The Variation in the Traits Ameliorated by Inhibitors of JAK1/2, TGF-β, P-Selectin, and CXCR1/CXCR2 in the Gata1low Model Suggests That Myelofibrosis Should Be Treated by These Drugs in Combination
Abstract
:1. Introduction
2. Results
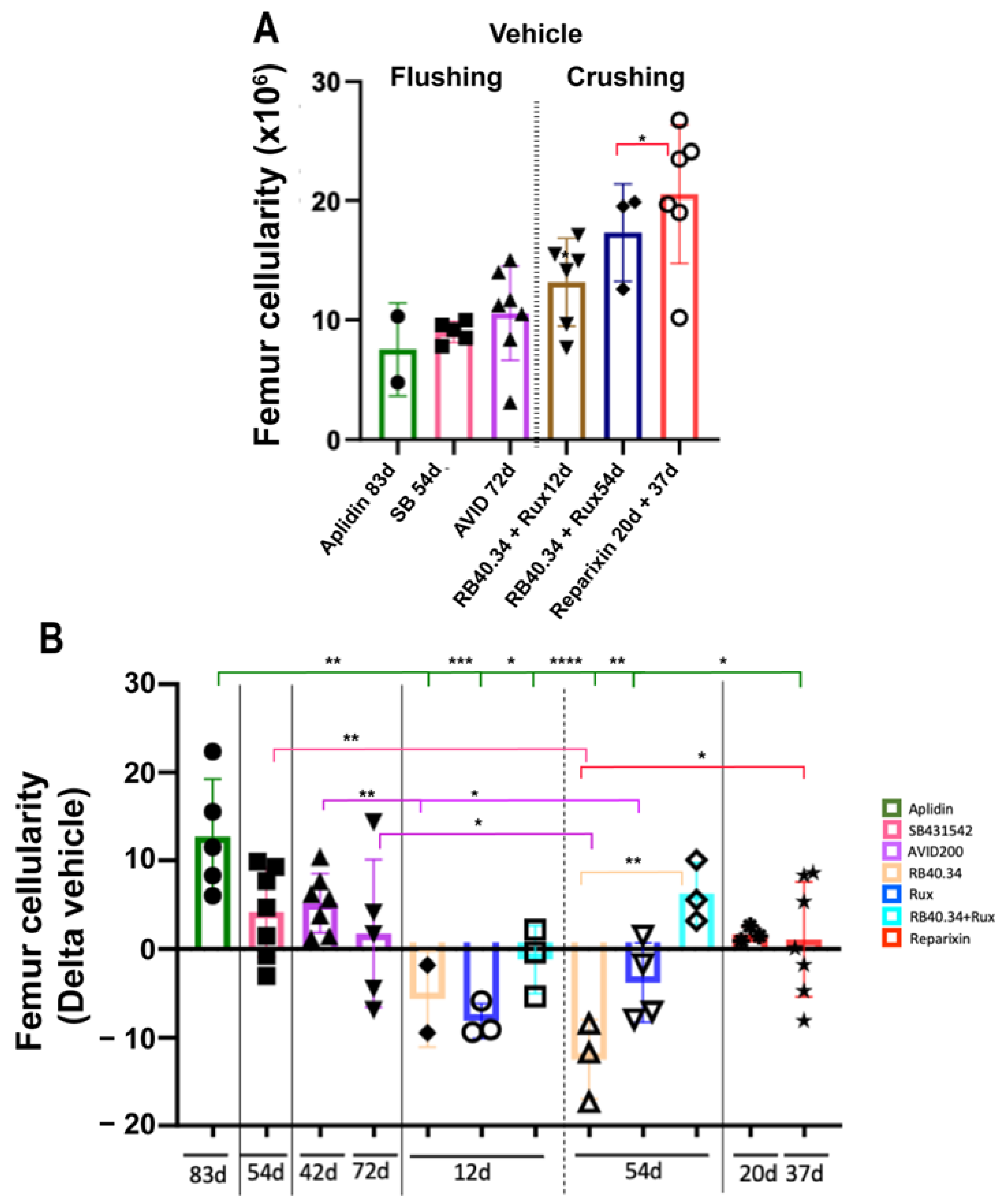
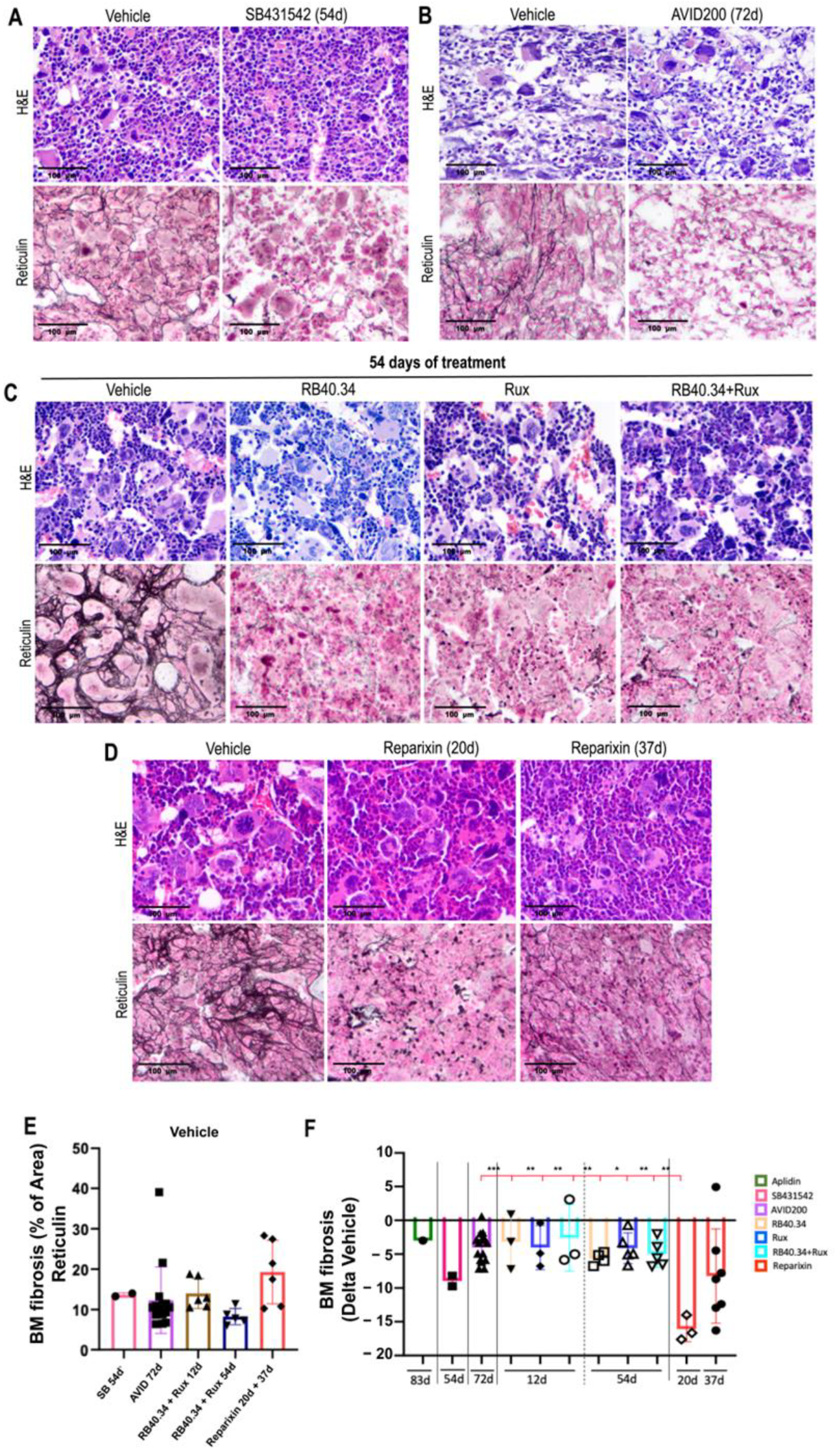
2.1. Effects on Cellularity, Fibrosis, and Neo-Angiogenesis in Bone Marrow
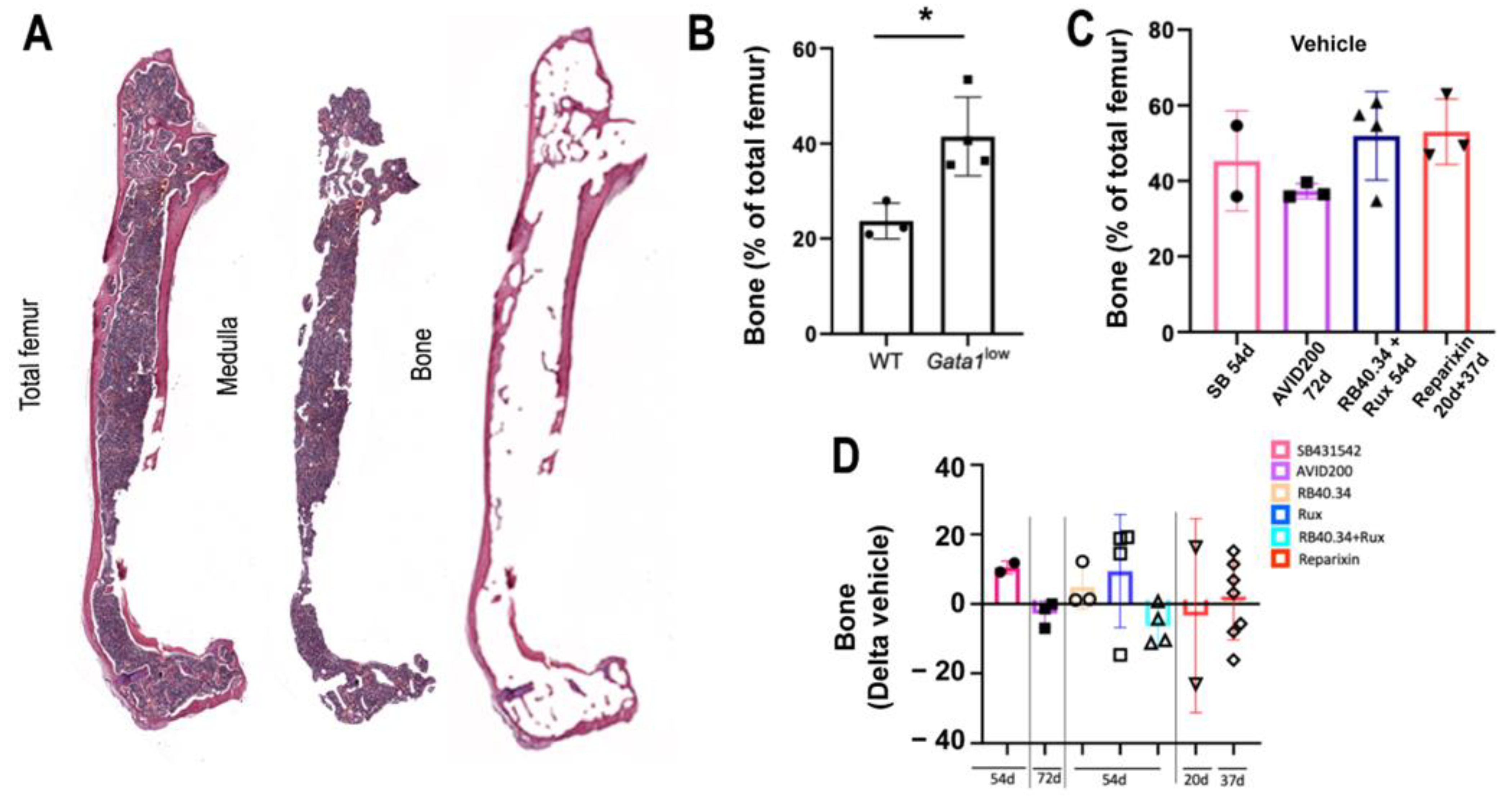
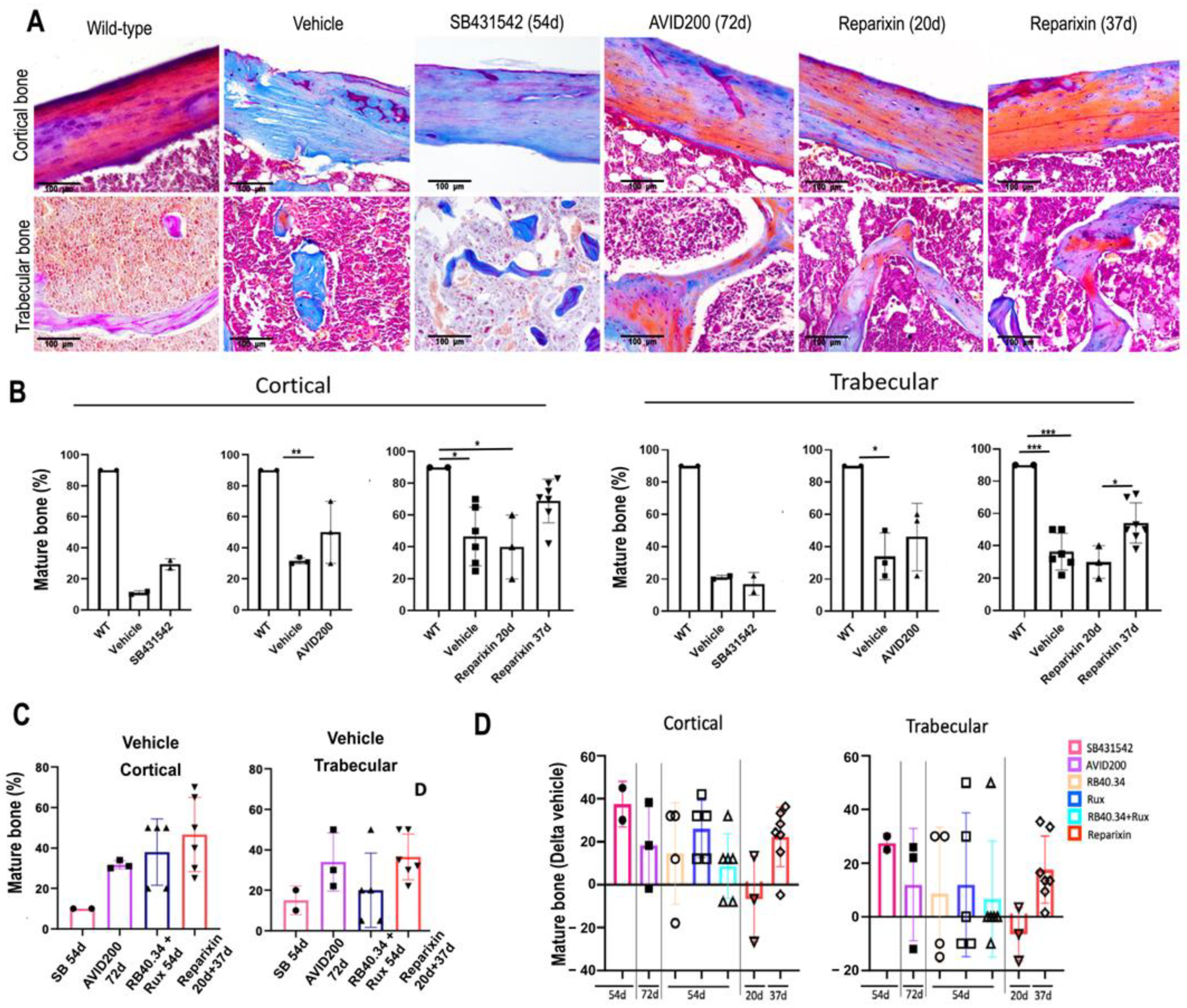
2.2. Effects on Deposition and Maturation of Bone
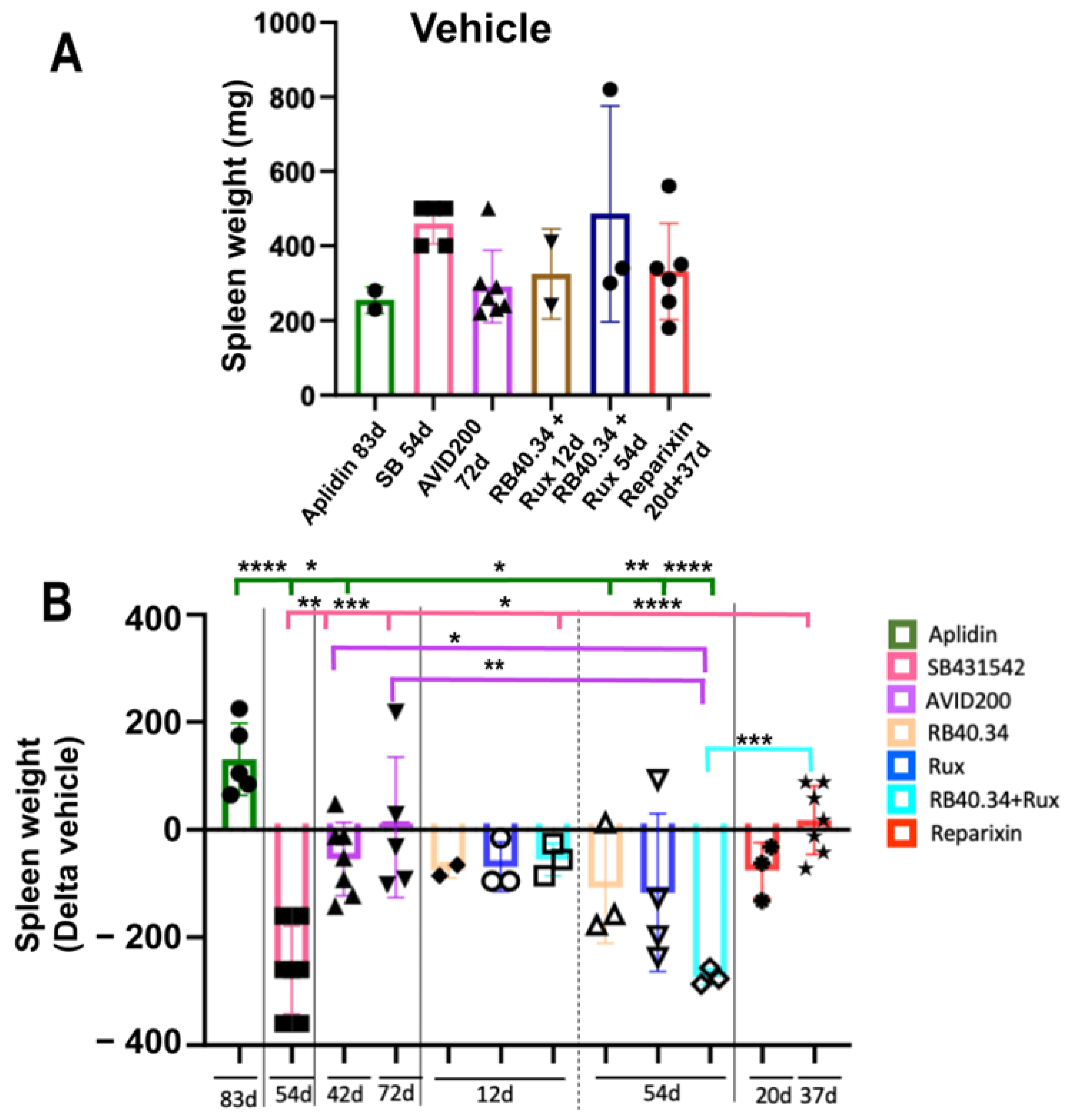
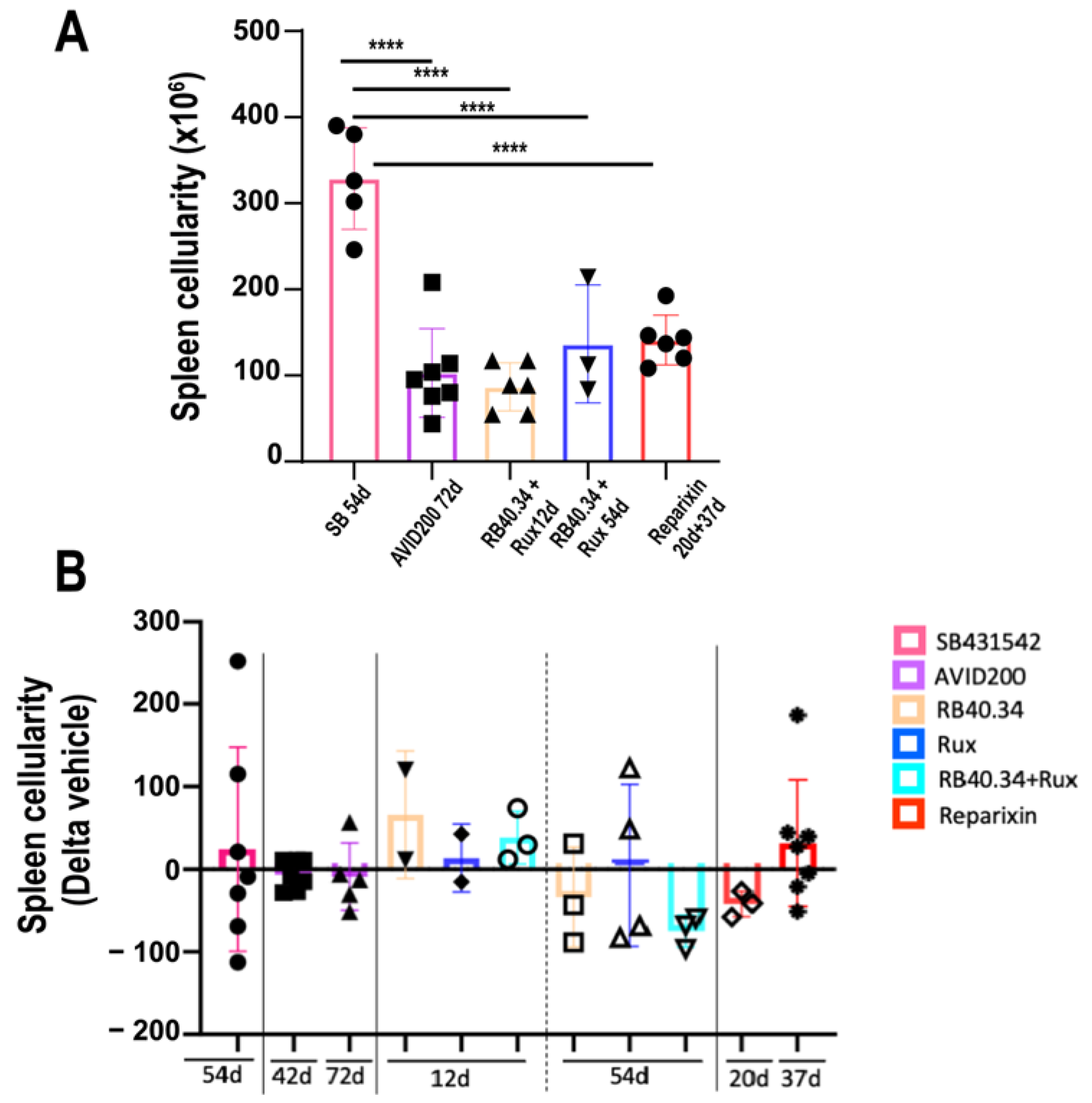
2.3. Effects on Size and Fibrosis of Spleen
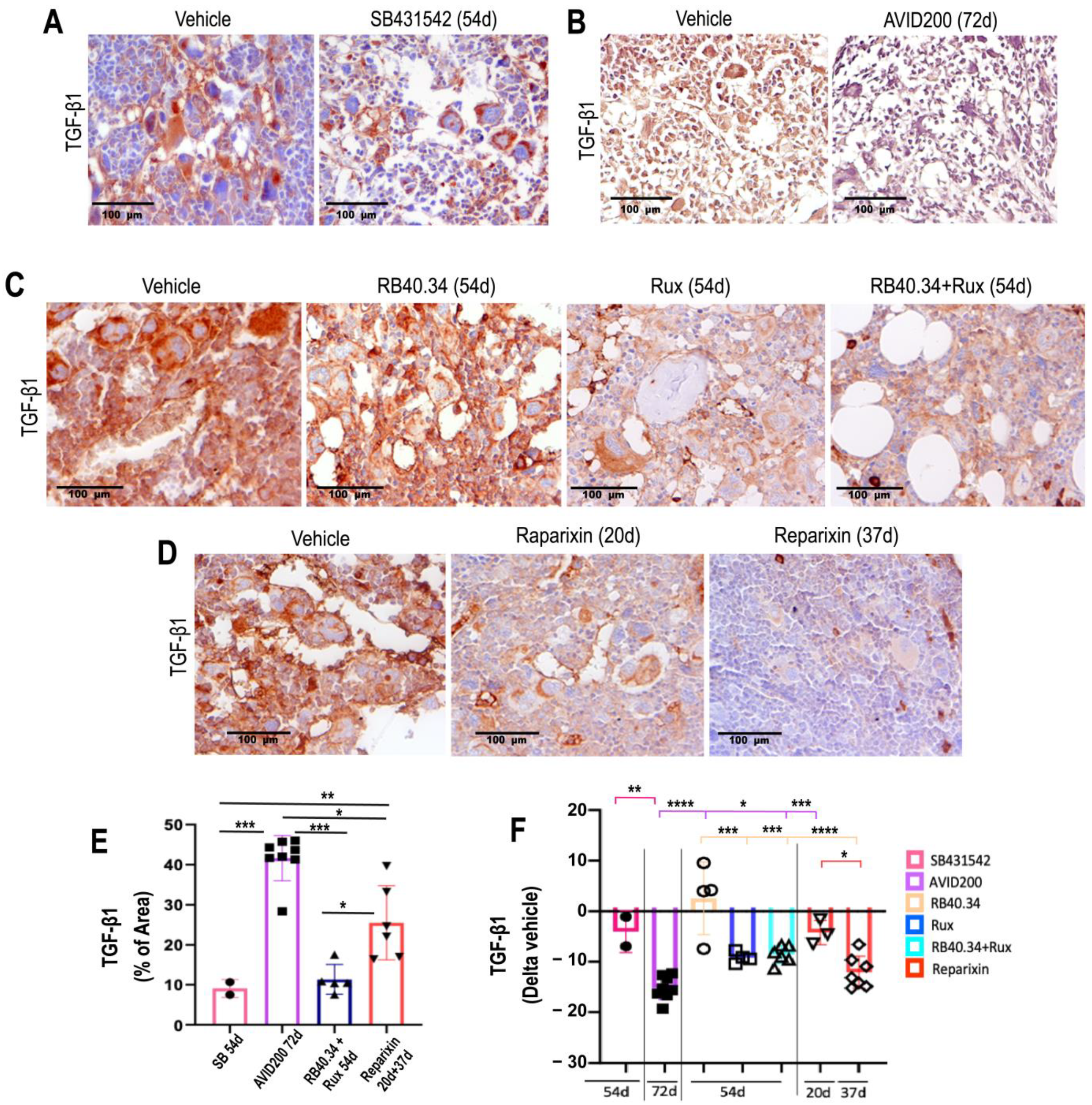
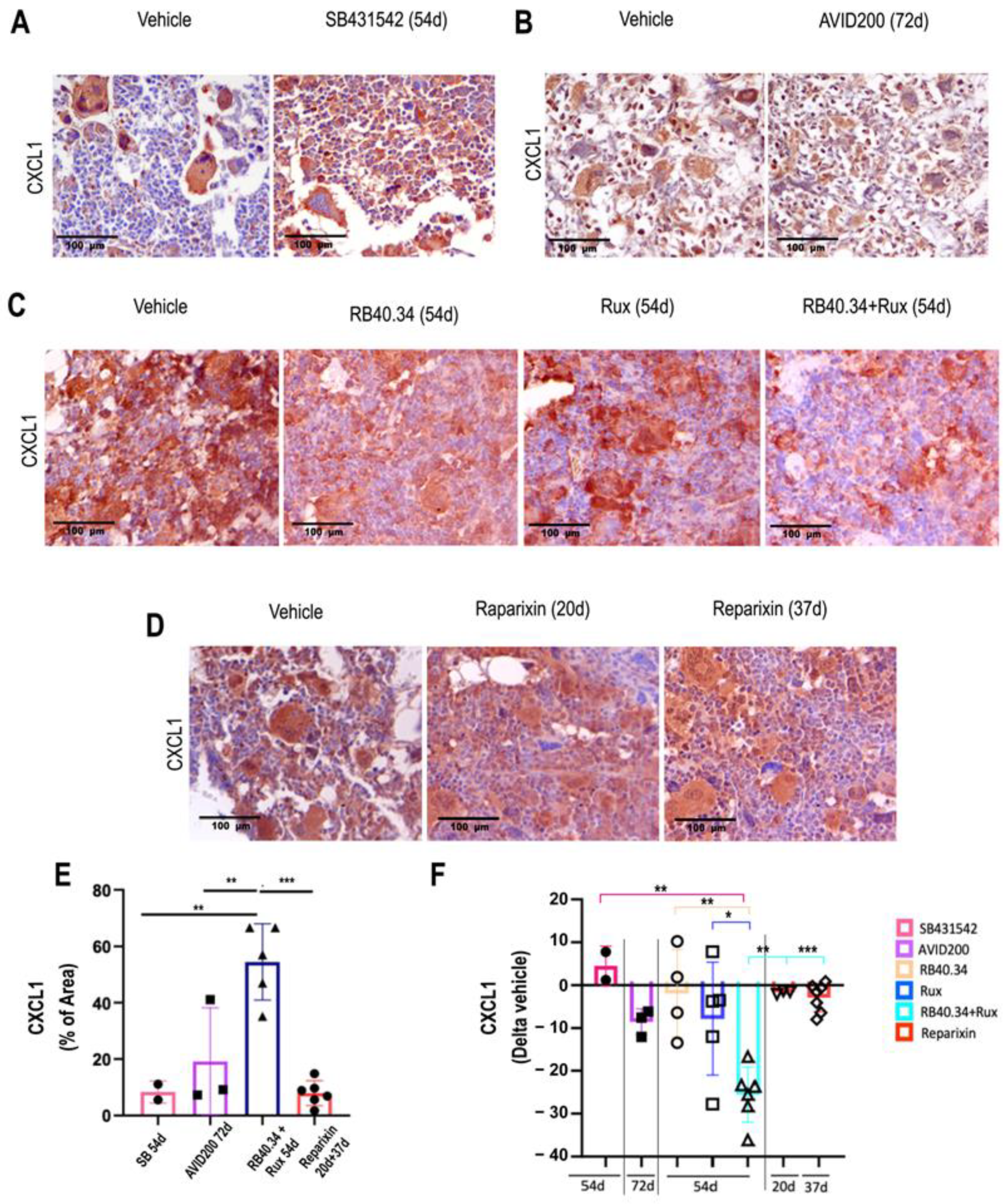
2.4. Effects on TGF-β and CXCL1 Contents in Bone Marrow
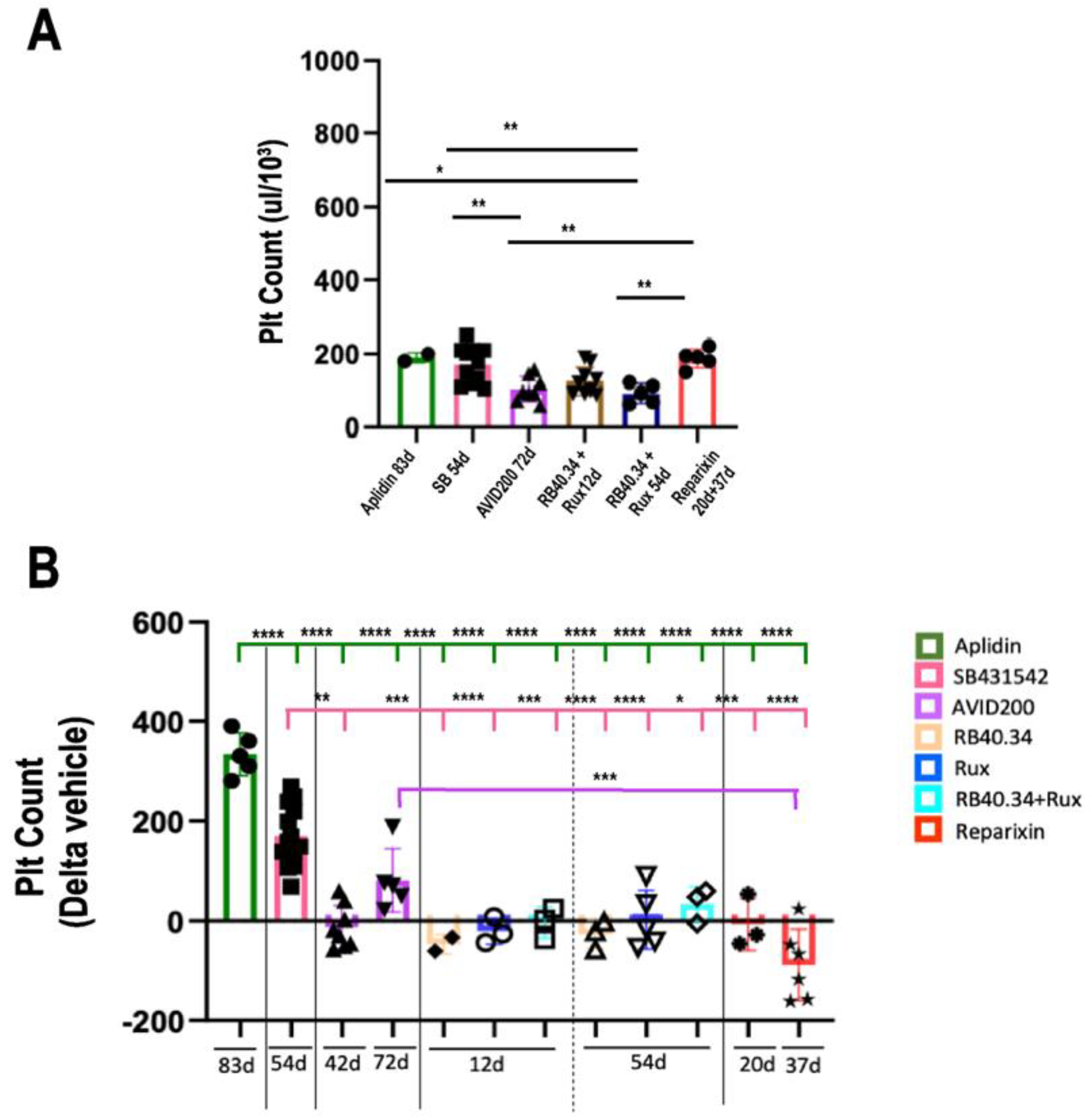
2.5. Effects on Platelet Counts
2.6. Effects on Megakaryocyte Maturation
3. Discussion
4. Materials and Methods
4.1. Mice
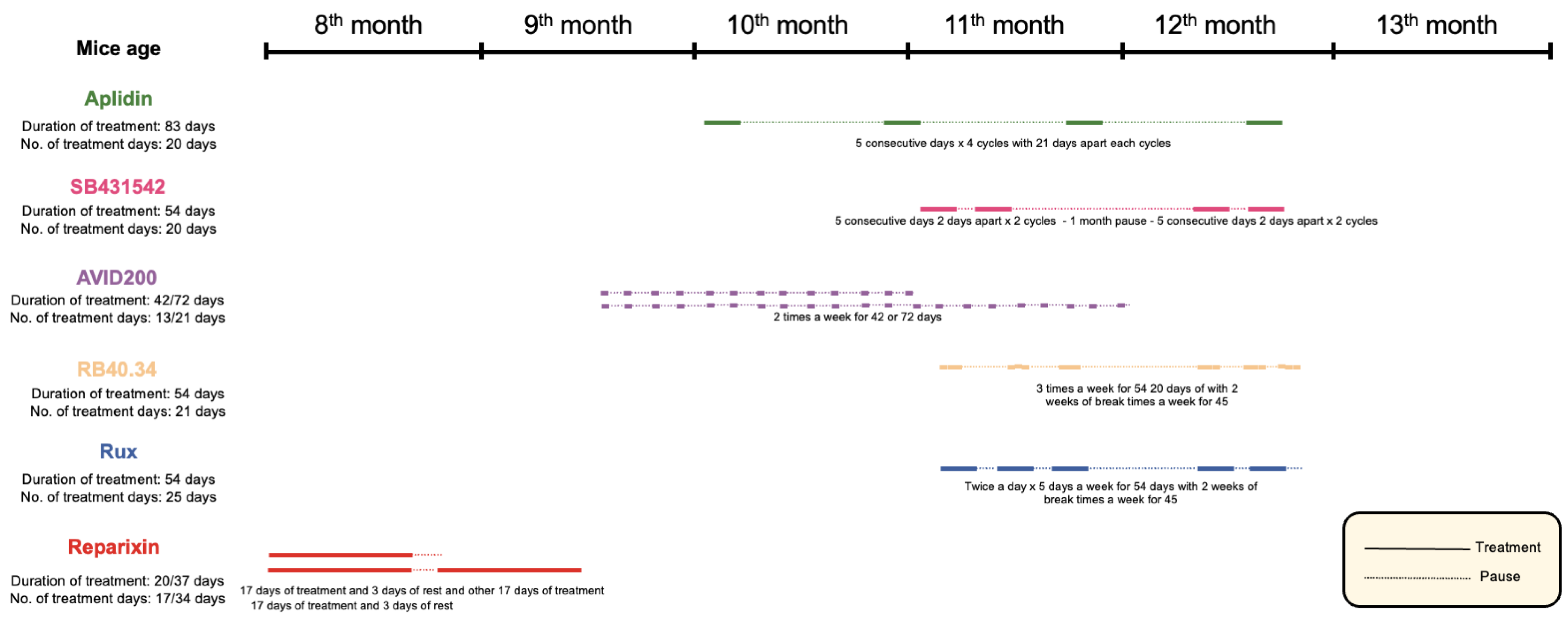
4.2. Mice Treatments
4.3. Endpoints
4.3.1. Blood Determinations
4.3.2. Histological and Immunohistochemical Analyses
4.3.3. Immunofluorescence and Confocal Microscopy
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tefferi, A. Primary Myelofibrosis: 2023 Update on Diagnosis, Risk-stratification, and Management. Am. J. Hematol. 2023, 98, 801–821. [Google Scholar] [CrossRef] [PubMed]
- Grinfeld, J.; Nangalia, J.; Green, A.R. Molecular Determinants of Pathogenesis and Clinical Phenotype in Myeloproliferative Neoplasms. Haematologica 2017, 102, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Marcellino, B.K.; Verstovsek, S.; Mascarenhas, J. The Myelodepletive Phenotype in Myelofibrosis: Clinical Relevance and Therapeutic Implication. Clin. Lymphoma Myeloma Leuk. 2020, 20, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.; Hoffman, R. A Comprehensive Review and Analysis of the Effect of Ruxolitinib Therapy on the Survival of Patients with Myelofibrosis. Blood 2013, 121, 4832–4837. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.; Vannucchi, A.M.; Kiladjian, J.-J.; Al-Ali, H.K.; Sirulnik, A.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.S.; Passamonti, F.; et al. Three-Year Efficacy, Safety, and Survival Findings from COMFORT-II, a Phase 3 Study Comparing Ruxolitinib with Best Available Therapy for Myelofibrosis. Blood 2013, 122, 4047–4053. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, A.M.; Kantarjian, H.M.; Kiladjian, J.-J.; Gotlib, J.; Cervantes, F.; Mesa, R.A.; Sarlis, N.J.; Peng, W.; Sandor, V.; Gopalakrishna, P.; et al. A Pooled Analysis of Overall Survival in COMFORT-I and COMFORT-II, 2 Randomized Phase III Trials of Ruxolitinib for the Treatment of Myelofibrosis. Haematologica 2015, 100, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Jacquelin, S.; Kramer, F.; Mullally, A.; Lane, S.W. Murine Models of Myelofibrosis. Cancers 2020, 12, 2381. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Merchant, D.; Mahmud, N.; Ishii, T.; Zhao, Y.; Hu, W.; Bruno, E.; Barosi, G.; Xu, M.; Hoffman, R. Pivotal Contributions of Megakaryocytes to the Biology of Idiopathic Myelofibrosis. Blood 2007, 110, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, A.M.; Pancrazzi, A.; Guglielmelli, P.; Di Lollo, S.; Bogani, C.; Baroni, G.; Bianchi, L.; Migliaccio, A.R.; Bosi, A.; Paoletti, F. Abnormalities of GATA-1 in Megakaryocytes from Patients with Idiopathic Myelofibrosis. Am. J. Pathol. 2005, 167, 849–858. [Google Scholar] [CrossRef]
- Zingariello, M.; Verachi, P.; Gobbo, F.; Martelli, F.; Falchi, M.; Mazzarini, M.; Valeri, M.; Sarli, G.; Marinaccio, C.; Melo-Cardenas, J.; et al. Resident Self-Tissue of Proinflammatory Cytokines Rather than Their Systemic Levels Correlates with Development of Myelofibrosis in Gata1low Mice. Biomolecules 2022, 12, 234. [Google Scholar] [CrossRef]
- Messina, G.; Blasi, C.; La Rocca, S.A.; Pompili, M.; Calconi, A.; Grossi, M. P27Kip1 Acts Downstream of N-Cadherin-Mediated Cell Adhesion to Promote Myogenesis beyond Cell Cycle Regulation. Mol. Biol. Cell 2005, 16, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Rodrigues, N.; Dombkowski, D.; Stier, S.; Scadden, D.T. Stem Cell Repopulation Efficiency but Not Pool Size Is Governed by P27kip1. Nat. Med. 2000, 6, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Tefferi, A.; Guglielmelli, P.; Bogani, C.; Bartalucci, N.; Rodríguez, J.; Extremera, S.; Pérez, I.; Alfaro, V.; Vannucchi, A.M. Evaluation of Plitidepsin in Patients with Primary Myelofibrosis and Post Polycythemia Vera/Essential Thrombocythemia Myelofibrosis: Results of Preclinical Studies and a Phase II Clinical Trial. Blood Cancer J. 2015, 5, e286. [Google Scholar] [CrossRef] [PubMed]
- Mcdevitt, M.A.; Fujiwara, Y.; Shivdasani, R.A.; Orkin, S.H. An Upstream, DNase I Hypersensitive Region of the Hematopoietic-Expressed Transcription Factor GATA-1 Gene Confers Developmental Specificity in Transgenic Mice. Proc. Natl. Acad. Sci. USA 1997, 94, 7976–7981. [Google Scholar] [CrossRef] [PubMed]
- Shivdasani, R.A.; Fujiwara, Y.; McDevitt, M.A.; Orkin, S.H. A Lineage-Selective Knockout Establishes the Critical Role of Transcription Factor GATA-1 in Megakaryocyte Growth and Platelet Development. EMBO J. 1997, 16, 3965–3973. [Google Scholar] [CrossRef] [PubMed]
- Zingariello, M.; Ruggeri, A.; Martelli, F.; Marra, M.; Sancillo, L.; Ceglia, I.; Rana, R.A.; Migliaccio, A.R. A Novel Interaction between Megakaryocytes and Activated Fibrocytes Increases TGF-β Bioavailability in the Gata1(Low) Mouse Model of Myelofibrosis. Am. J. Blood Res. 2015, 5, 34–61. [Google Scholar] [PubMed]
- Zingariello, M.; Martelli, F.; Ciaffoni, F.; Masiello, F.; Ghinassi, B.; D’Amore, E.; Massa, M.; Barosi, G.; Sancillo, L.; Li, X.; et al. Characterization of the TGF-B1 Signaling Abnormalities in the Gata1low Mouse Model of Myelofibrosis. Blood 2013, 121, 3345–3363. [Google Scholar] [CrossRef]
- Spangrude, G.J.; Lewandowski, D.; Martelli, F.; Marra, M.; Zingariello, M.; Sancillo, L.; Alba Rana, R.; Rita Migliaccio, A. P-Selectin Sustains Extramedullary Hematopoiesis in the Gata1low Model of Myelofibrosis. Stem Cells 2016, 34, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Zingariello, M.; Martelli, F.; Verachi, P.; Bardelli, C.; Gobbo, F.; Mazzarini, M.; Migliaccio, A.R. Novel Targets to Cure Primary Myelofibrosis from Studies on Gata1 Low Mice. IUBMB Life 2020, 72, 131–141. [Google Scholar] [CrossRef]
- Verrucci, M.; Pancrazzi, A.; Aracil, M.; Martelli, F.; Guglielmelli, P.; Zingariello, M.; Ghinassi, B.; D’Amore, E.; Jimeno, J.; Vannucchi, A.M.; et al. CXCR4-independent Rescue of the Myeloproliferative Defect of the Gata1 Low Myelofibrosis Mouse Model by Aplidin®. J. Cell Physiol. 2010, 225, 490–499. [Google Scholar] [CrossRef]
- Varricchio, L.; Iancu-Rubin, C.; Upadhyaya, B.; Zingariello, M.; Martelli, F.; Verachi, P.; Clementelli, C.; Denis, J.-F.; Rahman, A.H.; Tremblay, G.; et al. TGF-Β1 Protein Trap AVID200 Beneficially Affects Hematopoiesis and Bone Marrow Fibrosis in Myelofibrosis. JCI Insight 2021, 6, e145651. [Google Scholar] [CrossRef] [PubMed]
- Ataga, K.I.; Kutlar, A.; Kanter, J.; Liles, D.; Cancado, R.; Friedrisch, J.; Guthrie, T.H.; Knight-Madden, J.; Alvarez, O.A.; Gordeuk, V.R.; et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 429–439. [Google Scholar] [CrossRef]
- Verachi, P.; Gobbo, F.; Martelli, F.; Martinelli, A.; Sarli, G.; Dunbar, A.; Levine, R.L.; Hoffman, R.; Massucci, M.T.; Brandolini, L.; et al. The CXCR1/CXCR2 Inhibitor Reparixin Alters the Development of Myelofibrosis in the Gata1low Mice. Front. Oncol. 2022, 12, 853484. [Google Scholar] [CrossRef] [PubMed]
- Zingariello, M.; Sancillo, L.; Martelli, F.; Ciaffoni, F.; Marra, M.; Varricchio, L.; Rana, R.A.; Zhao, C.; Crispino, J.D.; Migliaccio, A.R. The Thrombopoietin/MPL Axis Is Activated in the Gata1 Low Mouse Model of Myelo Fi Brosis and Is Associated with a Defective RPS14 Signature. Blood Cancer J. 2017, 7, e572. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Bianchi, L.; Cellai, C.; Paoletti, F.; Rana, R.A.; Lorenzini, R.; Migliaccio, G.; Migliaccio, A.R. Development of Myelofibrosis in Mice Genetically Impaired for GATA-1 Expression (GATA-1low Mice). Blood 2002, 100, 1123–1132. [Google Scholar] [CrossRef]
- Verachi, P.; Gobbo, F.; Martelli, F.; Falchi, M.; di Virgilio, A.; Sarli, G.; Wilke, C.; Bruederle, A.; Prahallad, A.; Arciprete, F.; et al. Preclinical Studies on the Use of a P-Selectin-Blocking Monoclonal Antibody to Halt Progression of Myelofibrosis in the Gata1 Mouse Model. Exp. Hematol. 2023, 117, 43–61. [Google Scholar] [CrossRef]
- Kuter, D.J.; Bain, B.; Mufti, G.; Bagg, A.; Hasserjian, R.P. Bone Marrow Fibrosis: Pathophysiology and Clinical Significance of Increased Bone Marrow Stromal Fibres. Br. J. Haematol. 2007, 139, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.; Kvasnicka, H.M.; Facchetti, F.; Franco, V.; van der Walt, J.; Orazi, A. European Consensus on Grading Bone Marrow Fibrosis and Assessment of Cellularity. Haematologica 2005, 90, 1128–1132. [Google Scholar] [PubMed]
- Karagianni, A.; Ravid, K. Myeloproliferative Disorders and Their Effects on Bone Homeostasis: The Role of Megakaryocytes. Blood 2022, 139, 3127–3137. [Google Scholar] [CrossRef]
- Kacena, M.A.; Shivdasani, R.A.; Wilson, K.; Xi, Y.; Troiano, N.; Nazarian, A.; Gundberg, C.M.; Bouxsein, M.L.; Lorenzo, J.A.; Horowitz, M.C. Megakaryocyte-Osteoblast Interaction Revealed in Mice Deficient in Transcription Factors GATA-1 and NF-E2. J. Bone Miner. Res. 2004, 19, 652–660. [Google Scholar] [CrossRef]
- Garimella, R.; Kacena, M.A.; Tague, S.E.; Wang, J.; Horowitz, M.C.; Anderson, H.C. Expression of Bone Morphogenetic Proteins and Their Receptors in the Bone Marrow Megakaryocytes of GATA-1low Mice: A Possible Role in Osteosclerosis. J. Histochem. Cytochem. 2007, 55, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Stavnichuk, M.; Komarova, S.V. Megakaryocyte-Bone Cell Interactions: Lessons from Mouse Models of Experimental Myelofibrosis and Related Disorders. Am. J. Physiol.-Cell Physiol. 2022, 322, C177–C184. [Google Scholar] [CrossRef]
- Tremblay, D.; Mascarenhas, J. Pharmacotherapeutic Advances for Splenomegaly in Myelofibrosis. Expert. Opin. Pharmacother. 2023, 24, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, H.K.; Griesshammer, M.; le Coutre, P.; Waller, C.F.; Liberati, A.M.; Schafhausen, P.; Tavares, R.; Giraldo, P.; Foltz, L.; Raanani, P.; et al. Safety and Efficacy of Ruxolitinib in an Open-Label, Multicenter, Single-Arm Phase 3b Expanded-Access Study in Patients with Myelofibrosis: A Snapshot of 1144 Patients in the JUMP Trial. Haematologica 2016, 101, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Centurione, L.; Di Baldassarre, A.; Zingariello, M.; Bosco, D.; Gatta, V.; Rana, R.A.; Langella, V.; Di Virgilio, A.; Vannucchi, A.M.; Migliaccio, A.R. Increased and Pathologic Emperipolesis of Neutrophils within Megakaryocytes Associated with Marrow Fibrosis in GATA-1low Mice. Blood 2004, 104, 3573–3580. [Google Scholar] [CrossRef] [PubMed]
- Blank, U.; Karlsson, S. TGF-β Signaling in the Control of Hematopoietic Stem Cells. Blood 2015, 125, 3542–3550. [Google Scholar] [CrossRef] [PubMed]
- Gostynska, S.; Venkatesan, T.; Subramani, K.; Cortez, B.; Robertson, A.; Subrahmanian, S.; Dube, P.; Ahamed, J. Megakaryocyte/Platelet-Derived TGF-Β1 Inhibits Megakaryopoiesis in Bone Marrow by Regulating Thrombopoietin Production in Liver. Blood Adv. 2022, 6, 3321–3328. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.; Hoffman, R. Ruxolitinib: The First FDA Approved Therapy for the Treatment of Myelofibrosis. Clin. Cancer Res. 2012, 18, 3008–3014. [Google Scholar] [CrossRef] [PubMed]
- Arana Yi, C.; Tam, C.S.; Verstovsek, S. Efficacy and Safety of Ruxolitinib in the Treatment of Patients with Myelofibrosis. Future Oncol. 2015, 11, 719–733. [Google Scholar] [CrossRef]
- Poppenborg, F.; Röth, A.; Yamamoto, R.; Reinhardt, C.; Alashkar, F. Crizanlizumab in Adult Patients with Sickle Cell Disease (SCD): A Retrospective German Analysis. Front. Hematol. 2024, 3, 1374181. [Google Scholar] [CrossRef]
- Kanter, J.; Brown, R.C.; Norris, C.; Nair, S.M.; Kutlar, A.; Manwani, D.; Shah, N.; Tanaka, C.; Bodla, S.; Sanchez-Olle, G.; et al. Pharmacokinetics, Pharmacodynamics, Safety, and Efficacy of Crizanlizumab in Patients with Sickle Cell Disease. Blood Adv. 2023, 7, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Voltz, C.; Stain, M.; Lapveteläinen, T.; Urach, S.; Lähteenvuo, J.; Penttilä, K.; Gisselbrecht, C.; Enzmann, H.; Pignatti, F. The European Medicines Agency Review of Crizanlizumab for the Prevention of Recurrent Vaso-Occlusive Crises in Patients with Sickle Cell Disease. Hemasphere 2021, 5, e604. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kim, J.-H.; Lu, W.; Williams, D.M.; Kim, J.; Cope, L.; Rampal, R.K.; Koche, R.P.; Xian, L.; Luo, L.Z.; et al. HMGA1 Chromatin Regulators Induce Transcriptional Networks Involved in GATA2 and Proliferation during MPN Progression. Blood 2022, 139, 2797–2815. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.; Kosiorek, H.E.; Bhave, R.; Palmer, J.M.; Kuykendall, A.T.; Mesa, R.A.; Rampal, R.; Gerds, A.T.; Yacoub, A.; Pettit, K.M.; et al. Treatment of Myelofibrosis Patients with the TGF-β 1/3 Inhibitor AVID200 (MPN-RC 118) Induces a Profound Effect on Platelet Production. Blood 2021, 138, 142. [Google Scholar] [CrossRef]
- Santini, V.; Valcárcel, D.; Platzbecker, U.; Komrokji, R.S.; Cleverly, A.L.; Lahn, M.M.; Janssen, J.; Zhao, Y.; Chiang, A.; Giagounidis, A.; et al. Phase II Study of the ALK5 Inhibitor Galunisertib in Very Low-, Low-, and Intermediate-Risk Myelodysplastic Syndromes. Clin. Cancer Res. 2019, 25, 6976–6985. [Google Scholar] [CrossRef] [PubMed]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted Drug Delivery Strategies for Precision Medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Gao, X.; Xu, C.; Asada, N.; Frenette, P.S. The Hematopoietic Stem Cell Niche: From Embryo to Adult. Development 2018, 145, dev139691. [Google Scholar] [CrossRef]
- Baccin, C.; Al-Sabah, J.; Velten, L.; Helbling, P.M.; Grünschläger, F.; Hernández-Malmierca, P.; Nombela-Arrieta, C.; Steinmetz, L.M.; Trumpp, A.; Haas, S. Combined Single-Cell and Spatial Transcriptomics Reveal the Molecular, Cellular and Spatial Bone Marrow Niche Organization. Nat. Cell Biol. 2020, 22, 38–48. [Google Scholar] [CrossRef]
- Coutu, D.L.; Kokkaliaris, K.D.; Kunz, L.; Schroeder, T. Three-Dimensional Map of Nonhematopoietic Bone and Bone-Marrow Cells and Molecules. Nat. Biotechnol. 2017, 35, 1202–1210. [Google Scholar] [CrossRef]
- Psaila, B.; Mead, A.J. Single-Cell Approaches Reveal Novel Cellular Pathways for Megakaryocyte and Erythroid Differentiation. Blood 2019, 133, 1427–1435. [Google Scholar] [CrossRef]
- He, N.; Zhang, L.; Cui, J.; Li, Z. Bone Marrow Vascular Niche: Home for Hematopoietic Stem Cells. Bone Marrow Res. 2014, 2014, 128436. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar Niches Maintain Haematopoietic Stem Cell Quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Arranz, L.; Sánchez-Aguilera, A.; Martín-Pérez, D.; Isern, J.; Langa, X.; Tzankov, A.; Lundberg, P.; Muntión, S.; Tzeng, Y.S.; Lai, D.M.; et al. Neuropathy of Haematopoietic Stem Cell Niche Is Essential for Myeloproliferative Neoplasms. Nature 2014, 512, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Borikar, S.; Bell, R.; Kuffler, L.; Philip, V.; Trowbridge, J.J. Progressive Alterations in Multipotent Hematopoietic Progenitors Underlie Lymphoid Cell Loss in Aging. J. Exp. Med. 2016, 213, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.H.; del Toro, R.; Rivera-Torres, J.; Rak, J.; Korn, C.; García-García, A.; Macías, D.; González-Gómez, C.; del Monte, A.; Wittner, M.; et al. Remodeling of Bone Marrow Hematopoietic Stem Cell Niches Promotes Myeloid Cell Expansion during Premature or Physiological Aging. Cell Stem Cell 2019, 25, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Kokkaliaris, K.D.; Kunz, L.; Cabezas-Wallscheid, N.; Christodoulou, C.; Renders, S.; Camargo, F.; Trumpp, A.; Scadden, D.T.; Schroeder, T. Adult Blood Stem Cell Localization Reflects the Abundance of Reported Bone Marrow Niche Cell Types and Their Combinations. Blood 2020, 136, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Mazzarini, M.; Arciprete, F.; Picconi, O.; Valeri, M.; Verachi, P.; Martelli, F.; Migliaccio, A.R.; Falchi, M.; Zingariello, M. Single Cell Analysis of the Localization of the Hematopoietic Stem Cells within the Bone Marrow Architecture Identifies Niche-Specific Proliferation Dynamics. Front. Med. 2023, 10, 1166758. [Google Scholar] [CrossRef]
- Yao, J.-C.; Oetjen, K.A.; Wang, T.; Xu, H.; Abou-Ezzi, G.; Krambs, J.R.; Uttarwar, S.; Duncavage, E.J.; Link, D.C. TGF-β Signaling in Myeloproliferative Neoplasms Contributes to Myelofibrosis without Disrupting the Hematopoietic Niche. J. Clin. Investig. 2022, 132, e154092. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, A.; Kim, D.; Lu, M.; Farina, M.; Bowman, R.L.; Yang, J.L.; Park, Y.C.; Karzai, A.; Xiao, W.; Zaroogian, Z.; et al. CXCL8/CXCR2 Signaling Mediates Bone Marrow Fibrosis and Represents a Therapeutic Target in Myelofibrosis. Blood 2023, 141, 2508–2519. [Google Scholar] [CrossRef]
- Jenkins, R.G.; Moore, B.B.; Chambers, R.C.; Eickelberg, O.; Königshoff, M.; Kolb, M.; Laurent, G.J.; Nanthakumar, C.B.; Olman, M.A.; Pardo, A.; et al. An Official American Thoracic Society Workshop Report: Use of Animal Models for the Preclinical Assessment of Potential Therapies for Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2017, 56, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.C.; Burbury, K.; Lehmann, T.; Ross, D.M.; Reiter, A.; Gupta, V.; Harrison, C.; Kiladjian, J.-J.; Vannucchi, A.M.; Wondergem, M.; et al. Adore: A Randomized, Open-Label, Phase 1/2 Open-Platform Study Evaluating Safety and Efficacy of Novel Ruxolitinib Combinations in Patients with Myelofibrosis. Blood 2020, 136, 52–53. [Google Scholar] [CrossRef]
- Belletti, B.; Pellizzari, I.; Berton, S.; Fabris, L.; Wolf, K.; Lovat, F.; Schiappacassi, M.; D’Andrea, S.; Nicoloso, M.S.; Lovisa, S.; et al. P27kip1 Controls Cell Morphology and Motility by Regulating Microtubule-Dependent Lipid Raft Recycling. Mol. Cell Biol. 2010, 30, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Beignet, J.; Loirand, G.; Sauzeau, V. Rac1 as a Therapeutic Anticancer Target: Promises and Limitations. Biochem. Pharmacol. 2022, 203, 115180. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Published | Performed for This Study | Not Available |
|---|---|---|---|
| Femur | |||
| Cellularity (total cell counts of a femur) | All treatments | ||
| Fibrosis (reticulin staining) | Aplidin | Confirmed ex novo for all the other drugs | |
| Vessel density (IHC for CD34+ staining) | SB431542, AVID200, RB40.34, and Rux | Reparixin | Aplidin |
| Bone area (computer-assisted evaluation of track images) | SB431542, AVID200, RB40.34, Rux, and Reparixin | Aplidin | |
| Bone maturation (Mallory trichrome staining) | SB431542, AVID200, RB40.34, Rux, and Reparixin | Aplidin | |
| TGF-β1/CXCL1 content (IHC with specific antibodies) | SB431542, AVID200, RB40.34, Rux, and Reparixin | Aplidin | |
| Spleen | |||
| Weight | All treatments | ||
| Cellularity | All treatments | Aplidin | |
| Fibrosis (reticulin staining) | SB431542, AVID200, RB40.34, Rux, and Reparixin | Aplidin | |
| Thrombopoiesis | |||
| Platelet counts | All treatments | ||
| Total MK number in BM (IF with CD42b) | SB431542, AVID200, RB40.34, Rux, and Reparixin | Aplidin | |
| GATA1 content in MKs (double IF for GATA1 and CD42b) | SB431542, AVID200, RB40.34, Rux, and Reparixin | Aplidin | |
| Treatment | Femur Cellularity (p-Value vs. Vehicle) | BM Fibrosis (p-Value vs. Vehicle) | No. of Vessels (p-Value vs. Vehicle) | Bone Area (p-Value vs. Vehicle) | Mature Cortical Bone (p-Value vs. Vehicle) | Mature Trabecular Bone (p-Value vs. Vehicle) | Spleen Weight (p-Value vs. Vehicle) | Spleen Cellularity (p-Value vs. Vehicle) | Fibrosis Spleen (p-Value vs. Vehicle) | TGF-β1 (p-Value vs. Vehicle) | CXCL1 (p-Value vs. Vehicle) | Platelet Count (p-Value vs. Vehicle) | GATA + MKs (p-Value vs. Vehicle) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aplidin (83d) | p < 0.05 | p < 0.05 | p < 0.05 | \ | \ | \ | ns | \ | \ | p < 0.05 | \ | p < 0.05 | p < 0.05 |
| SB431542 (54d) | p < 0.05 | p < 0.05 | p < 0.05 | ns | ns | ns | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | ns | p < 0.0001 | ns |
| AVID200 (42d) | p < 0.05 | \ | \ | \ | \ | \ | ns | ns | \ | \ | \ | p < 0.05 | \ |
| AVID200 (72d) | p < 0.05 | p < 0.001 | p < 0.001 | ns | ns | ns | ns | ns | ns | p < 0.001 | ns | p < 0.01 | p < 0.05 |
| RB40.34 (12d) | ns | ns | \ | \ | \ | \ | ns | ns | \ | \ | \ | ns | \ |
| RB40.34 (54d) | ns | p < 0.05 | p < 0.001 | ns | ns | ns | ns | ns | p < 0.05 | ns | ns | ns | ns |
| Rux (12d) | ns | ns | \ | \ | \ | \ | ns | ns | \ | \ | \ | ns | \ |
| Rux (54d) | ns | ns | p < 0.001 | ns | ns | ns | ns | ns | p < 0.001 | p < 0.05 | ns | ns | p < 0.0001 |
| RB40.34 + Rux (12d) | ns | ns | \ | \ | \ | \ | ns | ns | \ | \ | \ | ns | \ |
| RB40.34 + Rux (54d) | p < 0.05 | p < 0.05 | p < 0.001 | ns | ns | ns | ns | ns | p < 0.001 | p < 0.05 | p < 0.01 | ns | ns |
| Reparixin (20d) | ns | p < 0.05 | ns | ns | ns | ns | ns | ns | p < 0.05 | p < 0.05 | ns | ns | p < 0.001 |
| Reparixin (37d) | ns | ns | p < 0.05 | ns | ns | ns | ns | ns | ns | p < 0.0001 | ns | ns | ns |
| Endpoints | Most Effective Treatments (in Order of Efficacy) |
|---|---|
| Femur | |
| Cellularity | Aplidin |
| Fibrosis | Reparixin 20 days |
| Vessel density | Reparixin 37 days SB431542 54 days |
| Bone area | RB40.34 + Rux 54 days |
| Mature cortical bone | SB431542 54 days Rux 54 days Reparixin 37 days |
| Mature trabecular bone | SB431542 54 days Reparixin 37 days |
| TGF-β1 | AVID200 Reparixin 37 days |
| CXCL1 | RB40.34 + Rux 54 days |
| Spleen | |
| Weight | SB431542 54 days RB40.34 + Rux 54 days |
| Cellularity | RB40.34 + Rux 54 days |
| Fibrosis | Rux 54 days RB40.34 + Rux 54 days |
| Thrombopoiesis | |
| Platelet counts | Aplidin SB431542 54 days AVID200 |
| Total MK number in BM | None |
| GATA1 content in MKs | AVID200, Rux 54 days, and Reparixin 20 days but only SB431542 in the polylobated cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobbo, F.; Martelli, F.; Di Virgilio, A.; Demaria, E.; Sarli, G.; Migliaccio, A.R. The Variation in the Traits Ameliorated by Inhibitors of JAK1/2, TGF-β, P-Selectin, and CXCR1/CXCR2 in the Gata1low Model Suggests That Myelofibrosis Should Be Treated by These Drugs in Combination. Int. J. Mol. Sci. 2024, 25, 7703. https://doi.org/10.3390/ijms25147703
Gobbo F, Martelli F, Di Virgilio A, Demaria E, Sarli G, Migliaccio AR. The Variation in the Traits Ameliorated by Inhibitors of JAK1/2, TGF-β, P-Selectin, and CXCR1/CXCR2 in the Gata1low Model Suggests That Myelofibrosis Should Be Treated by These Drugs in Combination. International Journal of Molecular Sciences. 2024; 25(14):7703. https://doi.org/10.3390/ijms25147703
Chicago/Turabian StyleGobbo, Francesca, Fabrizio Martelli, Antonio Di Virgilio, Elena Demaria, Giuseppe Sarli, and Anna Rita Migliaccio. 2024. "The Variation in the Traits Ameliorated by Inhibitors of JAK1/2, TGF-β, P-Selectin, and CXCR1/CXCR2 in the Gata1low Model Suggests That Myelofibrosis Should Be Treated by These Drugs in Combination" International Journal of Molecular Sciences 25, no. 14: 7703. https://doi.org/10.3390/ijms25147703







