Pharmacotherapeutic Considerations on Telomere Biology: The Positive Effect of Pharmacologically Active Substances on Telomere Length
Abstract
:1. Introduction
2. Molecular Mechanisms of Ageing
3. Pharmacologically Active Substances Acting on the Cardiovascular System
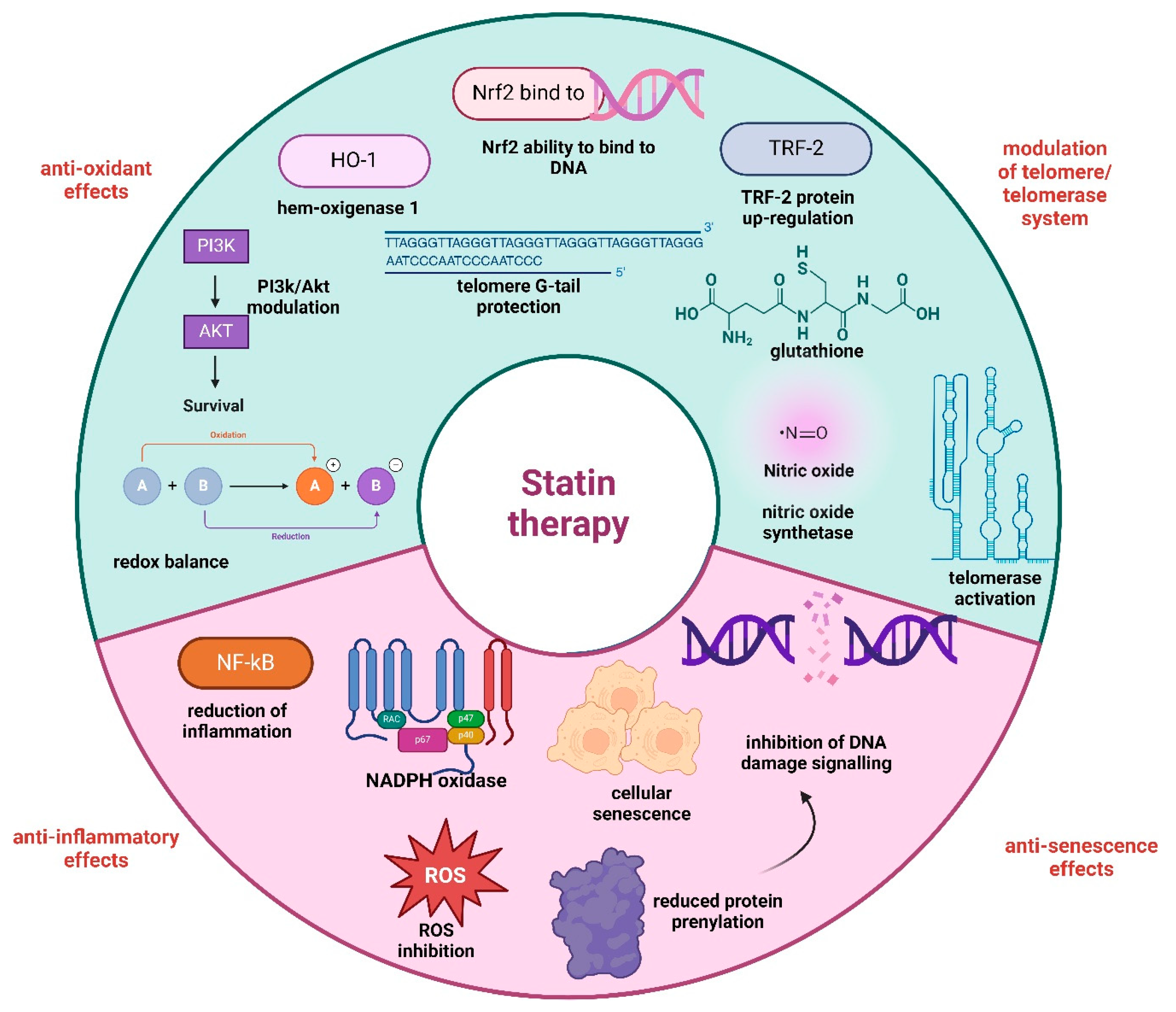
3.1. Statins
3.2. Calcium Channel Blockers (CCB)
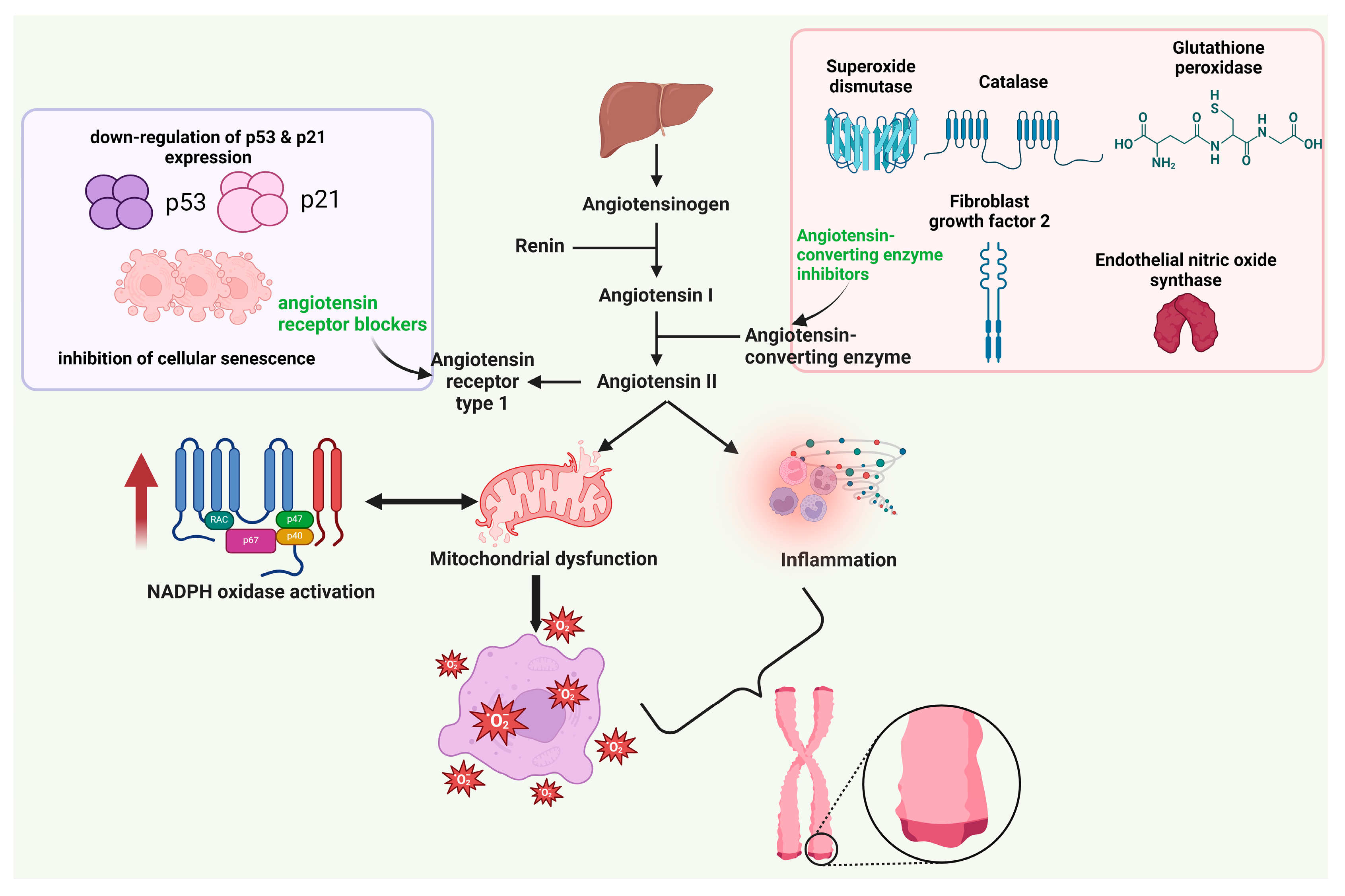
3.3. Agents Acting on the Renin-Angiotensin System (RAS)
4. Pharmacologically Active Substances Used in Diabetes
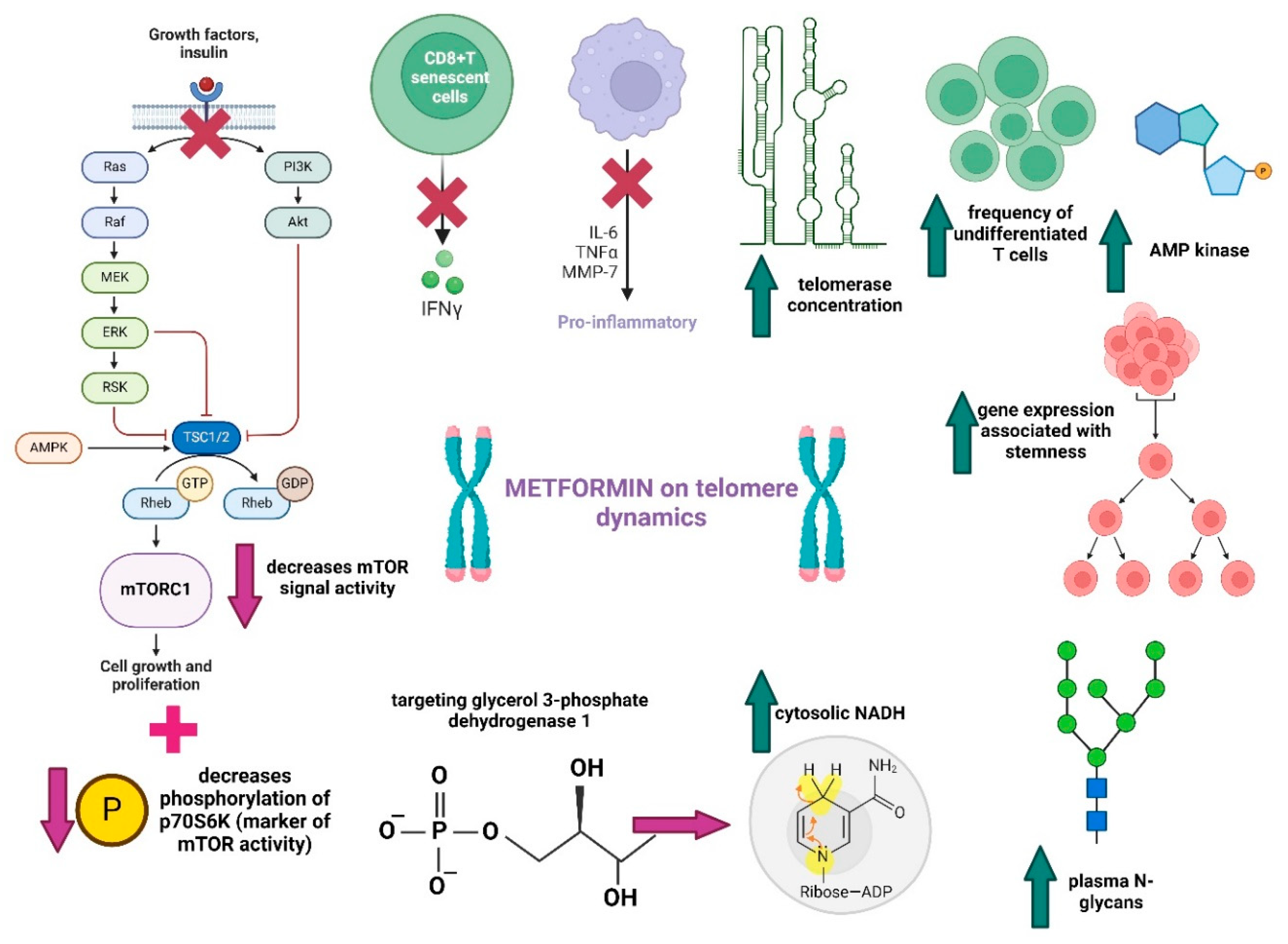
4.1. Biguanides
4.2. Dipeptidyl Peptidase-4 Inhibitors (DPP-4i)
5. Pharmacologically Active Substances Acting on the Central Nervous System
5.1. Antipsychotics
5.2. Melatonin
6. Pharmacologically Active Substances Acting as Modulators of the Genital System
6.1. Hormone Replacement Therapy
6.2. Antigonadotropins (Danazol)
7. Probiotics
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Revy, P.; Kannengiesser, C.; Bertuch, A.A. Genetics of Human Telomere Biology Disorders. Nat. Rev. Genet. 2023, 24, 86–108. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Renieri, E.; Tsoukalas, D.; Buga, A.; Sarandi, E.; Vakonaki, E.; Fragkiadaki, P.; Alegakis, A.; Nikitovic, D.; Calina, D.; et al. A Novel Nutraceutical Formulation Increases Telomere Length and Activates Telomerase Activity in Middle-aged Rats. Mol. Med. Rep. 2023, 28, 232. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, D.; Buga, A.; Docea, A.; Sarandi, E.; Mitrut, R.; Renieri, E.; Spandidos, D.; Rogoveanu, I.; Cercelaru, L.; Niculescu, M.; et al. Reversal of Brain Aging by Targeting Telomerase: A Nutraceutical Approach. Int. J. Mol. Med. 2021, 48, 199. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere Dysfunction in Ageing and Age-Related Diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Renieri, E.; Vakonaki, E.; Karzi, V.; Fragkiadaki, P.; Tsatsakis, A.M. Telomere Length: Associations with Nutrients and Xenobiotics. In Toxicological Risk Assessment and Multi-System Health Impacts from Exposure; Elsevier: Amsterdam, The Netherlands, 2021; pp. 295–306. [Google Scholar]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, Health, and Hallmarks of Aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, J.; Mihara, K.; Bhattacharjee, D.; Mukherjee, M. Telomere Length as a Potential Biomarker of Coronary Artery Disease. Indian J. Med. Res. 2017, 145, 730. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Carroll, L.; Joglekar, M.V.; Januszewski, A.S.; Wong, K.K.; Hardikar, A.A.; Jenkins, A.J.; Ma, R.C.W. Diabetes, Metabolic Disease, and Telomere Length. Lancet Diabetes Endocrinol. 2021, 9, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Fragkiadaki, P.; Nikitovic, D.; Kalliantasi, K.; Sarandi, E.; Thanasoula, M.; Stivaktakis, P.; Nepka, C.; Spandidos, D.; Theodoros, T.; Tsatsakis, A. Telomere Length and Telomerase Activity in Osteoporosis and Osteoarthritis (Review). Exp. Ther. Med. 2019, 19, 1626–1632. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, B.; Gispert, J.D.; Guigo, R.; Navarro, A.; Vilor-Tejedor, N.; Crous-Bou, M. Genetically Predicted Telomere Length and Its Relationship with Neurodegenerative Diseases and Life Expectancy. Comput. Struct. Biotechnol. J. 2022, 20, 4251–4256. [Google Scholar] [CrossRef]
- Pousa, P.A.; Souza, R.M.; Melo, P.H.M.; Correa, B.H.M.; Mendonça, T.S.C.; Simões-e-Silva, A.C.; Miranda, D.M. Telomere Shortening and Psychiatric Disorders: A Systematic Review. Cells 2021, 10, 1423. [Google Scholar] [CrossRef]
- Vasilopoulos, E.; Fragkiadaki, P.; Kalliora, C.; Fragou, D.; Docea, A.; Vakonaki, E.; Tsoukalas, D.; Calina, D.; Buga, A.; Georgiadis, G.; et al. The Association of Female and Male Infertility with Telomere Length (Review). Int. J. Mol. Med. 2019, 44, 375–389. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Oikonomopoulou, T.; Nikolouzakis, T.; Vakonaki, E.; Tzatzarakis, M.; Flamourakis, M.; Renieri, E.; Fragkiadaki, P.; Iliaki, E.; Bachlitzanaki, M.; et al. Role of Telomere Length in Human Carcinogenesis (Review). Int. J. Oncol. 2023, 63, 78. [Google Scholar] [CrossRef]
- Unni, E. Medicine Use in Chronic Diseases. Pharmacy 2023, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement. ACS Chem. Neurosci. 2024, 15, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.; Veronese, N.; Barbagallo, M. Magnesium and the Hallmarks of Aging. Nutrients 2024, 16, 496. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, Lifestyle, Cancer, and Aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, A.; O’Loghlen, A. Cellular Senescence and Ageing: Mechanisms and Interventions. Front. Aging 2022, 3, 866718. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular Mechanisms of Aging and Anti-Aging Strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef]
- Mc Auley, M.T.; Guimera, A.M.; Hodgson, D.; Mcdonald, N.; Mooney, K.M.; Morgan, A.E.; Proctor, C.J. Modelling the Molecular Mechanisms of Aging. Biosci. Rep. 2017, 37, BSR20160177. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic Regulation of Aging: Implications for Interventions of Aging and Diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.-W.; Lee, S.-J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef] [PubMed]
- Gopenath, T.S.; Shreshtha, S.; Basalingappa, K.M. Telomerase Reactivation for Anti-Aging. In Anti-Aging Drug Discovery on the Basis of Hallmarks of Aging; Elsevier: Amsterdam, The Netherlands, 2022; pp. 113–125. [Google Scholar]
- Razgonova, M.; Zakharenko, A.; Golokhvast, K.; Thanasoula, M.; Sarandi, E.; Nikolouzakis, K.; Fragkiadaki, P.; Tsoukalas, D.; Spandidos, D.; Tsatsakis, A. Telomerase and Telomeres in Aging Theory and Chronographic Aging Theory (Review). Mol. Med. Rep. 2020, 22, 1679–1694. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, D.; Fragkiadaki, P.; Docea, A.; Alegakis, A.; Sarandi, E.; Thanasoula, M.; Spandidos, D.; Tsatsakis, A.; Razgonova, M.; Calina, D. Discovery of Potent Telomerase Activators: Unfolding New Therapeutic and Anti-Aging Perspectives. Mol. Med. Rep. 2019, 20, 3701–3708. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Yu, Y.; Li, D.; Zhang, Y.; Zhang, K.; Tong, J.; Yang, K.; Jia, S. Alternative Lengthening of Telomeres and Mediated Telomere Synthesis. Cancers 2022, 14, 2194. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Arora, R.; Azzalin, C.M. The Alternative Lengthening of Telomeres Mechanism Jeopardizes Telomere Integrity If Not Properly Restricted. Proc. Natl. Acad. Sci. USA 2022, 119, e2208669119. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular Senescence: A Key Therapeutic Target in Aging and Diseases. J. Clin. Investig. 2022, 132, 158450. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Wang, C.-Y. Telomere Attrition and Clonal Hematopoiesis of Indeterminate Potential in Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 9867. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Richardson, G.; Haendeler, J.; Altschmied, J.; Andrés, V.; Spyridopoulos, I. Telomerase as a Therapeutic Target in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, Z.; Su, X.; Da, M.; Yang, Z.; Duan, W.; Mo, X. Association between Leucocyte Telomere Length and Cardiovascular Disease in a Large General Population in the United States. Sci. Rep. 2020, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte Telomere Length and Risk of Cardiovascular Disease: Systematic Review and Meta-Analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-K.; Wang, C.-Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Paolisso, G. The Association between Statins and Telomere Shortening. Clin. Lipidol. 2014, 9, 311–315. [Google Scholar] [CrossRef]
- Zhuang, X.-D.; Liao, L.-Z.; Guo, Y.; Li, Y.; Liao, X.-X.; Hu, X.; Du, Z.-M. Rationale and Design of RETAIN Study: Rosuvastatin Effect on Telomere–Telomerase System in Acute Coronary Syndrome Patients Undergoing Percutaneous Coronary Intervention. Int. J. Cardiol. 2015, 184, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Martynowicz, H.; Gać, P.; Kornafel-Flak, O.; Filipów, S.; Łaczmański, Ł.; Sobieszczańska, M.; Mazur, G.; Porȩba, R. The Relationship Between the Effectiveness of Blood Pressure Control and Telomerase Reverse Transcriptase Concentration, Adipose Tissue Hormone Concentration and Endothelium Function in Hypertensives. Hear Lung Circ. 2020, 29, e200–e209. [Google Scholar] [CrossRef]
- Vasan, R.S.; Demissie, S.; Kimura, M.; Cupples, L.A.; Rifai, N.; White, C.; Wang, T.J.; Gardner, J.P.; Cao, X.; Benjamin, E.J.; et al. Association of Leukocyte Telomere Length with Circulating Biomarkers of the Renin-Angiotensin-Aldosterone System. Circulation 2008, 117, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Nemtsova, V.; Bondar, T.; Shalimova, A. Effect of Achieving Blood Pressure Targets on the Relative Telomere Length in Hypertensive Patients with and without Type 2 Diabetes Mellitus. Arter. Hypertens. 2020, 24, 61–66. [Google Scholar] [CrossRef]
- Harvey, A.; Montezano, A.C.; Touyz, R.M. Vascular Biology of Ageing—Implications in Hypertension. J. Mol. Cell. Cardiol. 2015, 83, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Dayar, E.; Pechanova, O. Targeted Strategy in Lipid-Lowering Therapy. Biomedicines 2022, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and Cons. Med. Clin. 2018, 150, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Morofuji, Y.; Nakagawa, S.; Ujifuku, K.; Fujimoto, T.; Otsuka, K.; Niwa, M.; Tsutsumi, K. Beyond Lipid-Lowering: Effects of Statins on Cardiovascular and Cerebrovascular Diseases and Cancer. Pharmaceuticals 2022, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Rawat, U.; Kumar, P.; Mittal, P. Pleotropic Effects of Statins: The Dilemma of Wider Utilization of Statin. Egypt. Hear J. 2023, 75, 1. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Long, A.; Wang, J.; Wang, X. Effects of Oral Atorvastatin on Inflammatory Markers and Postoperative Delirium in Elderly Patients with Hip Fracture Surgery. Farmacia 2022, 70, 944–953. [Google Scholar] [CrossRef]
- Nose, D.; Shiga, Y.; Takahashi, R.; Yamamoto, Y.; Suematsu, Y.; Kuwano, T.; Sugihara, M.; Kanda, M.; Tahara, H.; Miura, S. Association Between Telomere G-Tail Length and Coronary Artery Disease or Statin Treatment in Patients with Cardiovascular Risks—A Cross-Sectional Study—. Circ. Rep. 2023, 5, 338–347. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Banach, M.; Penson, P.E.; Jamialahmadi, T.; Sahebkar, A. Statin-Induced Nitric Oxide Signaling: Mechanisms and Therapeutic Implications. J. Clin. Med. 2019, 8, 2051. [Google Scholar] [CrossRef]
- Mansouri, A.; Reiner, Ž.; Ruscica, M.; Tedeschi-Reiner, E.; Radbakhsh, S.; Bagheri Ekta, M.; Sahebkar, A. Antioxidant Effects of Statins by Modulating Nrf2 and Nrf2/HO-1 Signaling in Different Diseases. J. Clin. Med. 2022, 11, 1313. [Google Scholar] [CrossRef]
- Jang, H.J.; Hong, E.M.; Kim, M.; Kim, J.H.; Jang, J.; Park, S.W.; Byun, H.W.; Koh, D.H.; Choi, M.H.; Kae, S.H.; et al. Simvastatin Induces Heme Oxygenase-1 via NF-E2-Related Factor 2 (Nrf2) Activation through ERK and PI3K/Akt Pathway in Colon Cancer. Oncotarget 2016, 7, 46219–46229. [Google Scholar] [CrossRef]
- Sugimoto, M.; Ko, R.; Goshima, H.; Koike, A.; Shibano, M.; Fujimori, K. Formononetin Attenuates H2O2-Induced Cell Death through Decreasing ROS Level by PI3K/Akt-Nrf2-Activated Antioxidant Gene Expression and Suppressing MAPK-Regulated Apoptosis in Neuronal SH-SY5Y Cells. Neurotoxicology 2021, 85, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Mazzanti, I.; Abbatecola, M.A.; Recchioni, R.; Marcheselli, F.; Procopio, D.A.; Antonicelli, R. Telomere/Telomerase System: A New Target of Statins Pleiotropic Effect? Curr. Vasc. Pharmacol. 2012, 10, 216–224. [Google Scholar] [CrossRef]
- Zaky, M.Y.; Fan, C.; Zhang, H.; Sun, X.-F. Unraveling the Anticancer Potential of Statins: Mechanisms and Clinical Significance. Cancers 2023, 15, 4787. [Google Scholar] [CrossRef]
- Tan, X.W.; Fong, A.Y.Y. Telomere and Telomerase Biology in Cardiovascular Disease: A State-of-the-Art Review and Outlook. J. Asian Pacific Soc. Cardiol. 2023, 2, e46. [Google Scholar] [CrossRef]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the Evidence for the Efficacy and Safety of Statin Therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef]
- Cheng, J. Statin Therapy Decreased the Recurrence Frequency of Atrial Fibrillation after Electrical Cardioversion: A Meta-Analysis. Med. Sci. Monit. 2014, 20, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A. Statins and Telomere Length: Risk Assessment and Management for Coronary Heart Disease. Expert Opin. Ther. Targets 2007, 11, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Bennaceur, K.; Atwill, M.; Al Zhrany, N.; Hoffmann, J.; Keavney, B.; Breault, D.; Richardson, G.; von Zglinicki, T.; Saretzki, G.; Spyridopoulos, I. Atorvastatin Induces T Cell Proliferation by a Telomerase Reverse Transcriptase (TERT) Mediated Mechanism. Atherosclerosis 2014, 236, 312–320. [Google Scholar] [CrossRef]
- Strazhesko, I.D.; Tkacheva, O.N.; Akasheva, D.U.; Dudinskaya, E.N.; Plokhova, E.V.; Pykhtina, V.S.; Kruglikova, A.S.; Kokshagina, N.V.; Sharashkina, N.V.; Agaltsov, M.V.; et al. Atorvastatin Therapy Modulates Telomerase Activity in Patients Free of Atherosclerotic Cardiovascular Diseases. Front. Pharmacol. 2016, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- McKeever, R.; Hamilton, R. Calcium Channel Blockers; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Elliott, W.J.; Ram, C.V.S. Calcium Channel Blockers. J. Clin. Hypertens. 2011, 13, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, N.; Zhou, M.; Guo, J.; Zhu, C.; Zhou, J.; Ma, M.; He, L. Calcium Channel Blockers versus Other Classes of Drugs for Hypertension. Cochrane Database Syst. Rev. 2022, 2022, CD003654. [Google Scholar] [CrossRef]
- Haller, H. Effective Management of Hypertension with Dihydropyridine Calcium Channel Blocker-Based Combination Therapy in Patients at High Cardiovascular Risk. Int. J. Clin. Pract. 2008, 62, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Yamaguchi, T.; Sakakibara, Y.; Taguchi, K.; Maeda, M.; Kuzuya, M.; Hattori, Y. ENOS-Dependent Antisenscence Effect of a Calcium Channel Blocker in Human Endothelial Cells. PLoS ONE 2014, 9, e88391. [Google Scholar] [CrossRef] [PubMed]
- Godfraind, T. Calcium-Channel Modulators for Cardiovascular Disease. Expert Opin. Emerg. Drugs 2006, 11, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, X.; Wang, Y.; Sjölander, A.; Johnell, K.; Thambisetty, M.; Ferrucci, L.; Reynolds, C.A.; Finkel, D.; Jylhävä, J.; et al. Longitudinal Associations between Use of Antihypertensive, Antidiabetic, and Lipid-Lowering Medications and Biological Aging. GeroScience 2023, 45, 2065–2078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Li, R.X.; Yang, Y.Y.; Chen, Y.; Yang, S.J.; Li, J.; Fu, L.; Hui, R.T.; Zhang, W.L. P1693The Longitudinal Associations between Telomere Attrition and the Effects of Blood Pressure Lowering and Antihypertensive Treatment. Eur. Heart J. 2019, 40, ehz748.0448. [Google Scholar] [CrossRef]
- Ramalingam, L.; Menikdiwela, K.; LeMieux, M.; Dufour, J.M.; Kaur, G.; Kalupahana, N.; Moustaid-Moussa, N. The Renin Angiotensin System, Oxidative Stress and Mitochondrial Function in Obesity and Insulin Resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1106–1114. [Google Scholar] [CrossRef]
- Maranduca, M.A.; Cosovanu, M.A.; Clim, A.; Pinzariu, A.C.; Filip, N.; Drochioi, I.C.; Vlasceanu, V.I.; Timofte, D.V.; Nemteanu, R.; Plesa, A.; et al. The Renin-Angiotensin System: The Challenge behind Autoimmune Dermatological Diseases. Diagnostics 2023, 13, 3398. [Google Scholar] [CrossRef]
- Yi, W.; Chen, F.; Zhang, H.; Tang, P.; Yuan, M.; Wen, J.; Wang, S.; Cai, Z. Role of Angiotensin II in Aging. Front. Aging Neurosci. 2022, 14, 1002138. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-K.; Lin, M.-H.; Wang, C.-Y. Telomeres as Therapeutic Targets in Heart Disease. JACC Basic Transl. Sci. 2019, 4, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.M. Angiotensin Receptor Blockers Are Not Just for Hypertension Anymore. Physiology 2021, 36, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Kim, S.; Jo, Y.; Kim, Y.; Ye, B.S.; Yu, Y.M. Neuroprotective Effect of Angiotensin II Receptor Blockers on the Risk of Incident Alzheimer’s Disease: A Nationwide Population-Based Cohort Study. Front. Aging Neurosci. 2023, 15, 1137197. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.R.; Anderson, S. The Aging Kidney: Physiological Changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Aoulad Fares, D.; Wiegel, R.E.; Eggink, A.J.; van Meurs, J.B.J.; Willemsen, S.P.; Danser, A.H.J.; Steegers-Theunissen, R.P.M. First-Trimester Maternal Renin-Angiotensin-Aldosterone System Activation and the Association with Maternal Telomere Length after Natural and IVF/ICSI Conceived Pregnancies: The Rotterdam Periconception Cohort. Hypertens. Pregnancy 2023, 42, 2238086. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Bartholome, R.; Peutz-Kootstra, C.J.; Smits, J.F.M.; Struijker-Boudier, H.A.J. Sustained Tubulo-Interstitial Protection in SHRs by Transient Losartan Treatment: An Effect of Decelerated Aging? Am. J. Hypertens. 2008, 21, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, L.; Li, Y. Change of Telomere Length in Angiotensin Ii-Induced Human Glomerular Mesangial Cell Senescence and the Protective Role of Losartan. Mol. Med. Rep. 2011, 4, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, F.; Tiitu, A.; Saijonmaa, O.; Forsblom, C.; Groop, P.-H. Telomere Length and Progression of Diabetic Nephropathy in Patients with Type 1 Diabetes. J. Intern. Med. 2010, 267, 278–286. [Google Scholar] [CrossRef]
- Ancion, A.; Tridetti, J.; Nguyen Trung, M.-L.; Oury, C.; Lancellotti, P. A Review of the Role of Bradykinin and Nitric Oxide in the Cardioprotective Action of Angiotensin-Converting Enzyme Inhibitors: Focus on Perindopril. Cardiol. Ther. 2019, 8, 179–191. [Google Scholar] [CrossRef]
- Obtułowicz, K.; Góralska, J.; Bogdali, A.; Dyga, W.; Obtułowicz, A.; Myszkowska, D.; Ziemianin, M.; Gruca, A.; Solnica, B.; Czarnobilska, E. Bradykinin and Oxidative Stress in Patients with Hereditary Angioedema Due to C1 Inhibitor Deficiency. Polish Arch. Intern. Med. 2020, 130, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Donnini, S.; Terzuoli, E.; Ziche, M.; Morbidelli, L. Sulfhydryl Angiotensin-Converting Enzyme Inhibitor Promotes Endothelial Cell Survival through Nitric-Oxide Synthase, Fibroblast Growth Factor-2, and Telomerase Cross-Talk. J. Pharmacol. Exp. Ther. 2010, 332, 776–784. [Google Scholar] [CrossRef] [PubMed]
- De Vries, N.; Prestes, P.; Raipuria, M.; Byars, S.; Allen, A.; Harrap, S.; Charchar, F. A15812 ANGIOTENSIN CONVERTING ENZYME INHIBITORS EPIGENETICALLY ATENUATE TELOMERE SHORTENING. J. Hypertens. 2018, 36, e85. [Google Scholar] [CrossRef]
- Akinnibosun, O.; Prestes, P.; De Vries, N.; Raipuria, M.; Byars, S.; Allen, A.; Harrap, S.; Charchar, F. Investigation of Telomere Involvement in the Legacy Effect of Angiotensin Converting Enzyme Inhibitors in Spontaneously Hypertensive Rats. Hear Lung Circ. 2023, 32, S130. [Google Scholar] [CrossRef]
- WHO. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 15 April 2020).
- Farmaki, P.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Savvanis, S.; Diamantis, E. Complications of the Type 2 Diabetes Mellitus. Curr. Cardiol. Rev. 2021, 16, 249–251. [Google Scholar] [CrossRef]
- Tilinca, M.C.; Antal, C.; Sălcudean, A.; Abălașei, B.L.; Fărcaș, R.M.; Groșan, A. New Directions in Pharmacological Treatment with SGLT-2 Inhibitor Molecules in the Light of Current Guidelines for Diabetes Mellitus, Heart Failure and Kidney Disease. Farmacia 2023, 71, 686–696. [Google Scholar] [CrossRef]
- Abdulsaied, R.A.; Jabbar, A.S.; Akar, T.K. Disorders of Pulmonary Function in Type 2 Diabetes Mellitus Patients with Different Types of Oral Hypoglycemic Medications: Metformin, Metformin Plus Thiazolidinedione, and Metformin Plus Sulfonylurea. Farmacia 2022, 70, 1050–1056. [Google Scholar] [CrossRef]
- Cheng, F.; Luk, A.O.; Shi, M.; Huang, C.; Jiang, G.; Yang, A.; Wu, H.; Lim, C.K.P.; Tam, C.H.T.; Fan, B.; et al. Shortened Leukocyte Telomere Length Is Associated with Glycemic Progression in Type 2 Diabetes: A Prospective and Mendelian Randomization Analysis. Diabetes Care 2022, 45, 701–709. [Google Scholar] [CrossRef]
- Chaithanya, V.; Kumar, J.; Leela, K.V.; Murugesan, R.; Angelin, M.; Satheesan, A. Impact of Telomere Attrition on Diabetes Mellitus and Its Complications. Diabetes Epidemiol. Manag. 2023, 12, 100174. [Google Scholar] [CrossRef]
- Qin, B. Can Antidiabetic Medications Affect Telomere Length in Patients with Type 2 Diabetes? A Mini-Review. Diabetes Metab. Syndr. Obes. 2023, 16, 3739–3750. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, X.; Cao, L.; Sun, Y.; Qiu, Y.; Zhang, Y.; Cao, R.; Covasa, M.; Zhong, L. Association between Telomere Length and Diabetes Mellitus: A Meta-Analysis. J. Int. Med. Res. 2016, 44, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- Baltzis, D.; Meimeti, E.; Grammatikopoulou, M.; Roustit, M.; Mavrogonatou, E.; Kletsas, D.; Efraimidou, S.; Manes, C.; Nikolouzakis, T.; Tsiaoussis, J.; et al. Assessment of Telomerase Activity in Leukocytes of Type 2 Diabetes Mellitus Patients Having or Not Foot Ulcer: Possible Correlation with Other Clinical Parameters. Exp. Ther. Med. 2018, 15, 3420–3424. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Jhun, B.S.; Yoon, Y. High-Glucose Stimulation Increases Reactive Oxygen Species Production Through the Calcium and Mitogen-Activated Protein Kinase-Mediated Activation of Mitochondrial Fission. Antioxid. Redox Signal. 2011, 14, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Cuevas Diaz, P.; Nicolini, H.; Nolasco-Rosales, G.A.; Juarez Rojop, I.; Tovilla-Zarate, C.A.; Rodriguez Sanchez, E.; Genis-Mendoza, A.D. Telomere Shortening in Three Diabetes Mellitus Types in a Mexican Sample. Biomedicines 2023, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Izumiyama-Shimomura, N.; Kimbara, Y.; Nakamura, K.; Ishikawa, N.; Aida, J.; Chiba, Y.; Mori, S.; Arai, T.; Aizawa, T.; et al. β-Cell Telomere Attrition in Diabetes: Inverse Correlation Between HbA1c and Telomere Length. J. Clin. Endocrinol. Metab. 2014, 99, 2771–2777. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Monickaraj, F.; Aravind, S.; Gokulakrishnan, K.; Sathishkumar, C.; Prabu, P.; Prabu, D.; Mohan, V.; Balasubramanyam, M. Accelerated Aging as Evidenced by Increased Telomere Shortening and Mitochondrial DNA Depletion in Patients with Type 2 Diabetes. Mol. Cell. Biochem. 2012, 365, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cui, W.; Zhang, D.; Wu, W.; Yang, Z. The Shortening of Leukocyte Telomere Length Relates to DNA Hypermethylation of LINE-1 in Type 2 Diabetes Mellitus. Oncotarget 2017, 8, 73964–73973. [Google Scholar] [CrossRef]
- Ojeda-Rodriguez, A.; Alcala-Diaz, J.F.; Rangel-Zuñiga, O.A.; Arenas-de Larriva, A.P.; Gutierrez-Mariscal, F.M.; Torres-Peña, J.D.; Mora-Ortiz, M.; Romero-Cabrera, J.L.; Luque, R.M.; Ordovas, J.M.; et al. Telomere Maintenance Is Associated with Type 2 Diabetes Remission in Response to a Long-Term Dietary Intervention without Non-Weight Loss in Patients with Coronary Heart Disease: From the CORDIOPREV Randomized Controlled Trial. Antioxidants 2024, 13, 125. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, H.; Ping, F.; Li, W.; Li, Y. Insulin Treatment Affects Leukocyte Telomere Length in Patients with Type 2 Diabetes: 6-Year Longitudinal Study. J. Diabetes Complicat. 2019, 33, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Herrmann, M.; Almer, G.; Zelzer, S.; Moeller, R.; Horejsi, R.; Renner, W. Telomere Shortening Associates with Elevated Insulin and Nuchal Fat Accumulation. Sci. Rep. 2020, 10, 6863. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Retzik-Stahr, C.; Singh, V.; Plomondon, R.; Anderson, V.; Rasouli, N. Should Metformin Remain the First-Line Therapy for Treatment of Type 2 Diabetes? Ther. Adv. Endocrinol. Metab. 2021, 12, 204201882098022. [Google Scholar] [CrossRef] [PubMed]
- Apostu, A.; Buzatu, R.; Cabuta, M.; Macasoi, I.; Dinu, S.; Iftode, A.; Mânea, H.C.; Gaita, D.I.; Chiriac, S.D. In Vitro Assessment of the Potential Cytotoxic Effect of Metformin on Colorectal Cancer Cells. Farmacia 2023, 71, 50–57. [Google Scholar] [CrossRef]
- Chen, S.; Gan, D.; Lin, S.; Zhong, Y.; Chen, M.; Zou, X.; Shao, Z.; Xiao, G. Metformin in Aging and Aging-Related Diseases: Clinical Applications and Relevant Mechanisms. Theranostics 2022, 12, 2722–2740. [Google Scholar] [CrossRef] [PubMed]
- Son, D.-H.; Park, W.-J.; Lee, Y.-J. Recent Advances in Anti-Aging Medicine. Korean J. Fam. Med. 2019, 40, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Sevim, C.; Taghizadehghalehjoughi, A.; Kara, M.; Nosyrev, A.E.; Nițulescu, G.M.; Margină, D.; Tsatsakis, A. Investigation of the Effects of Metformin on the miR21/PTEN/Akt Pathway in HT-29 Human Colorectal Adenocarcinoma Cell and HUVEC Co-Culture. Farmacia 2024, 72, 60–65. [Google Scholar]
- Mohammed, I.; Hollenberg, M.D.; Ding, H.; Triggle, C.R. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front. Endocrinol. 2021, 12, 718942. [Google Scholar] [CrossRef] [PubMed]
- Chrienova, Z.; Nepovimova, E.; Kuca, K. The Role of MTOR in Age-Related Diseases. J. Enzyme Inhib. Med. Chem. 2021, 36, 1678–1692. [Google Scholar] [CrossRef]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-Dose Metformin Targets the Lysosomal AMPK Pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on Mechanisms of Action and Repurposing Potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Birnbaum, M.J. Control of Gluconeogenesis by Metformin: Does Redox Trump Energy Charge? Cell Metab. 2014, 20, 197–199. [Google Scholar] [CrossRef]
- Luo, S.; Wong, I.C.K.; Chui, C.S.L.; Zheng, J.; Huang, Y.; Schooling, C.M.; Yeung, S.L.A. Effects of Putative Metformin Targets on Phenotypic Age and Leukocyte Telomere Length: A Mendelian Randomisation Study Using Data from the UK Biobank. Lancet Heal. Longev. 2023, 4, e337–e344. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.-C.; Zhang, J.-Q.; Zou, J.-Y.; Zhang, X.; Chen, W.-M.; Gu, Y.; Hong, H. The Effect of Metformin on Senescence of T Lymphocytes. Immun. Ageing 2023, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Vigili de Kreutzenberg, S.; Ceolotto, G.; Cattelan, A.; Pagnin, E.; Mazzucato, M.; Garagnani, P.; Borelli, V.; Bacalini, M.G.; Franceschi, C.; Fadini, G.P.; et al. Metformin Improves Putative Longevity Effectors in Peripheral Mononuclear Cells from Subjects with Prediabetes. A Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 686–693. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Florentin, M.; Kostapanos, M.S.; Papazafiropoulou, A.K. Role of Dipeptidyl Peptidase 4 Inhibitors in the New Era of Antidiabetic Treatment. World J. Diabetes 2022, 13, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.; Sharma, S.; Khan, Y. DPP-4 Inhibitors for Treating T2DM—Hype or Hope? An Analysis Based on the Current Literature. Front. Mol. Biosci. 2023, 10, 1130625. [Google Scholar] [CrossRef]
- Dudinskaya, E.; Matchekhina, L.; Strazhesko, I.; Tkacheva, O. Combined Vildagliptin + Metformin Therapy Can Increase Telomerase Activity in Patients with Type 2 Diabetes. Endocr. Abstr. 2021, 73, AEP196. [Google Scholar] [CrossRef]
- Vakonaki, E.; Tsiminikaki, K.; Plaitis, S.; Fragkiadaki, P.; Tsoukalas, D.; Katsikantami, I.; Vaki, G.; Tzatzarakis, M.; Spandidos, D.; Tsatsakis, A. Common Mental Disorders and Association with Telomere Length (Review). Biomed. Rep. 2018, 8, 111–116. [Google Scholar] [CrossRef]
- Yonezawa, K.; Kanegae, S.; Ozawa, H. Antipsychotics/Neuroleptics: Pharmacology and Biochemistry. In NeuroPsychopharmacotherapy; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–10. [Google Scholar]
- Polho, G.; Cardillo, G.; Kerr, D.; Chile, T.; Gattaz, W.; Forlenza, O.; Brentani, H.; De-Paula, V. Antipsychotics Preserve Telomere Length in Peripheral Blood Mononuclear Cells after Acute Oxidative Stress Injury. Neural Regen. Res. 2022, 17, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, H.; Du, X.; Zhou, J.; Yuan, L.; Ren, H.; Yang, X.; Zhang, G.; Chen, X. Circulating Brain-Derived Neurotrophic Factor, Antioxidant Enzymes Activities, and Mitochondrial DNA in Bipolar Disorder: An Exploratory Report. Front. Psychiatry 2020, 11, 514658. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Guller, V.; Berner, Y.N.; Tal, S. Are Atypical Antipsychotics Safer than Typical Antipsychotics for Treating Behavioral and Psychological Symptoms of Dementia? J. Nutr. Health Aging 2012, 16, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Len, X.; Pi, Z.; Hong, Z.; Feng, J.; Ma, C. Effect of Aripiprazole Combined with Olanzapine on the Clinical Efficacy of Schizophrenia. Farmacia 2022, 70, 550–556. [Google Scholar] [CrossRef]
- Caruso, G.; Grasso, M.; Fidilio, A.; Tascedda, F.; Drago, F.; Caraci, F. Antioxidant Properties of Second-Generation Antipsychotics: Focus on Microglia. Pharmaceuticals 2020, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; Fiorani, M.; Canonico, B.; De Matteis, R.; Guidarelli, A.; Montanari, M.; Buffi, G.; Coppo, L.; Arnér, E.S.J.; Cantoni, O. Clozapine Suppresses NADPH Oxidase Activation, Counteracts Cytosolic H2O2, and Triggers Early Onset Mitochondrial Dysfunction during Adipogenesis of Human Liposarcoma SW872 Cells. Redox Biol. 2023, 67, 102915. [Google Scholar] [CrossRef]
- Ben-Azu, B.; del Re, E.C.; VanderZwaag, J.; Carrier, M.; Keshavan, M.; Khakpour, M.; Tremblay, M.-È. Emerging Epigenetic Dynamics in Gut-Microglia Brain Axis: Experimental and Clinical Implications for Accelerated Brain Aging in Schizophrenia. Front. Cell. Neurosci. 2023, 17, 1139357. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Jaramillo, N.; Rodríguez-Agudelo, Y.; Aviña-Cervantes, L.C.; Roberts, D.L.; Velligan, D.I.; Walss-Bass, C. Leukocyte Telomere Length in Hispanic Schizophrenia Patients under Treatment with Olanzapine. J. Psychiatr. Res. 2017, 90, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-Y.; Chang, H.-W.; Lin, C.-H.; Cho, C.-L. Short Telomeres in Patients with Chronic Schizophrenia Who Show a Poor Response to Treatment. J. Psychiatry Neurosci. 2008, 33, 244–247. [Google Scholar]
- Fernandez-Egea, E.; Bernardo, M.; Heaphy, C.M.; Griffith, J.K.; Parellada, E.; Esmatjes, E.; Conget, I.; Nguyen, L.; George, V.; Stoppler, H.; et al. Telomere Length and Pulse Pressure in Newly Diagnosed, Antipsychotic-Naive Patients with Nonaffective Psychosis. Schizophr. Bull. 2009, 35, 437–442. [Google Scholar] [CrossRef]
- Li, Z.; Hu, M.; Zong, X.; He, Y.; Wang, D.; Dai, L.; Dong, M.; Zhou, J.; Cao, H.; Lv, L.; et al. Association of Telomere Length and Mitochondrial DNA Copy Number with Risperidone Treatment Response in First-Episode Antipsychotic-Naïve Schizophrenia. Sci. Rep. 2015, 5, 18553. [Google Scholar] [CrossRef] [PubMed]
- Pippal, N.; Halder, S.; Srivastava, S.; Kar, R.; Gupta, R.; Anthonio, A.E. Correlation between Telomere Length and Efficacy of Oral and Long-Acting Injectable Antipsychotics on Severity and Cognitive Impairment of Schizophrenia. Int. J. Psychiatry Clin. Pract. 2022, 26, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hishimoto, A.; Otsuka, I.; Watanabe, Y.; Numata, S.; Yamamori, H.; Boku, S.; Horai, T.; Someya, T.; Ohmori, T.; et al. Longer Telomeres in Elderly Schizophrenia Are Associated with Long-Term Hospitalization in the Japanese Population. J. Psychiatr. Res. 2018, 103, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.K.; Rizzo, L.B.; Xavier, G.; Tempaku, P.F.; Ota, V.K.; Santoro, M.L.; Spíndola, L.M.; Moretti, P.S.; Mazzotti, D.R.; Gadelha, A.; et al. Leukocyte Telomere Length Variation in Different Stages of Schizophrenia. J. Psychiatr. Res. 2018, 96, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Köse Çinar, R. Telomere Length and HTERT in Mania and Subsequent Remission. Rev. Bras. Psiquiatr. 2017, 40, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Prabhu, V.V.; Nguyen, T.B.; Devi, S.M.; Chung, Y.-C. Longer Telomere Length of T Lymphocytes in Patients with Early and Chronic Psychosis. Clin. Psychopharmacol. Neurosci. 2017, 15, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Masters, A.; Pandi-Perumal, S.R.; Seixas, A.; Girardin, J.L.; McFarlane, S.I. Melatonin, the Hormone of Darkness: From Sleep Promotion to Ebola Treatment. Brain Disord. Ther. 2015, 04, 1000151. [Google Scholar] [CrossRef]
- Ţincu, R.C.; Ivan, A.S.; Cobilinschi, C.; Ţincu, I.F.; Macovei, R.A. 5-Hydroxytryptophan Dietary Supplementation in Post-Traumatic Stress Syndrome. Farmacia 2022, 70, 320–324. [Google Scholar] [CrossRef]
- Horodincu, L.; Solcan, C. Influence of Different Light Spectra on Melatonin Synthesis by the Pineal Gland and Influence on the Immune System in Chickens. Animals 2023, 13, 2095. [Google Scholar] [CrossRef]
- Poza, J.J.; Pujol, M.; Ortega-Albás, J.J.; Romero, O. Melatonina En Los Trastornos de Sueño. Neurología 2022, 37, 575–585. [Google Scholar] [CrossRef]
- Stancu, E.; Carata, A.; Tăerel, A.-E. From the History of Drugs: Oleum Jecoris Aselli, a Long Time Used Remedy. Farmacia 2015, 63, 776–780. [Google Scholar]
- Sharkey, K.M.; Fogg, L.F.; Eastman, C.I. Effects of Melatonin Administration on Daytime Sleep after Simulated Night Shift Work. J. Sleep Res. 2001, 10, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Ogundele, M.O.; Yemula, C. Management of Sleep Disorders among Children and Adolescents with Neurodevelopmental Disorders: A Practical Guide for Clinicians. World J. Clin. Pediatr. 2022, 11, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Smits, M.; Boss, M.; Miedema, I.; van Geijlswijk, I. Efficacy and Safety of Supplemental Melatonin for Delayed Sleep–Wake Phase Disorder in Children: An Overview. Sleep Med. X 2020, 2, 100022. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Smith, D.; Hardeland, R.; Yang, M.; Xu, H.; Zhang, L.; Yin, H.; Zhu, Q. Melatonin Receptor Genes in Vertebrates. Int. J. Mol. Sci. 2013, 14, 11208–11223. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and Health: Insights of Melatonin Action, Biological Functions, and Associated Disorders. Cell. Mol. Neurobiol. 2023, 43, 2437–2458. [Google Scholar] [CrossRef] [PubMed]
- Sehirli, A.O.; Sayıner, S.; Chukwunyere, U.; Serakinci, N. Role of Melatonin in Angiotensin and Aging. Molecules 2021, 26, 4666. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; de las Heras, N.; Lahera, V.; Tresguerres, J.A.F.; Reiter, R.J.; Manucha, W. Melatonin as an Anti-Aging Therapy for Age-Related Cardiovascular and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 888292. [Google Scholar] [CrossRef] [PubMed]
- Erdem, Y.; Altunay, İ.; Özkur, E.; Şekerlisoy, G.; Karabay, E.; Özdemir, F.; Çerman, A. The Association between Melatonin Levels and Sleep Quality in Patients with Pruritus: A Potential Biomarker on a Candidate Future Treatment. Indian J. Dermatol. 2021, 66, 609–615. [Google Scholar] [CrossRef]
- Sabot, D.; Lovegrove, R.; Stapleton, P. The Association between Sleep Quality and Telomere Length: A Systematic Literature Review. Brain Behav. Immun.-Health 2023, 28, 100577. [Google Scholar] [CrossRef]
- Hasannia, E.; Derakhshanpour, F.; Vakili, M.A. Effects of Melatonin on Salivary Levels of Cortisol and Sleep Quality of Hemodialysis Patients: A Randomized Clinical Trial. Iran. J. Psychiatry 2021, 16, 305. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lou, D.; Zhang, D. Melatonin Alleviates Age-Associated Endothelial Injury of Atherosclerosis via Regulating Telomere Function. J. Inflamm. Res. 2021, 14, 6799–6812. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Fu, A.; Hoffman, A.E.; Zheng, T.; Zhu, Y. Melatonin Enhances DNA Repair Capacity Possibly by Affecting Genes Involved in DNA Damage Responsive Pathways. BMC Cell Biol. 2013, 14, 1–8. [Google Scholar] [CrossRef]
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular Mechanism of Estrogen–Estrogen Receptor Signaling. Reprod. Med. Biol. 2017, 16, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Mal, R.; Magner, A.; David, J.; Datta, J.; Vallabhaneni, M.; Kassem, M.; Manouchehri, J.; Willingham, N.; Stover, D.; Vandeusen, J.; et al. Estrogen Receptor Beta (ERβ): A Ligand Activated Tumor Suppressor. Front. Oncol. 2020, 10, 587386. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Ghafouri-Fard, S.; Najafi, S.; Kallenbach, J.; Keramatfar, E.; Atri Roozbahani, G.; Heidari Horestani, M.; Hussen, B.M.; Baniahmad, A. Hormonal Regulation of Telomerase Activity and HTERT Expression in Steroid-Regulated Tissues and Cancer. Cancer Cell Int. 2022, 22, 258. [Google Scholar] [CrossRef] [PubMed]
- Motlani, V.; Motlani, G.; Pamnani, S.; Sahu, A.; Acharya, N. Changed Endocrinology in Postmenopausal Women: A Comprehensive View. Cureus 2023, 15, e51287. [Google Scholar] [CrossRef] [PubMed]
- Tire, B.; Ozturk, S. Potential Effects of Assisted Reproductive Technology on Telomere Length and Telomerase Activity in Human Oocytes and Early Embryos. J. Ovarian Res. 2023, 16, 130. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kroenke, C.H.; Epel, E.; Kenna, H.A.; Wolkowitz, O.M.; Blackburn, E.; Rasgon, N.L. Greater Endogenous Estrogen Exposure Is Associated with Longer Telomeres in Postmenopausal Women at Risk for Cognitive Decline. Brain Res. 2011, 1379, 224–231. [Google Scholar] [CrossRef]
- Park, J.; Hu, R.; Qian, Y.; Xiong, S.; El-Sabbagh, A.S.; Ibrahim, M.; Wang, J.; Xu, Z.; Chen, Z.; Song, Q.; et al. Estrogen Counteracts Age-Related Decline in Beige Adipogenesis through the NAMPT-Regulated ER Stress Response. Nat. Aging 2024, 4, 839–853. [Google Scholar] [CrossRef]
- Gu, D.; Li, J.; Little, J.; Li, H.; Zhang, X. Associations between Serum Sex Hormone Concentrations and Telomere Length among U.S. Adults, 1999–2002. J. Nutr. Health Aging 2020, 24, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Marais, G.A.B.; Gaillard, J.-M.; Vieira, C.; Plotton, I.; Sanlaville, D.; Gueyffier, F.; Lemaitre, J.-F. Sex Gap in Aging and Longevity: Can Sex Chromosomes Play a Role? Biol. Sex Differ. 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Fait, T. Menopause Hormone Therapy: Latest Developments and Clinical Practice. Drugs Context 2019, 8, 212551. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, H. Pharmacology of Estrogens and Progestogens: Influence of Different Routes of Administration. Climacteric 2005, 8, 3–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-C.; Im, J.-A.; Kim, J.-H.; Lee, H.-R.; Shim, J.-Y. Effect of Long-Term Hormone Therapy on Telomere Length in Postmenopausal Women. Yonsei Med. J. 2005, 46, 471. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.; Li, H.; Jones, M.E.E.; Pinto, A.R.; van Sinderen, M.; Drummond, A.; Simpson, E.R.; Liu, J.-P. Estrogen Deficiency Reversibly Induces Telomere Shortening in Mouse Granulosa Cells and Ovarian Aging in Vivo. Protein Cell 2011, 2, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Kresovich, J.K.; Parks, C.G.; Sandler, D.P.; Taylor, J.A. Reproductive History and Blood Cell Telomere Length. Aging 2018, 10, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Entringer, S.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Buss, C.; Simhan, H.N.; Wadhwa, P.D. Maternal Estriol Concentrations in Early Gestation Predict Infant Telomere Length. J. Clin. Endocrinol. Metab. 2015, 100, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.; Jones, M.E.; Li, H.; Pinto, A.R.; Simpson, E.R.; Liu, J.-P. Estrogen Deficiency Leads to Telomerase Inhibition, Telomere Shortening and Reduced Cell Proliferation in the Adrenal Gland of Mice. Cell Res. 2008, 18, 1141–1150. [Google Scholar] [CrossRef]
- Shen, J.; Terry, M.B.; Liao, Y.; Gurvich, I.; Wang, Q.; Senie, R.T.; Santella, R.M. Genetic Variation in Telomere Maintenance Genes, Telomere Length and Breast Cancer Risk. PLoS ONE 2012, 7, e44308. [Google Scholar] [CrossRef]
- Duckworth, A.; Ruth, K.S.; Prague, J.K.; Russell, A.-M.; Almond, H.; Conway, J.; Beaumont, R.N.; Wood, A.R.; Martin, S.; Lunnon, K.; et al. Study of the Associations between Short Telomeres, Sex Hormones and Pulmonary Fibrosis. medRxiv 2022. [Google Scholar] [CrossRef]
- Calado, R.T.; Yewdell, W.T.; Wilkerson, K.L.; Regal, J.A.; Kajigaya, S.; Young, N.S. Sex Hormones Modulate the Length of Telomeres of Normal and Telomerase-Mutant Leukocytes through the Estrogen Receptor Pathway. Blood 2006, 108, 182. [Google Scholar] [CrossRef]
- WHO. Endometriosis. Available online: https://www.who.int/news-room/fact-sheets/detail/endometriosis (accessed on 8 May 2024).
- Ilhan, M.; Battal, A.; Kaptaner, B.; Dogan, A.; Donmez, F.; Yilmaz, M.A.; Eroglu, H. Exploring the Ameliorative Effects of Hypericum scabrum L. on a Surgically-Induced Endometriosis Rat Model and Its Phytochemical Profiling by LC-MS/MS. Farmacia 2023, 71, 710–721. [Google Scholar] [CrossRef]
- Ashfaq, S.; Can, A. Danazol; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Townsley, D.M.; Dumitriu, B.; Liu, D.; Biancotto, A.; Weinstein, B.; Chen, C.; Hardy, N.; Mihalek, A.D.; Lingala, S.; Kim, Y.J.; et al. Danazol Treatment for Telomere Diseases. N. Engl. J. Med. 2016, 374, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.C.; Lutzky, V.P.; Apte, S.H.; Godbolt, D.; Feenstra, J.; Mackintosh, J. Successful Treatment of Telomeropathy-related Interstitial Lung Disease with Immunosuppression and Danazol. Respirol. Case Rep. 2020, 8, e00607. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Oriz, I.; Kohls, G.; Iglesias, C.; Polonio, A.M.; Chico-Sordo, L.; Toribio, M.; Meseguer, M.; Varela, E.; Pellicer, A.; García-Velasco, J.A. A Randomized Controlled Intervention Trial with Danazol to Improve Telomeric and Fertility Parameters in Women with Diminished Ovarian Reserve: A Pilot Study. Women’s Health Rep. 2023, 4, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Assis, V.; de Sousa Neto, I.V.; Ribeiro, F.M.; de Cassia Marqueti, R.; Franco, O.L.; da Silva Aguiar, S.; Petriz, B. The Emerging Role of the Aging Process and Exercise Training on the Crosstalk between Gut Microbiota and Telomere Length. Int. J. Environ. Res. Public Health 2022, 19, 7810. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Voicu, S.N.; Scărlătescu, A.I.; Apetroaei, M.-M.; Nedea, M.I.; Blejan, I.E.; Udeanu, D.I.; Velescu, B.Ș.; Ghica, M.; Nedea, O.A.; Cobelschi, C.P.; et al. Evaluation of Neuro-Hormonal Dynamics after the Administration of Probiotic Microbial Strains in a Murine Model of Hyperthyroidism. Nutrients 2024, 16, 1077. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of Antibiotics on the Human Microbiome and Consequences for Host Health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef] [PubMed]
- Chitulea, P.; Gherai, R.; Cheta, C.; Marin Negru, T. The Role of Intravaginal Prebiotics in Controlling the Evolution of Uncomplicated Bacterial and Fungal Vaginal Infections. Farmacia 2022, 70, 545–549. [Google Scholar] [CrossRef]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the Balance: Antibiotic Effects on Host–Microbiota Mutualism. Nat. Rev. Microbiol. 2011, 9, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Velescu, B.Ș.; Ilie, M.I.; Amzăr, A.I.; Lupașcu, R.E.; Marandiuc, I.M.; Apetroaei, M.-M.; Arsene, A.L.; Blejan, E.I.; Nedea, O.A.; Fistos, T.; et al. Development and Experimental Evaluation of Some Silver Nanoparticles with Antimicrobial Potential. Processes 2023, 11, 1212. [Google Scholar] [CrossRef]
- Strasser, B.; Wolters, M.; Weyh, C.; Krüger, K.; Ticinesi, A. The Effects of Lifestyle and Diet on Gut Microbiota Composition, Inflammation and Muscle Performance in Our Aging Society. Nutrients 2021, 13, 2045. [Google Scholar] [CrossRef] [PubMed]
- Vyas, C.M.; Ogata, S.; Reynolds, C.F.; Mischoulon, D.; Chang, G.; Cook, N.R.; Manson, J.E.; Crous-Bou, M.; De Vivo, I.; Okereke, O.I. Telomere Length and Its Relationships with Lifestyle and Behavioural Factors: Variations by Sex and Race/Ethnicity. Age Ageing 2021, 50, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Kleniewska, P.; Pawliczak, R. Antioxidative Activity of Probiotics. Arch. Med. Sci. 2021, 17, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Amzăr, A.I.; Udeanu, D.I.; Pițuru, M.T.; Hîrjău, M.; Popa, D.E.; Velescu, B.Ș.; Letiția, A. Signalling Through the Microbiota-Gut-Brain Triade. Farmacia 2022, 70, 402–409. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Li, M.; Wang, W.; Liu, Z.; Xi, C.; Huang, X.; Liu, J.; Huang, J.; Tian, D.; et al. Efficacy of Probiotics on Stress in Healthy Volunteers: A Systematic Review and Meta-analysis Based on Randomized Controlled Trials. Brain Behav. 2020, 10, e01699. [Google Scholar] [CrossRef]
- Messaoudi, M.; Violle, N.; Bisson, J.-F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial Psychological Effects of a Probiotic Formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in Healthy Human Volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Makarenko, M.S.; Chistyakov, V.A.; Usatov, A.V.; Mazanko, M.S.; Prazdnova, E.V.; Bren, A.B.; Gorlov, I.F.; Komarova, Z.B.; Chikindas, M.L. The Impact of Bacillus Subtilis KATMIRA1933 Supplementation on Telomere Length and Mitochondrial DNA Damage of Laying Hens. Probiotics Antimicrob. Proteins 2019, 11, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, S.; Reichenbach, P.; Marjavaara, L.; Nilsson, A.K.; Lingner, J.; Chabes, A.; Rothstein, R.; Chang, M. Telomere Length Homeostasis Responds to Changes in Intracellular DNTP Pools. Genetics 2013, 193, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Lew, L.C.; Hor, Y.Y.; Jaafar, M.H.; Lau, A.S.Y.; Ong, J.S.; Chuah, L.O.; Yap, K.P.; Azzam, G.; Azlan, A.; Liong, M.T. Lactobacilli Modulated AMPK Activity and Prevented Telomere Shortening in Ageing Rats. Benef. Microbes 2019, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Zheng, T.; Zhang, C.; Song, X.; Chen, J.; Shi, Y.; You, J.; Cheng, G.; Xiong, J. Yogurt and Streptococcus Thermophilus Metabolites Ameliorated Telomere Attrition in D-galactose-induced Ageing Mice and t -BHP-challenged HepG2 Cells. Int. J. Food Sci. Technol. 2020, 55, 2509–2516. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kurihara, S.; Kibe, R.; Ashida, H.; Benno, Y. Longevity in Mice Is Promoted by Probiotic-Induced Suppression of Colonic Senescence Dependent on Upregulation of Gut Bacterial Polyamine Production. PLoS ONE 2011, 6, e23652. [Google Scholar] [CrossRef]
- Hunsche, C.; Cruces, J.; De la Fuente, M. Improvement of Redox State and Functions of Immune Cells as Well as of Behavioral Response in Aged Mice After Two-Week Supplementation of Fermented Milk with Probiotics. Curr. Microbiol. 2019, 76, 1278–1289. [Google Scholar] [CrossRef]


| Methods | Study Groups | Drug Studied | Conclusion | Reference |
|---|---|---|---|---|
| TL by qPCR | G1: 170 Hispanic patients with SCZ (with antipsychotic therapy) G2: 126 Hispanic healthy controls | Low-risk antipsychotics; atypical antipsychotics (clozapine; olanzapine) | Compared to G2 and G1, using medium and low-risk antipsychotics, G1, with atypical antipsychotics, which cause metabolic syndrome, had severe TL erosion. Olanzapine promotes TL shortening significantly than clozapine. | [133] |
| Southern blot analysis of mean length of terminal restriction fragment | G1: 34 patients with SCZ that responded well to treatment G2: 35 patients with SCZ that did not respond well to treatment G3: 76 healthy controls | Antipsychotics (analysis conducted regarding treatment adherence) | The subsequent cellular malfunction could contribute to the gradual decline in treatment-resistant SCZ. TL shortening in G2. | [134] |
| Telomere DNA and PP | G1: antipsychotic treatment-naive SCZ patients G2: control subjects | Antipsychotics (analysis conducted regarding protective effects of drugs after treatment initiation) | Prior to antipsychotic treatment, patients with psychosis had a reduction in telomere DNA content and an elevation in PP. | [135] |
| TL and mtDNA copy number | G1: 89 patients with 8 weeks on antipsychotic terapy (divided into G1(a)-responders and G1(b)-non-responders) G2: 144 controls | Risperidone | Before risperidone initiation, the TL in G1 was average, but mtDNA was lower than in G2. After risperidone initiation, G1(a), compared to G1(B), had longer TL and lower mtDNA TL and mtDNA could predict response to antipsychotic treatment. | [136] |
| TL by qPCR | G1: 30 SCZ patients with long-acting injectable antipsychotics G2: 30 SCZ patients with oral atypical antipsychotics | Long-acting injectable antipsychotics; oral atypical antipsychotics | TL might be able to predict how antipsychotic drugs function in SCZ patients. | [137] |
| Negative SCZ symptoms are predicted by shorter TL. | ||||
| TL by qPCR | 1241 SCZ patients | Antipsychotics | Antipsychotic medication had no effect on TL | [138] |
| 1042 controls | ||||
| TL by multiplex qPCR | 81 antipsychotic naïve patients 173 SCZ patients 173 healthy controls | Antipsychotics | SCZ patients had longer TL than healthy individuals Patients with non-remitted SCZ exhibited a longer TL than those with remitted SCZ. No effect of antipsychotic medication on TL. | [139] |
| leukocytes subjected to H2O2; treated for 7 days with antipsychotics; TL by RT-PCR | Healthy individuals | Aripiprazole; haloperidol; clozapine | Aripiprazole and haloperidol treatment increased TL by 23% and 20% after hydrogen peroxide stimulation | [126] |
| qPCR for TL and hTERT gene expression, brain neurotrophic factor by ELISA | 20 male SCZ patients 20 healthy controls | Antipsychotics | SCZ patients had shorter TL than controls. SCZ patients’ TL increased after antipsychotic treatment. Late-stage patients exhibited a shorter TL than early-stage patients and controls. hTERT gene expression was upregulated during mania and remission. | [140] |
| TL by qPCR | SCZ patients with early duration of illness (≤5 years) SCZ patients with chronic duration of illness (≥5 years) healthy individuals | Chlorpromazine | Patients with early and chronic psychosis exhibited a considerably prolonged TL in comparison to healthy control subjects. Insignificant correlation between chlorpromazine-equivalent dosages and TL. | [141] |
| Methods | Study Groups | Studied Molecule | Mechanism and Conclusions | Reference |
|---|---|---|---|---|
| TL by qPCR | G1: 65 women on HT for >5 years G2: 65 women matched in age HT-naive | Oestrogen and progesterone | G1 exhibited greater TL compared to G2. Long-term HT inhibit TL shortening. | [170] |
| TL by qFISH | Mice | Oestrogen-replacement therapy (ORT) | ORT resulted in elevated levels of TERT gene expression, enhanced telomerase activity, elongated TL, and stimulated ovarian tissue growth. Oestrogen insufficiency or excessive activity can lead to the ageing of ovarian tissue or the development of tumours, respectively, via influencing the remodelling of telomeres through oestrogen control. | [171] |
| TL by qPCR | 1048 women in Sister Study | Oestrogen and progesterone | No association between HT and TL. Reproductive histories that indicate higher levels of naturally occurring oestrogen were linked to shorter TL. | [172] |
| TL by qPCR | 100 newborns from expecting mothers recruited in early pregnancy (tracked prospectively from intrauterine life to early childhood) | Endogenous E3 | An elevation in maternal E3 concentration during the initial stages of pregnancy was linked to a 14.42% rise in child TL. | [173] |
| TRAP for telomerase activity; qPCR for TERT gene expression | G1: female rats G2: female rats with a placebo | 21-day release oestrogen formulation | Without oestrogen, the TERT gene’s expression and telomerase activity were decreased. Oestrogen insufficiency leads to a notable reduction in the TL in the adrenal cortex. Oestrogen for 3 weeks restores TERT gene expression, telomerase activity, and cell proliferation. | [174] |
| TL by qPCR | G1: 333 breast cancer sister-sets G2: 409 unaffected sisters | HT | Shortened TL in unaffected sisters showed a statistically significant correlation with HT-naïve sisters. An inverse relationship was detected between the duration of HT and the TL. | [175] |
| TL by qPCR | G1: 415 females with IPF G2: 718 males with IPF G3: 204,321 healthy females G4: 174,254 healthy males | Endogenous sex hormones (analysis for the prospective role of HT in telomere maintenance) | Females who experienced early menopause and premature ovarian failure had a greater likelihood of developing IPF. The prevalence of IPF in males was correlated with the levels of bioavailable testosterone in the blood and the stages of the disease. Both males and females showed a correlation between lower levels of sex hormones and shorter TL. Elevated levels of sex hormones provide a protective effect against the development and advancement of IPF, potentially by decelerating the process of TL shortening. Hormonal supplements can potentially delay or prevent the start of diseases in those at risk of telomere-associated IPF and enhance the prognosis of the disease. | [176] |
| telomerase activity by TRAP; TERT by qPCR | G1: healthy subjects G2: subjects with TERT mutations | androgens; E2 | Both drugs increased telomerase activity in G1 in a dose-dependent manner, which was associated with higher amounts of TERT mRNA. Sex hormones activate the expression of TERT by binding to the oestrogen receptor, which can then interact with certain regions in the promoter region of the TERT gene. | [177] |
| Methods | Study Groups | Studied Molecule | Mechanism and Conclusion | Reference |
|---|---|---|---|---|
| TL and mtDNA by qPCR | G1: hisex brown hens (experimental group) G: hisex brown hens (control group) | 0.1% probiotic supplementation (Bacillus subtilis) | No significant effect on TL A DNA-protective activity, an inhibition in mtDNA damage by oxidative stress reduction | [200] |
| TL by qFISH | G1: 16 healthy individuals (experimental group) G2: 31 individuals (control group) | Nutraceutical supplementation (a mixture of various probiotics and vitamins) | The TL measures in G1 were 844 and 953 bases greater than those in G2. Positive impacts on TL by decreasing oxidative stress and inflammation. | [201] |
| TL by qPCR; AMPK expression | Rats | D-galactose to induce ageing symptoms + groups treated with a statin, L. reuteri, L. fermentum, L. plantarum | Statin, L. plantarum, L. fermentum, and L. reuteri substantially decreased TL shortening and elevated AMPK subunit-α1 expression. Statin and L. fermentum treatment significantly reduced plasma lipid peroxidation compared to control. Rats given L. plantarum showed higher levels of AMPK subunit-α2 compared to control. Lactobacillus probiotics were shown to have strain-dependent efficacy in alleviating age-related impairment. | [202] |
| TL by RT-PCR | 7 groups with 8 mice per group | Milk, yoghurt, S. thermophilus metabolites and L. rhamnosus metabolites | The yoghurt and S. thermophilus group had much longer TL in leucocytes and liver. When t-BHP-challenged HepG2 cells were exposed to digested yoghurt and S. thermophilus, the senescence index was reduced, and the TL was increased compared to the control. The yoghurt and metabolites of S. thermophilus exhibited antioxidative properties, but the milk and metabolites of L. rhamnosus had minimal impact on TL and oxidative stress. | [203] |
| DNA microarrays for ageing gene expression; HPLC, ELISA, PCR for inflammation markers | G1: mice (experimental group) G2: mice (control group) | B. lactis (LKM512) | G1 presented a decrease in the expression of genes linked with ageing and inflammation. Gene expression levels in G1 were similar to those in 10-month-old mice that were not treated (considered younger). G1 had a longer lifespan due to reduced chronic, low-level inflammation in the colon. | [204] |
| Oxidative stress parameters | Mice | Fermented milk (L. bulgaricus, L. casei, S. thermophillus) | The administration of fermented milk enriched with probiotics for a duration of two weeks resulted in enhanced behaviour, including increased muscular strength, exploratory activity, and reduced anxiety-like behaviour, in addition to better redox status and functionality of peritoneal immune cells in elderly mice. | [205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apetroaei, M.-M.; Fragkiadaki, P.; Velescu, B.Ș.; Baliou, S.; Renieri, E.; Dinu-Pirvu, C.E.; Drăgănescu, D.; Vlăsceanu, A.M.; Nedea, M.I.; Udeanu, D.I.; et al. Pharmacotherapeutic Considerations on Telomere Biology: The Positive Effect of Pharmacologically Active Substances on Telomere Length. Int. J. Mol. Sci. 2024, 25, 7694. https://doi.org/10.3390/ijms25147694
Apetroaei M-M, Fragkiadaki P, Velescu BȘ, Baliou S, Renieri E, Dinu-Pirvu CE, Drăgănescu D, Vlăsceanu AM, Nedea MI, Udeanu DI, et al. Pharmacotherapeutic Considerations on Telomere Biology: The Positive Effect of Pharmacologically Active Substances on Telomere Length. International Journal of Molecular Sciences. 2024; 25(14):7694. https://doi.org/10.3390/ijms25147694
Chicago/Turabian StyleApetroaei, Miruna-Maria, Persefoni Fragkiadaki, Bruno Ștefan Velescu, Stella Baliou, Elisavet Renieri, Cristina Elena Dinu-Pirvu, Doina Drăgănescu, Ana Maria Vlăsceanu, Marina Ionela (Ilie) Nedea, Denisa Ioana Udeanu, and et al. 2024. "Pharmacotherapeutic Considerations on Telomere Biology: The Positive Effect of Pharmacologically Active Substances on Telomere Length" International Journal of Molecular Sciences 25, no. 14: 7694. https://doi.org/10.3390/ijms25147694









