From Molecular Mechanisms to Clinical Therapy: Understanding Sepsis-Induced Multiple Organ Dysfunction
Abstract
1. Introduction
2. Definition of Sepsis
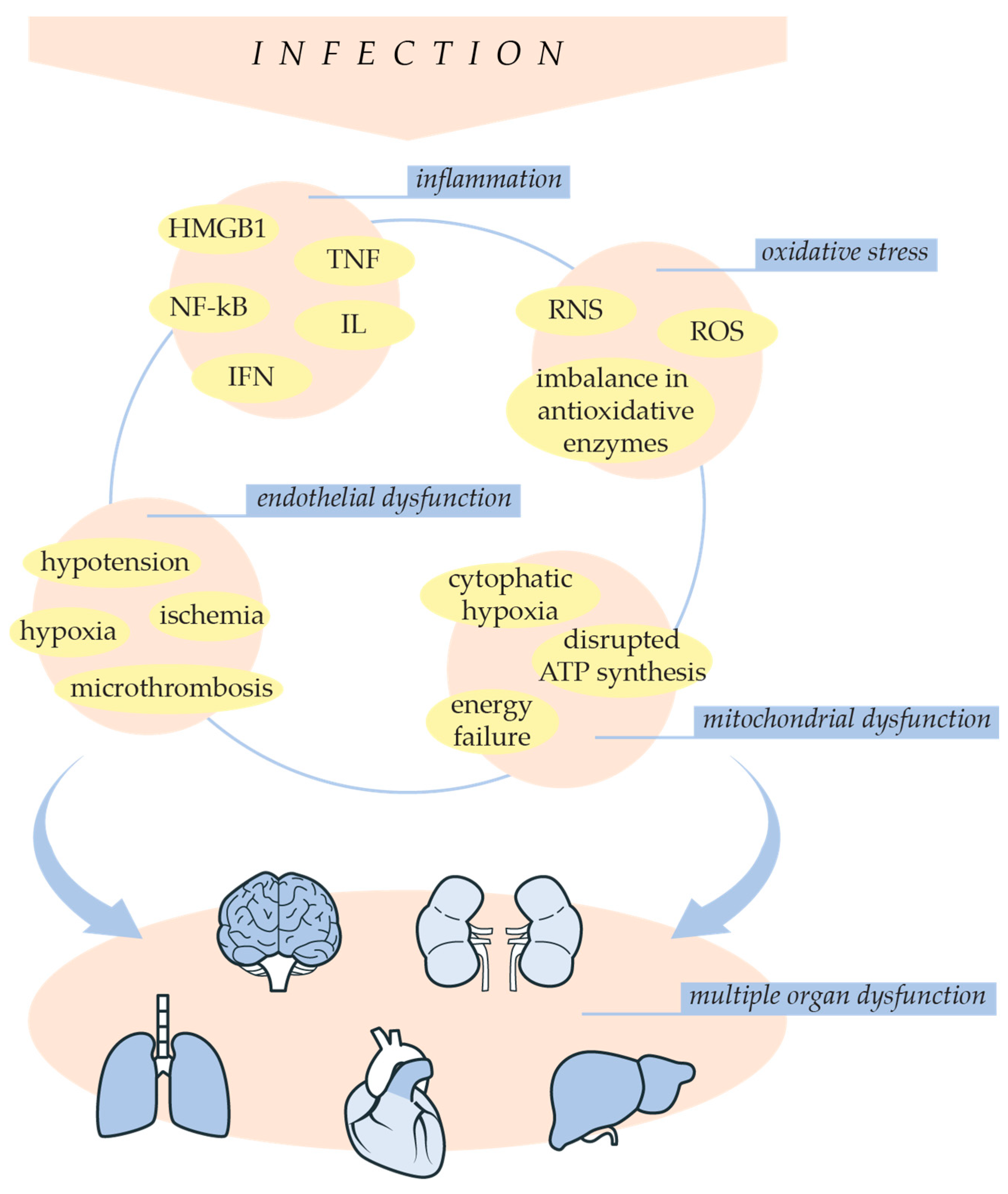
3. Pathophysiology of Sepsis
3.1. Inflammation and Oxidative Stress in Sepsis Development
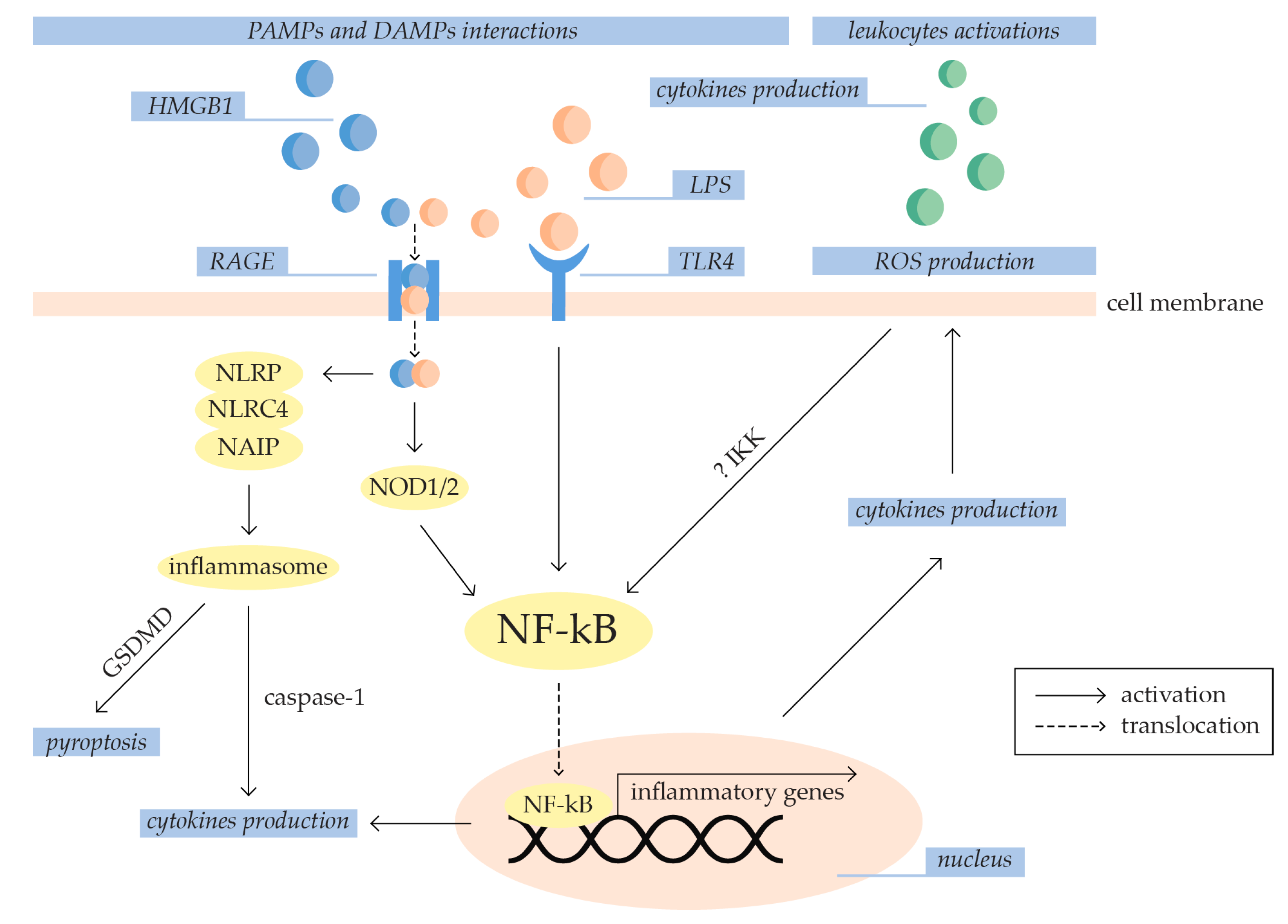
3.1.1. Inflammation
3.1.2. Oxidative Stress
3.2. Sepsis-Related Endothelial Dysfunction
4. Sepsis-Induced Multiple Organ Dysfunction
4.1. Liver Dysfunction
4.2. Cardiac Dysfunction
4.3. Acute Lung Injury (ALI)
4.4. Acute Kidney Injury (AKI)
5. Treatment Strategies for Sepsis
5.1. Early Diagnosis and Antimicrobial Therapy
5.2. Pharmacological Approaches in Clinical Practice of Sepsis Treatment
5.3. Metabolic Support
5.4. Septic Shock Treatment
6. Evaluating Sepsis Treatment
6.1. Experimental Models of Sepsis
6.2. Examination of Novel Therapeutical Approaches
6.2.1. Melatonin
6.2.2. Metformin
6.2.3. Palmitoylethanolamide (PEA)
6.2.4. Herbal Extracts
6.2.5. Modulating Gut Microbiota
7. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matot, I.; Sprung, C.L. Definition of Sepsis. Intensive Care Med. 2001, 27, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; Shankar-Hari, M.; Wiersinga, W.J. The Immunology of Sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef]
- Rello, J.; Valenzuela-Sánchez, F.; Ruiz-Rodriguez, M.; Moyano, S. Sepsis: A Review of Advances in Management. Adv. Ther. 2017, 34, 2393–2411. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and Mortality of Septic Shock in Europe and North America: A Systematic Review and Meta-Analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef]
- Gourd, N.M.; Nikitas, N. Multiple Organ Dysfunction Syndrome. J. Intensive Care Med. 2020, 35, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, C.; Jaimes, F. Focus: Death: Organ Dysfunction in Sepsis: An Ominous Trajectory from Infection to Death. Yale J. Biol. Med. 2019, 92, 629. [Google Scholar]
- Sun, J.; Zhang, J.; Wang, X.; Ji, F.; Ronco, C.; Tian, J.; Yin, Y. Gut-Liver Crosstalk in Sepsis-Induced Liver Injury. Crit. Care 2020, 24, 614. [Google Scholar] [CrossRef]
- Habimana, R.; Choi, I.; Cho, H.J.; Kim, D.; Lee, K.; Jeong, I. Sepsis-Induced Cardiac Dysfunction: A Review of Pathophysiology. Acute Crit. Care 2020, 35, 57–66. [Google Scholar] [CrossRef]
- Sun, B.; Lei, M.; Zhang, J.; Kang, H.; Liu, H.; Zhou, F. Acute Lung Injury Caused by Sepsis: How Does It Happen? Front. Med. 2023, 10, 1289194. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Priyanka, P.; Wang, S.; Smith, A.; Singbartl, K.; Palevsky, P.M.; Chawla, L.S.; Yealy, D.M.; Angus, D.C.; Kellum, J.A.; et al. Sepsis-Associated Acute Kidney Disease. Kidney Int. Rep. 2020, 5, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Majno, G. The Ancient Riddle of Σñψις (Sepsis). J. Infect. Dis. 1991, 163, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and Septic Shock. Nat. Rev. Dis. Prim. 2016, 2, 16045. [Google Scholar] [CrossRef]
- Guarino, M.; Perna, B.; Cesaro, A.E.; Maritati, M.; Spampinato, M.D.; Contini, C.; De Giorgio, R. 2023 Update on Sepsis and Septic Shock in Adult Patients: Management in the Emergency Department. J. Clin. Med. 2023, 12, 3188. [Google Scholar] [CrossRef]
- Deng, C.; Zhao, L.; Yang, Z.; Shang, J.; Wang, C.; Shen, M.; Jiang, S.; Li, T.; Di, W.; Chen, Y. Targeting HMGB1 for the Treatment of Sepsis and Sepsis-Induced Organ Injury. Acta Pharmacol. Sin. 2022, 43, 520–528. [Google Scholar] [CrossRef]
- Martín-Fernández, M.; Tamayo-Velasco, Á.; Aller, R.; Gonzalo-Benito, H.; Martínez-Paz, P.; Tamayo, E. Endothelial Dysfunction and Neutrophil Degranulation as Central Events in Sepsis Physiopathology. Int. J. Mol. Sci. 2021, 22, 6272. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine Storm and Sepsis Disease Pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; van der Poll, T. Immunopathophysiology of Human Sepsis. eBioMedicine 2022, 86, 104363. [Google Scholar] [CrossRef]
- Vénéreau, E.; Ceriotti, C.; Bianchi, M.E. DAMPs from Cell Death to New Life. Front. Immunol. 2015, 6, 422. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J. Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef]
- Choi, H.; Lee, J.Y.; Yoo, H.; Jeon, K. Bioinformatics Analysis of Gene Expression Profiles for Diagnosing Sepsis and Risk Prediction in Patients with Sepsis. Int. J. Mol. Sci. 2023, 24, 9362. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Feng, J.; Yang, Y.; Zhao, R.; Yu, Q.; Qin, H.; Wei, L.; Ji, P.; Li, H.; Wu, Z.; et al. Screening of Key Genes of Sepsis and Septic Shock Using Bioinformatics Analysis. J. Inflamm. Res. 2021, 14, 829–841. [Google Scholar] [CrossRef]
- Ow, C.P.C.; Trask-Marino, A.; Betrie, A.H.; Evans, R.G.; May, C.N.; Lankadeva, Y.R. Targeting Oxidative Stress in Septic Acute Kidney Injury: From Theory to Practice. J. Clin. Med. 2021, 10, 3798. [Google Scholar] [CrossRef]
- Prauchner, C.A. Oxidative Stress in Sepsis: Pathophysiological Implications Justifying Antioxidant Co-Therapy. Burns 2017, 43, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Nagar, H.; Piao, S.; Kim, C.-S. Role of Mitochondrial Oxidative Stress in Sepsis. Acute Crit. Care 2018, 33, 65–72. [Google Scholar] [CrossRef]
- Pashenkov, M.V.; Murugina, N.E.; Budikhina, A.S.; Pinegin, B.V. Synergistic Interactions between NOD Receptors and TLRs: Mechanisms and Clinical Implications. J. Leukoc. Biol. 2019, 105, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Platnich, J.M.; Muruve, D.A. NOD-like Receptors and Inflammasomes: A Review of Their Canonical and Non-Canonical Signaling Pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, J.-S.; Nahm, M.H. NOD-like Receptors in Infection, Immunity, and Diseases. Yonsei Med. J. 2016, 57, 5. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M.; Kayagaki, N. Dying Cells Fan the Flames of Inflammation. Science 2021, 374, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Nawroth, P.P. Modulation of the Vascular Endothelium during Infection-the Role of NF-κB Activation. Host Response Mech. Infect. Dis. 2003, 10, 86–105. [Google Scholar] [CrossRef]
- Biswas, R.; Bagchi, A. NFkB Pathway and Inhibition: An Overview. Comput. Mol. Biol. 2016, 6, 1–20. [Google Scholar] [CrossRef]
- Galley, H.F. Oxidative Stress and Mitochondrial Dysfunction in Sepsis. Br. J. Anaesth. 2011, 107, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Waghela, B.N.; Vaidya, F.U.; Agrawal, Y.; Santra, M.K.; Mishra, V.; Pathak, C. Molecular Insights of NADPH Oxidases and Its Pathological Consequences. Cell Biochem. Funct. 2021, 39, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Đurašević, S.; Ružičić, A.; Lakić, I.; Tosti, T.; Đurović, S.; Glumac, S.; Pejić, S.; Todorović, A.; Drakulić, D.; Stanković, S. The Effects of a Meldonium Pre-Treatment on the Course of the LPS-Induced Sepsis in Rats. Int. J. Mol. Sci. 2022, 23, 2395. [Google Scholar] [CrossRef] [PubMed]
- Schröder, K. NADPH Oxidases: Current Aspects and Tools. Redox Biol. 2020, 34, 101512. [Google Scholar] [CrossRef]
- Taylor, J.P.; Tse, H.M. The Role of NADPH Oxidases in Infectious and Inflammatory Diseases. Redox Biol. 2021, 48, 102159. [Google Scholar] [CrossRef] [PubMed]
- Mallat, J.; Rahman, N.; Hamed, F.; Hernandez, G.; Fischer, M.-O. Pathophysiology, Mechanisms, and Managements of Tissue Hypoxia. Anaesth. Crit. Care Pain Med. 2022, 41, 101087. [Google Scholar] [CrossRef]
- Salomão, R.; Ferreira, B.L.; Salomão, M.C.; Santos, S.S.; Azevedo, L.C.P.; Brunialti, M.K.C. Sepsis: Evolving Concepts and Challenges. Braz. J. Med. Biol. Res. 2019, 52, e8595. [Google Scholar] [CrossRef]
- Evans, C.E. Hypoxia and HIF Activation as a Possible Link between Sepsis and Thrombosis. Thromb. J. 2019, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Walborn, A.; Rondina, M.; Mosier, M.; Fareed, J.; Hoppensteadt, D. Endothelial Dysfunction Is Associated with Mortality and Severity of Coagulopathy in Patients with Sepsis and Disseminated Intravascular Coagulation. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619852163. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Dolmatova, E.V.; Wang, K.; Mandavilli, R.; Griendling, K.K. The Effects of Sepsis on Endothelium and Clinical Implications. Cardiovasc. Res. 2021, 117, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C. Sepsis and Septic Shock: Endothelial Molecular Pathogenesis Associated with Vascular Microthrombotic Disease. Thromb. J. 2019, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Carrick, M.M.; Mains, C.W.; Rael, L.T.; Slone, D.; Brody, E.N. Sepsis, Oxidative Stress, and Hypoxia: Are There Clues to Better Treatment? Redox Rep. 2015, 20, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Lelubre, C.; Vincent, J.-L. Mechanisms and Treatment of Organ Failure in Sepsis. Nat. Rev. Nephrol. 2018, 14, 417–427. [Google Scholar] [CrossRef]
- Đurašević, S.; Ružičić, A.; Lakić, I.; Tosti, T.; Đurović, S.; Glumac, S.; Pavlović, S.; Borković-Mitić, S.; Grigorov, I.; Stanković, S. The Effects of a Meldonium Pre-Treatment on the Course of the Faecal-Induced Sepsis in Rats. Int. J. Mol. Sci. 2021, 22, 9698. [Google Scholar] [CrossRef]
- Sun, G.-D.; Zhang, Y.; Mo, S.-S.; Zhao, M.-Y. Multiple Organ Dysfunction Syndrome Caused by Sepsis: Risk Factor Analysis. Int. J. Gen. Med. 2021, 14, 7159–7164. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, Y.-M.; Lu, Z.-Q. Mitochondrial Quality Control Mechanisms as Potential Therapeutic Targets in Sepsis-Induced Multiple Organ Failure. J. Mol. Med. 2019, 97, 451–462. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Sitkiewicz, D. Molecular Mechanisms of Organ Damage in Sepsis: An Overview. Braz. J. Infect. Dis. 2020, 24, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, J.; Tian, J.; Virzì, G.M.; Digvijay, K.; Cueto, L.; Yin, Y.; Rosner, M.H.; Ronco, C. Mitochondria in Sepsis-Induced AKI. J. Am. Soc. Nephrol. 2019, 30, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, Y.; Zheng, D.; Zhang, M.; Liu, H.; He, M.; Wu, Z. IL-27 Is Elevated in Sepsis with Acute Hepatic Injury and Promotes Hepatic Damage and Inflammation in the CLP Model. Cytokine 2020, 127, 154936. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Guo, J.; Li, X.; Wang, Y.; Wang, D.; Xiao, K.; Zhu, H.; Wang, X.; Hu, C.-A.A.; Zhang, G.; et al. Necroptosis Underlies Hepatic Damage in a Piglet Model of Lipopolysaccharide-Induced Sepsis. Front. Immunol. 2021, 12, 633830. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, S.M.; Singer, M. Pathophysiology of Sepsis-Induced Cardiomyopathy. Nat. Rev. Cardiol. 2021, 18, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Busch, K.; Kny, M.; Huang, N.; Klassert, T.E.; Stock, M.; Hahn, A.; Graeger, S.; Todiras, M.; Schmidt, S.; Chamling, B. Inhibition of the NLRP3/IL-1β Axis Protects against Sepsis-induced Cardiomyopathy. J. Cachexia Sarcopenia Muscle 2021, 12, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Kosyakovsky, L.B.; Angriman, F.; Katz, E.; Adhikari, N.K.; Godoy, L.C.; Marshall, J.C.; Ferreyro, B.L.; Lee, D.S.; Rosenson, R.S.; Sattar, N.; et al. Association between Sepsis Survivorship and Long-Term Cardiovascular Outcomes in Adults: A Systematic Review and Meta-Analysis. Intensive Care Med. 2021, 47, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xin, J.; Sun, Y.; Wang, X.; Sun, L.; Zhao, F.; Niu, C.; Liu, S. Mechanisms of Sepsis-Induced Acute Lung Injury and Advancements of Natural Small Molecules in Its Treatment. Pharmaceuticals 2024, 17, 472. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, P.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Chang, E.; Zingarelli, B.; Fan, H. Exosomes from Endothelial Progenitor Cells Improve Outcomes of the Lipopolysaccharide-Induced Acute Lung Injury. Crit. Care 2019, 23, 44. [Google Scholar] [CrossRef]
- Park, I.; Kim, M.; Choe, K.; Song, E.; Seo, H.; Hwang, Y.; Ahn, J.; Lee, S.-H.; Lee, J.H.; Jo, Y.H.; et al. Neutrophils Disturb Pulmonary Microcirculation in Sepsis-Induced Acute Lung Injury. Eur. Respir. J. 2019, 53, 1800786. [Google Scholar] [CrossRef]
- Pinheiro, K.H.E.; Azêdo, F.A.; Areco, K.C.N.; Laranja, S.M.R. Risk Factors and Mortality in Patients with Sepsis, Septic and Non Septic Acute Kidney Injury in ICU. Braz. J. Nephrol. 2019, 41, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Gómez, H.; Kellum, J.A. Chapter 90—Sepsis-Induced Acute Kidney Injury. In Critical Care Nephrology, 3rd ed.; Ronco, C., Bellomo, R., Kellum, J.A., Ricci, Z., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 524–533.e3. ISBN 978-0-323-44942-7. [Google Scholar]
- Janosevic, D.; Myslinski, J.; McCarthy, T.W.; Zollman, A.; Syed, F.; Xuei, X.; Gao, H.; Liu, Y.-L.; Collins, K.S.; Cheng, Y.-H.; et al. The Orchestrated Cellular and Molecular Responses of the Kidney to Endotoxin Define a Precise Sepsis Timeline. eLife 2021, 10, e62270. [Google Scholar] [CrossRef] [PubMed]
- Polat, G.; Ugan, R.A.; Cadirci, E.; Halici, Z. Sepsis and Septic Shock: Current Treatment Strategies and New Approaches. Eurasian J. Med. 2017, 49, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Minasyan, H. Sepsis and Septic Shock: Pathogenesis and Treatment Perspectives. J. Crit. Care 2017, 40, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.K.; Carey, P.D.; Byrne, K.; Sugerman, H.J.; Fowler, A.A. Sepsis-Induced Lung Injury and the Effects of Ibuprofen Pretreatment: Analysis of Early Alveolar Events via Repetitive Bronchoalveolar Lavage. Am. Rev. Respir. Dis. 1991, 143, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Wheeler, A.P.; Russell, J.A.; Schein, R.; Summer, W.R.; Steinberg, K.P.; Fulkerson, W.J.; Wright, P.E.; Christman, B.W.; Dupont, W.D. The Effects of Ibuprofen on the Physiology and Survival of Patients with Sepsis. N. Engl. J. Med. 1997, 336, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Beale, R.J.; Hollenberg, S.M.; Vincent, J.-L.; Parrillo, J.E. Vasopressor and Inotropic Support in Septic Shock: An Evidence-Based Review. Crit. Care Med. 2004, 32, S455–S465. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Van Der Poll, T. Thrombomodulin in Sepsis. Minerva Anestesiol. 2013, 79, 294–298. [Google Scholar] [PubMed]
- Wiedermann, C.J. Clinical Review: Molecular Mechanisms Underlying the Role of Antithrombin in Sepsis. Crit. Care 2006, 10, 209. [Google Scholar] [CrossRef]
- Li, X.; Ma, X. The Role of Heparin in Sepsis: Much More than Just an Anticoagulant. Br. J. Haematol. 2017, 179, 389–398. [Google Scholar] [CrossRef]
- Gao, F.; Linhartova, L.; Johnston, A.M.; Thickett, D. Statins and Sepsis. Br. J. Anaesth. 2008, 100, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Terblanche, M. Statins and Sepsis: Multiple Modifications at Multiple Levels. Int. J. Infect. Dis. 2008, 12, e6. [Google Scholar] [CrossRef][Green Version]
- Kotsaki, A.; Giamarellos-Bourboulis, E.J. Emerging Drugs for the Treatment of Sepsis. Expert Opin. Emerg. Drugs 2012, 17, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Alejandria, M.; Lansang, M.; Dans, L.; Mantaring, J., III. Intravenous Immunoglobulin for Treating Sepsis, Severe Sepsis and Septic Shock. Cochrane Database Syst. Rev. 2013, 2013, CD001090. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, X.; Zhang, F.; Xia, Y. Evaluation of the Effect of Intravenous Immunoglobulin Dosing on Mortality in Patients with Sepsis: A Network Meta-Analysis. Clin. Ther. 2019, 41, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Englert, J.A.; Rogers, A.J. Metabolism, Metabolomics, and Nutritional Support of Patients with Sepsis. Clin. Chest Med. 2016, 37, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L. Metabolic Support in Sepsis and Multiple Organ Failure: More Questions than Answers. Crit. Care Med. 2007, 35, S436–S440. [Google Scholar] [CrossRef] [PubMed]
- Todorović, Z.; Đurašević, S.; Stojković, M.; Grigorov, I.; Pavlović, S.; Jasnić, N.; Tosti, T.; Macut, J.B.; Thiemermann, C.; Đorđević, J. Lipidomics Provides New Insight into Pathogenesis and Therapeutic Targets of the Ischemia—Reperfusion Injury. Int. J. Mol. Sci. 2021, 22, 2798. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R. Hemoperfusion: Technical Aspects and State of the Art. Crit. Care 2022, 26, 135. [Google Scholar] [CrossRef]
- Ricci, Z.; Romagnoli, S.; Reis, T.; Bellomo, R.; Ronco, C. Hemoperfusion in the Intensive Care Unit. Intensive Care Med. 2022, 48, 1397–1408. [Google Scholar] [CrossRef]
- Cruz, D.N.; Antonelli, M.; Fumagalli, R.; Foltran, F.; Brienza, N.; Donati, A.; Malcangi, V.; Petrini, F.; Volta, G.; Pallavicini, F.M.B. Early Use of Polymyxin B Hemoperfusion in Abdominal Septic Shock: The EUPHAS Randomized Controlled Trial. JAMA 2009, 301, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Buras, J.A.; Holzmann, B.; Sitkovsky, M. Animal Models of Sepsis: Setting the Stage. Nat. Rev. Drug Discov. 2005, 4, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Zanotti-Cavazzoni, S.L.; Goldfarb, R.D. Animal Models of Sepsis. Crit. Care Clin. 2009, 25, 703–719. [Google Scholar] [CrossRef]
- Fink, M.P. Animal Models of Sepsis. Virulence 2014, 5, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Savi, F.F.; de Oliveira, A.; de Medeiros, G.F.; Bozza, F.A.; Michels, M.; Sharshar, T.; Dal-Pizzol, F.; Ritter, C. What Animal Models Can Tell Us about Long-Term Cognitive Dysfunction Following Sepsis: A Systematic Review. Neurosci. Biobehav. Rev. 2021, 124, 386–404. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Gong, C.; Fu, J.; Liu, X.; Bi, H.; Cheng, Y.; Liu, Y.; Tang, Y.; Wang, D. Evaluation of 2 Rat Models for Sepsis Developed by Improved Cecal Ligation/Puncture or Feces Intraperitoneal-Injection. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e919054-1. [Google Scholar] [CrossRef]
- Tsuchida, T.; Wada, T.; Mizugaki, A.; Oda, Y.; Kayano, K.; Yamakawa, K.; Tanaka, S. Protocol for a Sepsis Model Utilizing Fecal Suspension in Mice: Fecal Suspension Intraperitoneal Injection Model. Front. Med. 2022, 9, 765805. [Google Scholar] [CrossRef] [PubMed]
- Siempos, I.I.; Lam, H.C.; Ding, Y.; Choi, M.E.; Choi, A.M.K.; Ryter, S.W. Cecal Ligation and Puncture-Induced Sepsis as a Model To Study Autophagy in Mice. JoVE 2014, 84, e51066. [Google Scholar] [CrossRef]
- Srinivasan, V.; Mohamed, M.; Kato, H. Melatonin in Bacterial and Viral Infections with Focus on Sepsis: A Review. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012, 6, 30–39. [Google Scholar] [CrossRef]
- Biancatelli, R.M.L.C.; Berrill, M.; Mohammed, Y.H.; Marik, P.E. Melatonin for the Treatment of Sepsis: The Scientific Rationale. J. Thorac. Dis. 2020, 12, S54. [Google Scholar] [CrossRef]
- Escames, G.; Acuña-Castroviejo, D.; López, L.C.; Tan, D.; Maldonado, M.D.; Sánchez-Hidalgo, M.; León, J.; Reiter, R.J. Pharmacological Utility of Melatonin in the Treatment of Septic Shock: Experimental and Clinical Evidence. J. Pharm. Pharmacol. 2006, 58, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 896, Melatonin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/melatonin (accessed on 16 June 2024).
- An, R.; Zhao, L.; Xi, C.; Li, H.; Shen, G.; Liu, H.; Zhang, S.; Sun, L. Melatonin Attenuates Sepsis-Induced Cardiac Dysfunction via a PI3K/Akt-Dependent Mechanism. Basic Res. Cardiol. 2015, 111, 8. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nathan, D.; Maestroni, G.; Lustig, S.; Conti, A. Protective Effects of Melatonin in Mice Infected with Encephalitis Viruses. Arch. Virol. 1995, 140, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Baez-Ferrer, N.; Reiter, R.J.; Avanzas, P.; Hernandez-Vaquero, D. Melatonin and Cardioprotection in Humans: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021, 8, 635083. [Google Scholar] [CrossRef] [PubMed]
- Lochner, A.; Marais, E.; Huisamen, B. Melatonin and Cardioprotection against Ischaemia/Reperfusion Injury: What’s New? A Review. J. Pineal Res. 2018, 65, e12490. [Google Scholar] [CrossRef] [PubMed]
- Fink, T.; Glas, M.; Wolf, A.; Kleber, A.; Reus, E.; Wolff, M.; Kiefer, D.; Wolf, B.; Rensing, H.; Volk, T. Melatonin Receptors Mediate Improvements of Survival in a Model of Polymicrobial Sepsis. Crit. Care Med. 2014, 42, e22–e31. [Google Scholar] [CrossRef] [PubMed]
- Sewerynek, E.; Abe, M.; Reiter, R.J.; Barlow-Walden, L.R.; Chen, L.; McCabe, T.J.; Roman, L.J.; Diaz-Lopez, B. Melatonin Administration Prevents Lipopolysaccharide-induced Oxidative Damage in Phenobarbital-treated Animals. J. Cell. Biochem. 1995, 58, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Sewerynek, E.; Melchiorri, D.; Chen, L.; Reiter, R.J. Melatonin Reduces Both Basal and Bacterial Lipopolysaccharide-Induced Lipid Peroxidation in Vitro. Free Radic. Biol. Med. 1995, 19, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Şener, G.; Toklu, H.; Kapucu, C.; Ercan, F.; Erkanlı, G.; Kaçmaz, A.; Tilki, M.; Yeğen, B.Ç. Melatonin Protects Against Oxidative Organ Injury in a Rat Model of Sepsis. Surg. Today 2005, 35, 52–59. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Tsou, M.-Y.; Chen, T.-H.; Chen, S.-J.; Tsao, C.-M.; Wu, C.-C. Therapeutic Effects of Melatonin on Peritonitis-Induced Septic Shock with Multiple Organ Dysfunction Syndrome in Rats. J. Pineal Res. 2008, 45, 106–116. [Google Scholar] [CrossRef]
- Chen, L.; Han, Z.; Shi, Z.; Liu, C.; Lu, Q. Melatonin Alleviates Renal Injury in Mouse Model of Sepsis. Front. Pharmacol. 2021, 12, 697643. [Google Scholar] [CrossRef]
- Zhao, L.; An, R.; Yang, Y.; Yang, X.; Liu, H.; Yue, L.; Li, X.; Lin, Y.; Reiter, R.J.; Qu, Y. Melatonin Alleviates Brain Injury in Mice Subjected to Cecal Ligation and Puncture via Attenuating Inflammation, Apoptosis, and Oxidative Stress: The Role of SIRT1 Signaling. J. Pineal Res. 2015, 59, 230–239. [Google Scholar] [CrossRef]
- Du, J.; Song, Y.; Chen, M.; Du, Q. Protective Effects of Melatonin on Lung and Liver Injuries in a Rat Model of Acute Sepsis. Int. J. Clin. Exp. Med. 2018, 11, 11720–11731. [Google Scholar]
- Galley, H.F.; Lowes, D.A.; Allen, L.; Cameron, G.; Aucott, L.S.; Webster, N.R. Melatonin as a Potential Therapy for Sepsis: A Phase I Dose Escalation Study and an Ex Vivo Whole Blood Model under Conditions of Sepsis. J. Pineal Res. 2014, 56, 427–438. [Google Scholar] [CrossRef]
- Taher, A.; Shokoohmand, F.; Abdoli, E.; Mohammadi, Y.; Mehrpooya, M. A Pilot Study on the Melatonin Treatment in Patients with Early Septic Shock: Results of a Single-Center Randomized Controlled Trial. Irish J. Med. Sci. 2022, 191, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.; Duncan, L. Comparison of Chlorpropamide and Metformin Treatment on Weight and Blood-Glucose Response of Uncontrolled Obese Diabetics. Lancet 1968, 291, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Chen, H. Metformin: A Novel Weapon Against Inflammation. Front. Pharmacol. 2021, 12, 622262. [Google Scholar] [CrossRef] [PubMed]
- Bharath, L.P.; Nikolajczyk, B.S. The Intersection of Metformin and Inflammation. Am. J. Physiol.-Cell Physiol. 2021, 320, C873–C879. [Google Scholar] [CrossRef]
- Hassan, F.I.; Didari, T.; Khan, F.; Niaz, K.; Mojtahedzadeh, M.; Abdollahi, M. A Review on the Protective Effects of Metformin in Sepsis-Induced Organ Failure. Cell J. (Yakhteh) 2020, 21, 363–370. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Saisho, Y. Metformin and Inflammation: Its Potential beyond Glucose-Lowering Effect. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2015, 15, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4091, Metformin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/metformin (accessed on 16 June 2024).
- Yang, Q.; Zheng, J.; Chen, W.; Chen, X.; Wen, D.; Chen, W.; Xiong, X.; Zhang, Z. Association Between Preadmission Metformin Use and Outcomes in Intensive Care Unit Patients With Sepsis and Type 2 Diabetes: A Cohort Study. Front. Med. 2021, 8, 640785. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ding, X.; Li, L.; Wang, T.; Kan, Q.; Wang, L.; Sun, T. Association of Preadmission Metformin Use and Mortality in Patients with Sepsis and Diabetes Mellitus: A Systematic Review and Meta-Analysis of Cohort Studies. Crit. Care 2019, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Yang, H.; Chen, J.; Shi, M.; Ge, L.; Ge, X.; Zhu, G. Metformin Ameliorates Sepsis-Induced Brain Injury by Inhibiting Apoptosis, Oxidative Stress and Neuroinflammation via the PI3K/Akt Signaling Pathway. Oncotarget 2017, 8, 97977–97989. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Jang, H.J.; Nizamutdinova, I.T.; Kim, Y.M.; Lee, Y.S.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Metformin Inhibits HMGB1 Release in LPS-Treated RAW 264.7 Cells and Increases Survival Rate of Endotoxaemic Mice. Br. J. Pharmacol. 2011, 162, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Bo, A.; Li, R.; Liu, Y.; Li, W. Metformin Protects Septic Rats from Myocardial Injury. Int. J. Early Child. Spec. Educ. 2021, 30, 907–911. [Google Scholar] [CrossRef]
- Ghavimi, H.; Sheidaei, S.; Vaez, H.; Zolali, E.; Asgharian, P.; Hamishehkar, H. Metformin-Attenuated Sepsis-Induced Oxidative Damages: A Novel Role for Metformin. Iran. J. Basic Med. Sci. 2018, 21, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Song, H.; Zhang, X.; Song, G.; Wang, Y.; Ding, X.; Duan, X.; Li, L.; Sun, T.; Kan, Q. Metformin Attenuated Sepsis-Related Liver Injury by Modulating Gut Microbiota. Emerg. Microbes Infect. 2022, 11, 815–828. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Zhai, R.; Liang, H.; Song, G.; Yuan, Y.; Xu, Y.; Yan, Y.; Qiu, L.; Sun, T. Metformin Attenuated Sepsis-Associated Liver Injury and Inflammatory Response in Aged Mice. Bioengineered 2022, 13, 4598–4609. [Google Scholar] [CrossRef]
- Zhao, H.; Lyu, Y.; Zhai, R.; Sun, G.; Ding, X. Metformin Mitigates Sepsis-Related Neuroinflammation via Modulating Gut Microbiota and Metabolites. Front. Immunol. 2022, 13, 797312. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, S.; Niu, Y.; Duo, B.; Liu, Y.; Lu, Z.; Zhu, R. Effect of Metformin on Sepsis-Associated Acute Lung Injury and Gut Microbiota in Aged Rats with Sepsis. Front. Cell. Infect. Microbiol. 2023, 13, 1139436. [Google Scholar] [CrossRef]
- Qu, R.N.; Qu, W. Metformin Inhibits LPS-Induced Inflammatory Response in VSMCs by Regulating TLR4 and PPAR-γ. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4988–4995. [Google Scholar]
- Sun, J.; Huang, N.; Ma, W.; Zhou, H.; Lai, K. Protective Effects of Metformin on Lipopolysaccharide-induced Airway Epithelial Cell Injury via NF-κB Signaling Inhibition. Mol. Med. Rep. 2019, 19, 1817–1823. [Google Scholar] [CrossRef]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The Pharmacology of Palmitoylethanolamide and First Data on the Therapeutic Efficacy of Some of Its New Formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Redlich, S.; Ribes, S.; Schütze, S.; Nau, R. Palmitoylethanolamide Stimulates Phagocytosis of Escherichia Coli K1 by Macrophages and Increases the Resistance of Mice against Infections. J. Neuroinflamm. 2014, 11, 108. [Google Scholar] [CrossRef]
- Heide, E.C.; Bindila, L.; Post, J.M.; Malzahn, D.; Lutz, B.; Seele, J.; Nau, R.; Ribes, S. Prophylactic Palmitoylethanolamide Prolongs Survival and Decreases Detrimental Inflammation in Aged Mice With Bacterial Meningitis. Front. Immunol. 2018, 9, 02671. [Google Scholar] [CrossRef]
- D’Aloia, A.; Molteni, L.; Gullo, F.; Bresciani, E.; Artusa, V.; Rizzi, L.; Ceriani, M.; Meanti, R.; Lecchi, M.; Coco, S. Palmitoylethanolamide Modulation of Microglia Activation: Characterization of Mechanisms of Action and Implication for Its Neuroprotective Effects. Int. J. Mol. Sci. 2021, 22, 3054. [Google Scholar] [CrossRef]
- Sayd, A.; Antón, M.; Alén, F.; Caso, J.R.; Pavón, J.; Leza, J.C.; Rodríguez de Fonseca, F.; García-Bueno, B.; Orio, L. Systemic Administration of Oleoylethanolamide Protects from Neuroinflammation and Anhedonia Induced by LPS in Rats. Int. J. Neuropsychopharmacol. 2015, 18, pyu111. [Google Scholar] [CrossRef]
- D’Amico, R.; Monaco, F.; Siracusa, R.; Cordaro, M.; Fusco, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Cuzzocrea, S.; Di Paola, R. Ultramicronized Palmitoylethanolamide in the Management of Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. Int. J. Mol. Sci. 2021, 22, 11388. [Google Scholar] [CrossRef]
- Berdyshev, E.; Boichot, E.; Corbel, M.; Germain, N.; Lagente, V. Effects of Cannabinoid Receptor Ligands on LPS-Induced Pulmonary Inflammation in Mice. Life Sci. 1998, 63, PL125–PL129. [Google Scholar] [CrossRef]
- Hoareau, L.; Buyse, M.; Festy, F.; Ravanan, P.; Gonthier, M.-P.; Matias, I.; Petrosino, S.; Tallet, F.; D’Hellencourt, C.L.; Cesari, M.; et al. Anti-Inflammatory Effect of Palmitoylethanolamide on Human Adipocytes. Obesity 2009, 17, 431–438. [Google Scholar] [CrossRef]
- Uberti, F.; Ruga, S.; Farghali, M.; Galla, R.; Molinari, C. A Combination of α-Lipoic Acid (ALA) and Palmitoylethanolamide (PEA) Blocks Endotoxin-Induced Oxidative Stress and Cytokine Storm: A Possible Intervention for COVID-19. J. Diet. Suppl. 2023, 20, 133–155. [Google Scholar] [CrossRef]
- Usmani, J.; Khan, T.; Ahmad, R.; Sharma, M. Potential Role of Herbal Medicines as a Novel Approach in Sepsis Treatment. Biomed. Pharmacother. 2021, 144, 112337. [Google Scholar] [CrossRef]
- Yun, N.; Lee, C.-H.; Lee, S.-M. Protective Effect of Aloe Vera on Polymicrobial Sepsis in Mice. Food Chem. Toxicol. 2009, 47, 1341–1348. [Google Scholar] [CrossRef]
- Gao, H.; Ren, Y.; Liu, C. Aloe-Emodin Suppresses Oxidative Stress and Inflammation via a PI3K-Dependent Mechanism in a Murine Model of Sepsis. Evid.-Based Complement. Altern. Med. 2022, 2022, 9697887. [Google Scholar] [CrossRef]
- Li, C.-Y.; Suzuki, K.; Hung, Y.-L.; Yang, M.-S.; Yu, C.-P.; Lin, S.-P.; Hou, Y.-C.; Fang, S.-H. Aloe Metabolites Prevent LPS-Induced Sepsis and Inflammatory Response by Inhibiting Mitogen-Activated Protein Kinase Activation. Am. J. Chin. Med. 2017, 45, 847–861. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 12305761, Aloin A. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/aloin-a (accessed on 16 June 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 10207, Aloe Emodin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/aloe-emodin (accessed on 16 June 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 10168, Rhein. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/rhein (accessed on 16 June 2024).
- Yang, W.; Qiang, D.; Zhang, M.; Ma, L.; Zhang, Y.; Qing, C.; Xu, Y.; Zhen, C.; Liu, J.; Chen, Y.-H. Isoforskolin Pretreatment Attenuates Lipopolysaccharide-Induced Acute Lung Injury in Animal Models. Int. Immunopharmacol. 2011, 11, 683–692. [Google Scholar] [CrossRef]
- Du, X.; Shi, R.; Wang, Y.; Wu, W.; Sun, S.; Dai, Z.; Chen, C.; Weng, Z.; Li, X.; Liu, Q. Isoforskolin and Forskolin Attenuate Lipopolysaccharide-induced Inflammation through TLR4/MyD88/NF-κB Cascades in Human Mononuclear Leukocytes. Phytother. Res. 2019, 33, 602–609. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9549169, Isoforskolin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Isoforskolin (accessed on 16 June 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 47936, Forskolin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Forskolin (accessed on 16 June 2024).
- Liew, K.Y.; Hafiz, M.F.; Chong, Y.J.; Harith, H.H.; Israf, D.A.; Tham, C.L. A Review of Malaysian Herbal Plants and Their Active Constituents with Potential Therapeutic Applications in Sepsis. Evid.-Based Complement. Altern. Med. 2020, 2020, 8257817. [Google Scholar] [CrossRef]
- Ma, F.; Liu, F.; Ding, L.; You, M.; Yue, H.; Zhou, Y.; Hou, Y. Anti-Inflammatory Effects of Curcumin Are Associated with down Regulating microRNA-155 in LPS-Treated Macrophages and Mice. Pharm. Biol. 2017, 55, 1263–1273. [Google Scholar] [CrossRef]
- Karimi, A.; Ghodsi, R.; Kooshki, F.; Karimi, M.; Asghariazar, V.; Tarighat-Esfanjani, A. Therapeutic Effects of Curcumin on Sepsis and Mechanisms of Action: A Systematic Review of Preclinical Studies. Phytother. Res. 2019, 33, 2798–2820. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yang, M.; Sun, D.; Sun, S. Curcumin Protects Against Sepsis-Induced Acute Lung Injury in Rats. J. Surg. Res. 2012, 176, e31–e39. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 969516, Curcumin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/curcumin (accessed on 16 June 2024).
- Gu, J.; Ran, X.; Deng, J.; Zhang, A.; Peng, G.; Du, J.; Wen, D.; Jiang, B.; Xia, F. Glycyrrhizin Alleviates Sepsis-Induced Acute Respiratory Distress Syndrome via Suppressing of HMGB1/TLR9 Pathways and Neutrophils Extracellular Traps Formation. Int. Immunopharmacol. 2022, 108, 108730. [Google Scholar] [CrossRef]
- Zhao, F.; Fang, Y.; Deng, S.; Li, X.; Zhou, Y.; Gong, Y.; Zhu, H.; Wang, W. Glycyrrhizin Protects Rats from Sepsis by Blocking HMGB1 Signaling. BioMed Res. Int. 2017, 2017, 9719647. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 14982, Glycyrrhizin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/glycyrrhizin (accessed on 16 June 2024).
- Yang, F.; Li, J.; Lan, Y.; Lei, Y.; Zeng, F.; Huang, X.; Luo, X.; Liu, R. Potential Application of Ginseng in Sepsis:: Applications of Ginseng in Sepsis. J. Ginseng Res. 2023, 47, 353–358. [Google Scholar] [CrossRef]
- Kim, G.O.; Kim, N.; Song, G.Y.; Bae, J.-S. Inhibitory Activities of Rare Ginsenoside Rg4 on Cecal Ligation and Puncture-Induced Sepsis. Int. J. Mol. Sci. 2022, 23, 10836. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Luong, T.T.; Lee, S.Y.; Kim, G.L.; Kwon, H.; Lee, H.-G.; Park, C.-K.; Rhee, D.-K. Panax Ginseng Aqueous Extract Prevents Pneumococcal Sepsis in Vivo by Potentiating Cell Survival and Diminishing Inflammation. Phytomedicine 2015, 22, 1055–1061. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3086007, Ginsenosides. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/ginsenosides (accessed on 16 June 2024).
- Ku, S.-K.; Lee, I.-C.; Kim, J.A.; Bae, J.-S. Anti-Septic Effects of Pellitorine in HMGB1-Induced Inflammatory Responses In Vitro and In Vivo. Inflammation 2014, 37, 338–348. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Li, H.; Gu, L. Piperine Ameliorates Lipopolysaccharide-Induced Acute Lung Injury via Modulating NF-κB Signaling Pathways. Inflammation 2016, 39, 303–308. [Google Scholar] [CrossRef]
- Bae, G.-S.; Kim, M.-S.; Jung, W.-S.; Seo, S.-W.; Yun, S.-W.; Kim, S.G.; Park, R.-K.; Kim, E.-C.; Song, H.-J.; Park, S.-J. Inhibition of Lipopolysaccharide-Induced Inflammatory Responses by Piperine. Eur. J. Pharmacol. 2010, 642, 154–162. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 638024, Piperine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Piperine (accessed on 16 June 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5318516, Pellitorine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pellitorine (accessed on 16 June 2024).
- Liu, S.; Yu, X.; Hu, B.; Zou, Y.; Li, J.; Bo, L.; Deng, X. Salidroside Rescued Mice from Experimental Sepsis through Anti-Inflammatory and Anti-Apoptosis Effects. J. Surg. Res. 2015, 195, 277–283. [Google Scholar] [CrossRef]
- Guan, S.; Xiong, Y.; Song, B.; Song, Y.; Wang, D.; Chu, X.; Chen, N.; Huo, M.; Deng, X.; Lu, J. Protective Effects of Salidroside from Rhodiola Rosea on LPS-Induced Acute Lung Injury in Mice. Immunopharmacol. Immunotoxicol. 2012, 34, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.-C.; Chao, S.-C.; Wu, H.-Y.; Chiang, C.-L.; Wang, C.-C.; Liu, S.-H.; Weng, T.-I. Salidroside Ameliorates Sepsis-Induced Acute Lung Injury and Mortality via Downregulating NF-κB and HMGB1 Pathways through the Upregulation of SIRT1. Sci. Rep. 2017, 7, 12026. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 159278, Salidroside. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/salidroside (accessed on 16 June 2024).
- Lee, H.-H.; Shin, J.-S.; Lee, W.-S.; Ryu, B.; Jang, D.S.; Lee, K.-T. Biflorin, Isolated from the Flower Buds of Syzygium Aromaticum L., Suppresses LPS-Induced Inflammatory Mediators via STAT1 Inactivation in Macrophages and Protects Mice from Endotoxin Shock. J. Nat. Prod. 2016, 79, 711–720. [Google Scholar] [CrossRef]
- Magalhães, C.B.; Casquilho, N.V.; Machado, M.N.; Riva, D.R.; Travassos, L.H.; Leal-Cardoso, J.H.; Fortunato, R.S.; Faffe, D.S.; Zin, W.A. The Anti-Inflammatory and Anti-Oxidative Actions of Eugenol Improve Lipopolysaccharide-Induced Lung Injury. Respir. Physiol. Neurobiol. 2019, 259, 30–36. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Lu, Y.; Ma, C. Anti-Inflammatory Effects of Eugenol on Lipopolysaccharide-Induced Inflammatory Reaction in Acute Lung Injury via Regulating Inflammation and Redox Status. Int. Immunopharmacol. 2015, 26, 265–271. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3314, Eugenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/eugenol (accessed on 16 June 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 441959, Biflorin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/biflorin (accessed on 16 June 2024).
- Wali, A.F.; Rehman, M.U.; Raish, M.; Kazi, M.; Rao, P.G.M.; Alnemer, O.; Ahmad, P.; Ahmad, A. Zingerone [4-(3-Methoxy-4-Hydroxyphenyl)-Butan-2] Attenuates Lipopolysaccharide-Induced Inflammation and Protects Rats from Sepsis Associated Multi Organ Damage. Molecules 2020, 25, 5127. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Zhou, B.-W.; Yan, Z.-Z.; Zhao, J.; Zhao, B.-C.; Liu, W.-F.; Li, C.; Liu, K.-X. 6-Gingerol Attenuates Macrophages Pyroptosis via the Inhibition of MAPK Signaling Pathways and Predicts a Good Prognosis in Sepsis. Cytokine 2020, 125, 154854. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Luo, J.; Jie, J.; Liu, H.; Peng, L.; Bai, X.; Li, D. Zingerone Inhibits the Neutrophil Extracellular Trap Formation and Protects against Sepsis via Nrf2-Mediated ROS Inhibition. Oxidative Med. Cell. Longev. 2022, 2022, 3990607. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 442793, Gingerol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/gingerol (accessed on 16 June 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 31211, Zingerone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/zingerone (accessed on 16 June 2024).
- Salehi, B.; Staniak, M.; Czopek, K.; Stępień, A.; Dua, K.; Wadhwa, R.; Kumar Chellappan, D.; Sytar, O.; Brestic, M.; Ganesh Bhat, N.; et al. The Therapeutic Potential of the Labdane Diterpenoid Forskolin. Appl. Sci. 2019, 9, 4089. [Google Scholar] [CrossRef]
- Lokesh, B.; Deepa, R.; Divya, K. Medicinal Coleus (Coleus Forskohlii Briq): A Phytochemical Crop of Commercial Significance—Review. J. Pharmacogn. Phytochem. 2018, 7, 2856–2864. [Google Scholar]
- El-Agroudy, N.N.; El-Naga, R.N.; El-Razeq, R.A.; El-Demerdash, E. Forskolin, a Hedgehog Signalling Inhibitor, Attenuates Carbon Tetrachloride-induced Liver Fibrosis in Rats. Br. J. Pharmacol. 2016, 173, 3248–3260. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The Mucus and Mucins of the Goblet Cells and Enterocytes Provide the First Defense Line of the Gastrointestinal Tract and Interact with the Immune System. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus Barrier, Mucins and Gut Microbiota: The Expected Slimy Partners? Gut 2020, 69, 2232. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef] [PubMed]
- Adelman, M.W.; Woodworth, M.H.; Langelier, C.; Busch, L.M.; Kempker, J.A.; Kraft, C.S.; Martin, G.S. The Gut Microbiome’s Role in the Development, Maintenance, and Outcomes of Sepsis. Crit. Care 2020, 24, 278. [Google Scholar] [CrossRef]
- Niu, M.; Chen, P. Crosstalk between Gut Microbiota and Sepsis. Burn. Trauma 2021, 9, tkab036. [Google Scholar] [CrossRef]
- Sun, T.; Wang, L.; Zhang, H. Intestinal Microbiota in Sepsis. Intensive Care Res. 2022, 2, 1–7. [Google Scholar] [CrossRef]
- Agudelo-Ochoa, G.M.; Valdés-Duque, B.E.; Giraldo-Giraldo, N.A.; Jaillier-Ramírez, A.M.; Giraldo-Villa, A.; Acevedo-Castaño, I.; Yepes-Molina, M.A.; Barbosa-Barbosa, J.; Benítez-Paéz, A. Gut Microbiota Profiles in Critically Ill Patients, Potential Biomarkers and Risk Variables for Sepsis. Gut Microbes 2020, 12, 1707610. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, M.; Li, J.; Zhang, P.; Fan, H.; Hu, Q.; Han, M.; Su, L.; He, H.; Tong, Y.; et al. Classification of the Gut Microbiota of Patients in Intensive Care Units During Development of Sepsis and Septic Shock. Genom. Proteom. Bioinform. 2020, 18, 696–707. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Hu, S.; Wang, Y.; Zhang, L.; Yu, L.; Geng, F. Analysis of the Dynamic Changes in Gut Microbiota in Patients with Different Severity in Sepsis. BMC Infect. Dis. 2023, 23, 614. [Google Scholar] [CrossRef]




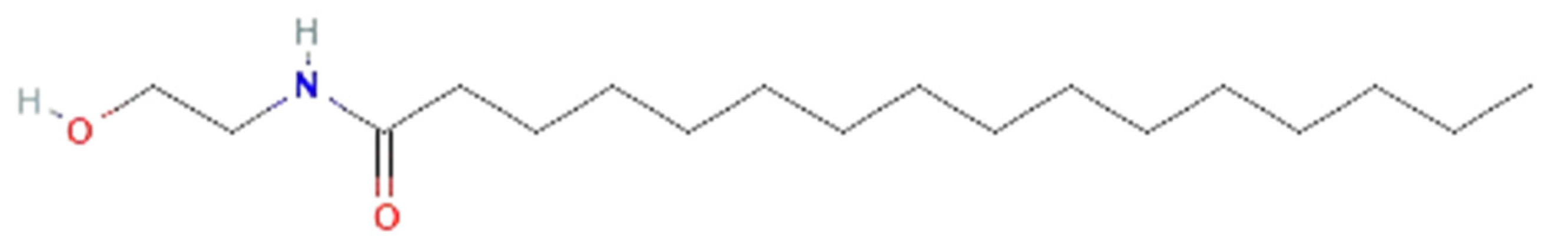
| Drug | Dose | Effects | References |
|---|---|---|---|
| Ibuprofen | 10 mg per kg | reduces fever, tachycardia, and lactic acid levels; attenuates cardiopulmonary response; decreases endothelial damage and inflammation | [66,67] |
| Noradrenaline | 5 μg per kg per minute | increases arterial pressure | [5,68] |
| Dopamine | 5–15 μg per kg per minute | increases arterial pressure | [5,68] |
| Vasopressin | 0.01–0.04 units per minute | increases arterial pressure | [5,68] |
| Heparin | 10,000–15,000 units per day | anticoagulation; modulates platelet activation, leukocyte and neutrophil recruitment, and LPS-induced release of cytokines | [64,71] |
| Thrombomodulin | 0.06 mg per kg per day | anticoagulation | [69] |
| Antithrombin | 6000 units per day | anticoagulation | [70] |
| Statins | 10–40 mg per day (pravastatin, rosuvastatin), 10–80 mg per day (simvastatin, atorvastatin), 20–80 mg per day (lovastatin, fluvastatin) | reduces platelet aggregation and leukocytes migration; decreases NO•, iNOS, NF-kB, and cytokines levels | [72,73] |
| IVIG | 7 mL per kg per day (Pentaglobin, Biotest Pharma), 0.6 g per kg per day (Polyglobin, Bayer Biological Products), 0.4 g per kg per day (Sandoglobulin, Sandoz Pharmaceutical Corp) | inactivates bacterial endotoxins; promotes phagocytosis; stimulates leukocytes; modulates cytokines release | [74,75,76] |
| Plant Species | Active Component | Chemical Structure | Effects | References |
|---|---|---|---|---|
| Aloe vera | aloin | 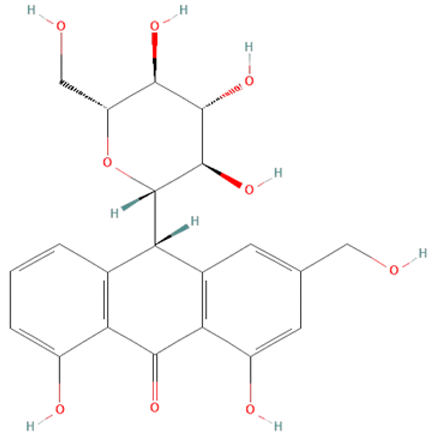 | decrease iNOS, NO•, IL-6, and TNF-α production, and ERK1/2 activation; increase SOD and GSH-Px activity | [139,140,141,142,143,144] |
| aloe-emodin |  | |||
| rhein |  | |||
| Coleus forskohlii | forskolin | 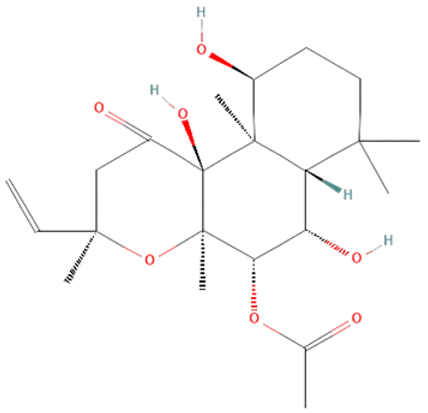 | increase cAMP levels, decrease TLR4, NF-kB, IL-1β, IL-6, and TNF-α levels | [145,146,147,148] |
| isoforskolin | 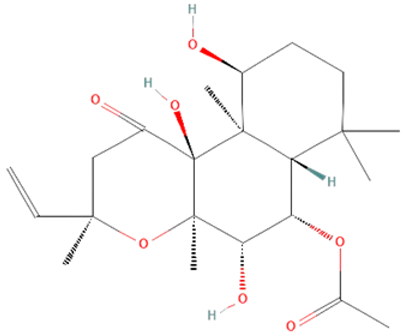 | |||
| Curcuma longa | curcumin |  | inhibits TLR4, NF-kB, iNOS, STAT1, and ERK1/2 activation; reduces ROS and cytokines levels; increases SOD levels | [149,150,151,152,153] |
| Glycyrrhiza glabra | glycyrrhizin | 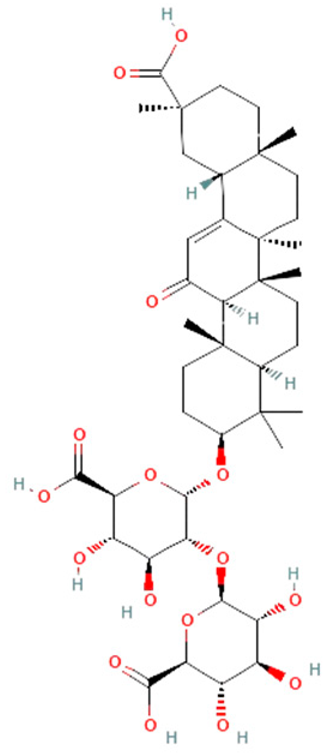 | reduces HMGB1, IL-1β, IL-6, and TNF-α | [154,155,156] |
| Panax ginseng | ginsenosides |  | reduce ROS, HMGB1, and cytokines levels; inhibit TLR4 and NF-kB expression | [157,158,159,160] |
| Piper nigrum | piperine | 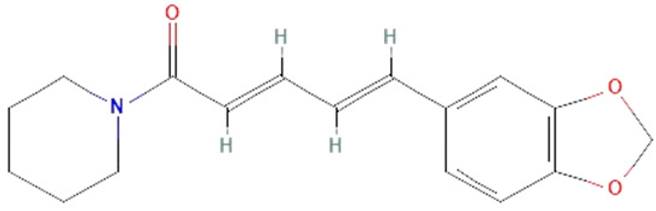 | decrease HMGB1, IL-1β, IL-6, TNF-α, and IFN levels, STAT1 activation, neutrophil infiltration, vascular permeability, and migration of leukocytes to the endothelium | [149,161,162,163,164,165] |
| pellitorine | 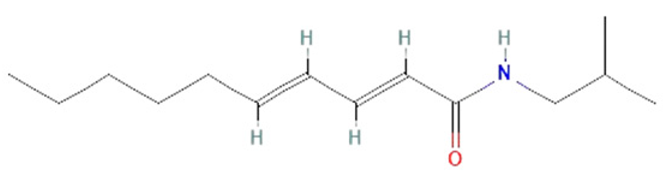 | |||
| Rhodiola rosea | salidroside |  | reduces HMGB1, iNOS, NO•, TNF-α, IL-6, and IL-1β production, and NF-kB activation | [166,167,168,169] |
| Syzygium aromaticum | eugenol |  | reduce levels of STAT1, NF-kB, NOXs, iNOS, NO•, IL-6, TNF-α, and IL-1β | [149,170,171,172,173,174] |
| biflorin | 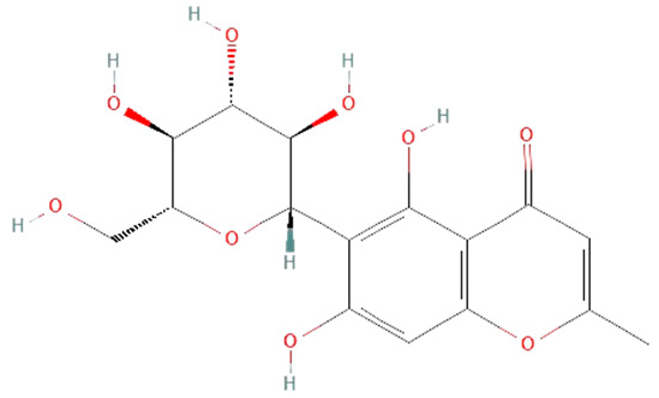 | |||
| Zingiber officinale | 6-gingerol | 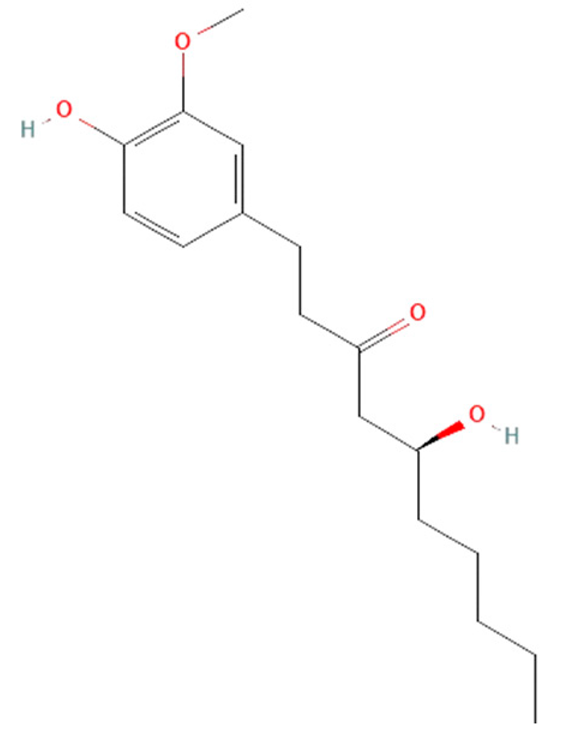 | reduce levels of ROS, HMGB1, TNF-α, IL-6, IL-1β, and IL-10 | [149,175,176,177,178,179] |
| zingerone | 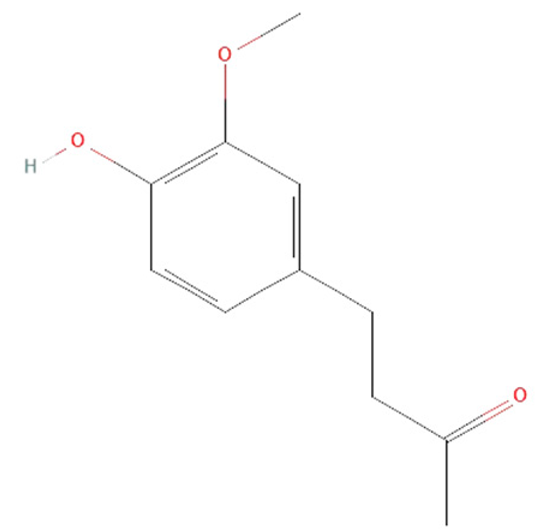 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srdić, T.; Đurašević, S.; Lakić, I.; Ružičić, A.; Vujović, P.; Jevđović, T.; Dakić, T.; Đorđević, J.; Tosti, T.; Glumac, S.; et al. From Molecular Mechanisms to Clinical Therapy: Understanding Sepsis-Induced Multiple Organ Dysfunction. Int. J. Mol. Sci. 2024, 25, 7770. https://doi.org/10.3390/ijms25147770
Srdić T, Đurašević S, Lakić I, Ružičić A, Vujović P, Jevđović T, Dakić T, Đorđević J, Tosti T, Glumac S, et al. From Molecular Mechanisms to Clinical Therapy: Understanding Sepsis-Induced Multiple Organ Dysfunction. International Journal of Molecular Sciences. 2024; 25(14):7770. https://doi.org/10.3390/ijms25147770
Chicago/Turabian StyleSrdić, Tijana, Siniša Đurašević, Iva Lakić, Aleksandra Ružičić, Predrag Vujović, Tanja Jevđović, Tamara Dakić, Jelena Đorđević, Tomislav Tosti, Sofija Glumac, and et al. 2024. "From Molecular Mechanisms to Clinical Therapy: Understanding Sepsis-Induced Multiple Organ Dysfunction" International Journal of Molecular Sciences 25, no. 14: 7770. https://doi.org/10.3390/ijms25147770
APA StyleSrdić, T., Đurašević, S., Lakić, I., Ružičić, A., Vujović, P., Jevđović, T., Dakić, T., Đorđević, J., Tosti, T., Glumac, S., Todorović, Z., & Jasnić, N. (2024). From Molecular Mechanisms to Clinical Therapy: Understanding Sepsis-Induced Multiple Organ Dysfunction. International Journal of Molecular Sciences, 25(14), 7770. https://doi.org/10.3390/ijms25147770











