpin precursors: Insights into miRNA processing and novel miRNA discovery. Nucleic Acids Res. 2013, 41, 3619–3634. [Google Scholar] [CrossRef] [PubMed]
- Flynt, A.S.; Greimann, J.C.; Chung, W.J.; Lima, C.D.; Lai, E.C. MicroRNA biogenesis via splicing and exosome-mediated trimming in Drosophila. Mol. Cell 2010, 38, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Schamberger, A.; Varady, G.; Fothi, A.; Orban, T.I. Posttranscriptional Regulation of the Human ABCG2 Multidrug Transporter Protein by Artificial Mirtrons. Genes 2021, 12, 1068. [Google Scholar] [CrossRef]
- Orlans, H.O.; McClements, M.E.; Barnard, A.R.; Martinez-Fernandez de la Camara, C.; MacLaren, R.E. Mirtron-mediated RNA knockdown/replacement therapy for the treatment of dominant retinitis pigmentosa. Nat. Commun. 2021, 12, 4934. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25, 1469. [Google Scholar] [CrossRef] [PubMed]
- Kwok, Z.H.; Zhang, B.; Chew, X.H.; Chan, J.J.; Teh, V.; Yang, H.; Kappei, D.; Tay, Y. Systematic Analysis of Intronic miRNAs Reveals Cooperativity within the Multicomponent FTX Locus to Promote Colon Cancer Development. Cancer Res. 2021, 81, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, V.N. Processing of intronic microRNAs. EMBO J. 2007, 26, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, N.; Fujita, M.; Ohno, M. Functional association of the Microprocessor complex with the spliceosome. Mol. Cell Biol. 2009, 29, 3243–3254. [Google Scholar] [CrossRef]
- Salim, U.; Kumar, A.; Kulshreshtha, R.; Vivekanandan, P. Biogenesis, characterization, and functions of mirtrons. Wiley Interdiscip. Rev. RNA 2022, 13, e1680. [Google Scholar] [CrossRef]
- Han, J.; Lee, Y.; Yeom, K.H.; Nam, J.W.; Heo, I.; Rhee, J.K.; Sohn, S.Y.; Cho, Y.; Zhang, B.T.; Kim, V.N. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 2006, 125, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, C.; Pianigiani, G.; Pagani, F. Cross talk between spliceosome and microprocessor defines the fate of pre-mRNA. Wiley Interdiscip. Rev. RNA 2014, 5, 647–658. [Google Scholar] [CrossRef]
- Rasschaert, P.; Figueroa, T.; Dambrine, G.; Rasschaert, D.; Laurent, S. Alternative splicing of a viral mirtron differentially affects the expression of other microRNAs from its cluster and of the host transcript. RNA Biol. 2016, 13, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Marasco, L.E.; Kornblihtt, A.R. The physiology of alternative splicing. Nat. Rev. Mol. Cell Biol. 2023, 24, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Riolo, G.; Cantara, S.; Ricci, C. What’s Wrong in a Jump? Prediction and Validation of Splice Site Variants. Methods Protoc. 2021, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Rogalska, M.E.; Vivori, C.; Valcarcel, J. Regulation of pre-mRNA splicing: Roles in physiology and disease, and therapeutic prospects. Nat. Rev. Genet. 2023, 24, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.H.; Rasko, J.E.J. Splice and Dice: Intronic microRNAs, Splicing and Cancer. Biomedicines 2021, 9, 1268. [Google Scholar] [CrossRef]
- Sun, Q.; Hao, Q.; Lin, Y.C.; Song, Y.J.; Bangru, S.; Arif, W.; Tripathi, V.; Zhang, Y.; Cho, J.H.; Freier, S.M.; et al. Antagonism between splicing and microprocessor complex dictates the serum-induced processing of lnc-MIRHG for efficient cell cycle reentry. RNA 2020, 26, 1603–1620. [Google Scholar] [CrossRef]
- Khanal, S.; de Cruz, M.; Strickland, B.; Mansfield, K.; Lai, E.C.; Flynt, A. A tailed mirtron promotes longevity in Drosophila. Nucleic Acids Res. 2024, 52, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Buccheri, V.; Pasulka, J.; Malik, R.; Loubalova, Z.; Taborska, E.; Horvat, F.; Roos Kulmann, M.I.; Jenickova, I.; Prochazka, J.; Sedlacek, R.; et al. Functional canonical RNAi in mice expressing a truncated Dicer isoform and long dsRNA. EMBO Rep. 2024, 25, 2896–2913. [Google Scholar] [CrossRef]
- Zapletal, D.; Taborska, E.; Pasulka, J.; Malik, R.; Kubicek, K.; Zanova, M.; Much, C.; Sebesta, M.; Buccheri, V.; Horvat, F.; et al. Structural and functional basis of mammalian microRNA biogenesis by Dicer. Mol. Cell 2022, 82, 4064–4079.e4013. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.C.; Kim, B.; Jang, H.; Kim, K.; Park, I.S.; Min, D.H.; Kim, V.N. Structural atlas of human primary microRNAs generated by SHAPE-MaP. Mol. Cell 2024, 84, 1158–1172.e1156. [Google Scholar] [CrossRef] [PubMed]
- Kolacsek, O.; Krizsik, V.; Schamberger, A.; Erdei, Z.; Apati, A.; Varady, G.; Mates, L.; Izsvak, Z.; Ivics, Z.; Sarkadi, B.; et al. Reliable transgene-independent method for determining Sleeping Beauty transposon copy numbers. Mob. DNA 2011, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gál, L.; Schamberger, A.; Wachtl, G.; Orbán, T.I. The Effect of Alternative Splicing Sites on Mirtron Formation and Arm Selection of Precursor microRNAs. Int. J. Mol. Sci. 2024, 25, 7643. https://doi.org/10.3390/ijms25147643
Gál L, Schamberger A, Wachtl G, Orbán TI. The Effect of Alternative Splicing Sites on Mirtron Formation and Arm Selection of Precursor microRNAs. International Journal of Molecular Sciences. 2024; 25(14):7643. https://doi.org/10.3390/ijms25147643
Chicago/Turabian StyleGál, Luca, Anita Schamberger, Gerda Wachtl, and Tamás I. Orbán. 2024. "The Effect of Alternative Splicing Sites on Mirtron Formation and Arm Selection of Precursor microRNAs" International Journal of Molecular Sciences 25, no. 14: 7643. https://doi.org/10.3390/ijms25147643
APA StyleGál, L., Schamberger, A., Wachtl, G., & Orbán, T. I. (2024). The Effect of Alternative Splicing Sites on Mirtron Formation and Arm Selection of Precursor microRNAs. International Journal of Molecular Sciences, 25(14), 7643. https://doi.org/10.3390/ijms25147643






