The Effect of Ascorbic Acid on Hepatic Ischaemia–Reperfusion Injury in Wistar Rats: An Experimental Study
Abstract
1. Introduction
2. Results
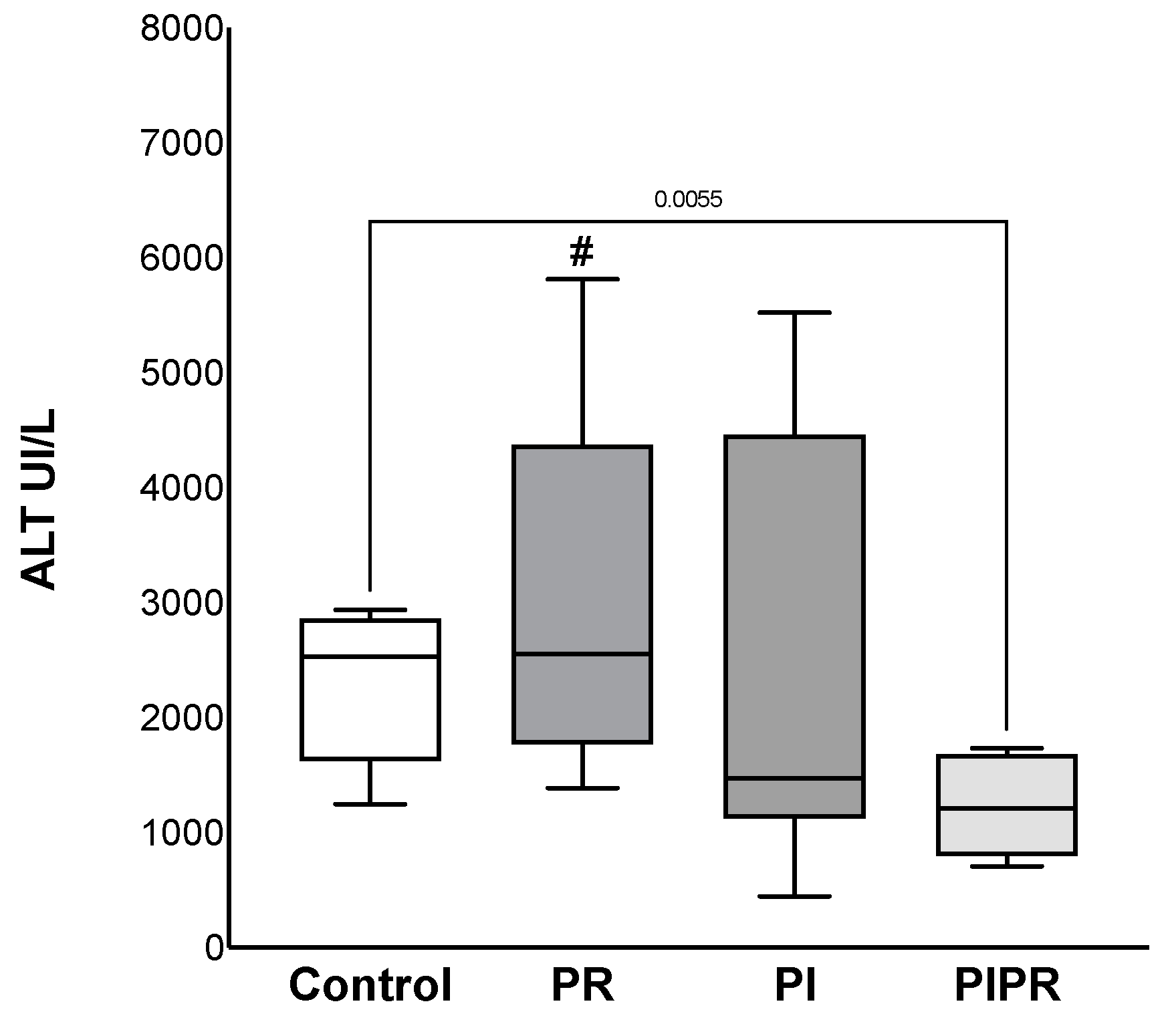
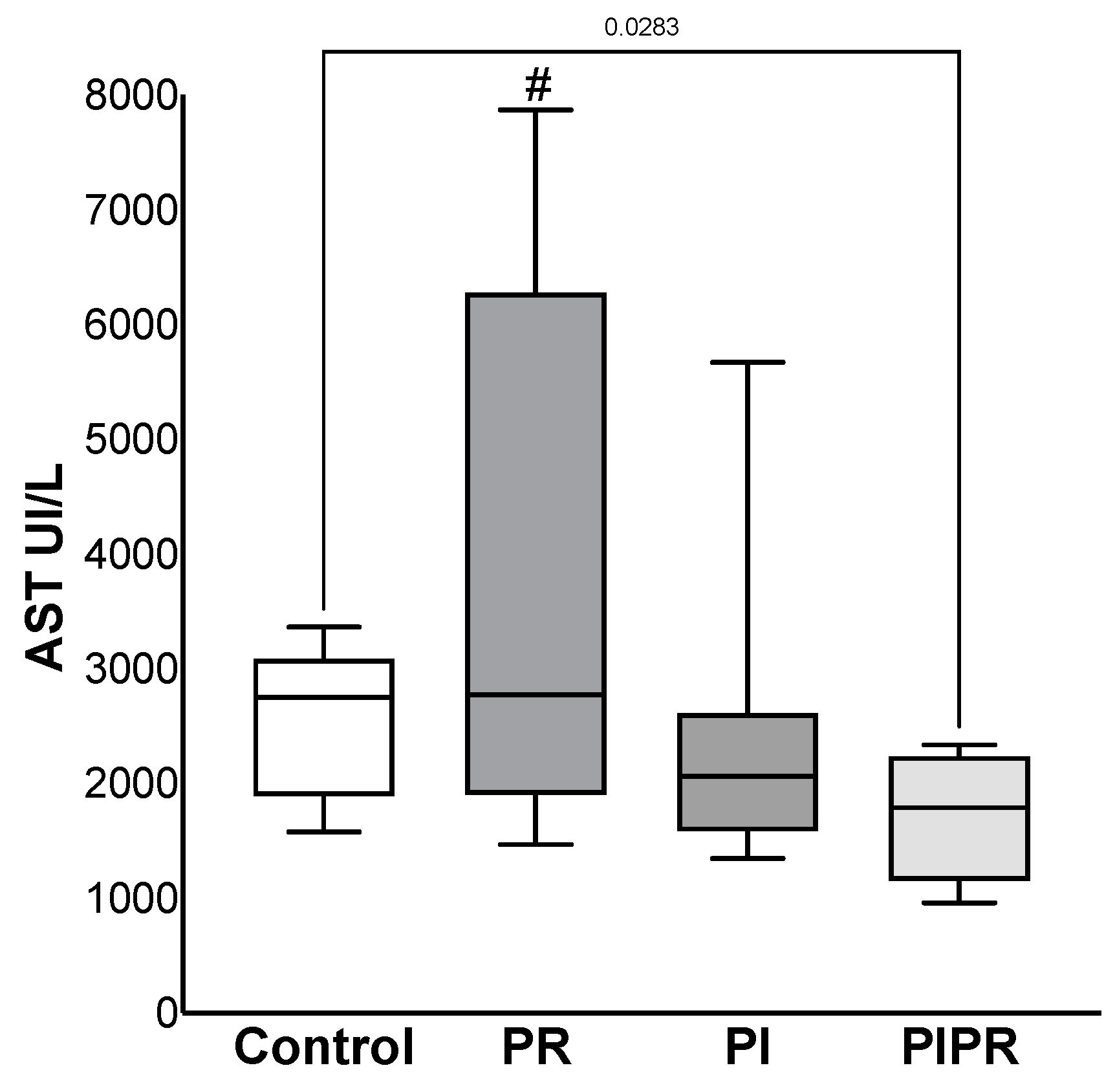
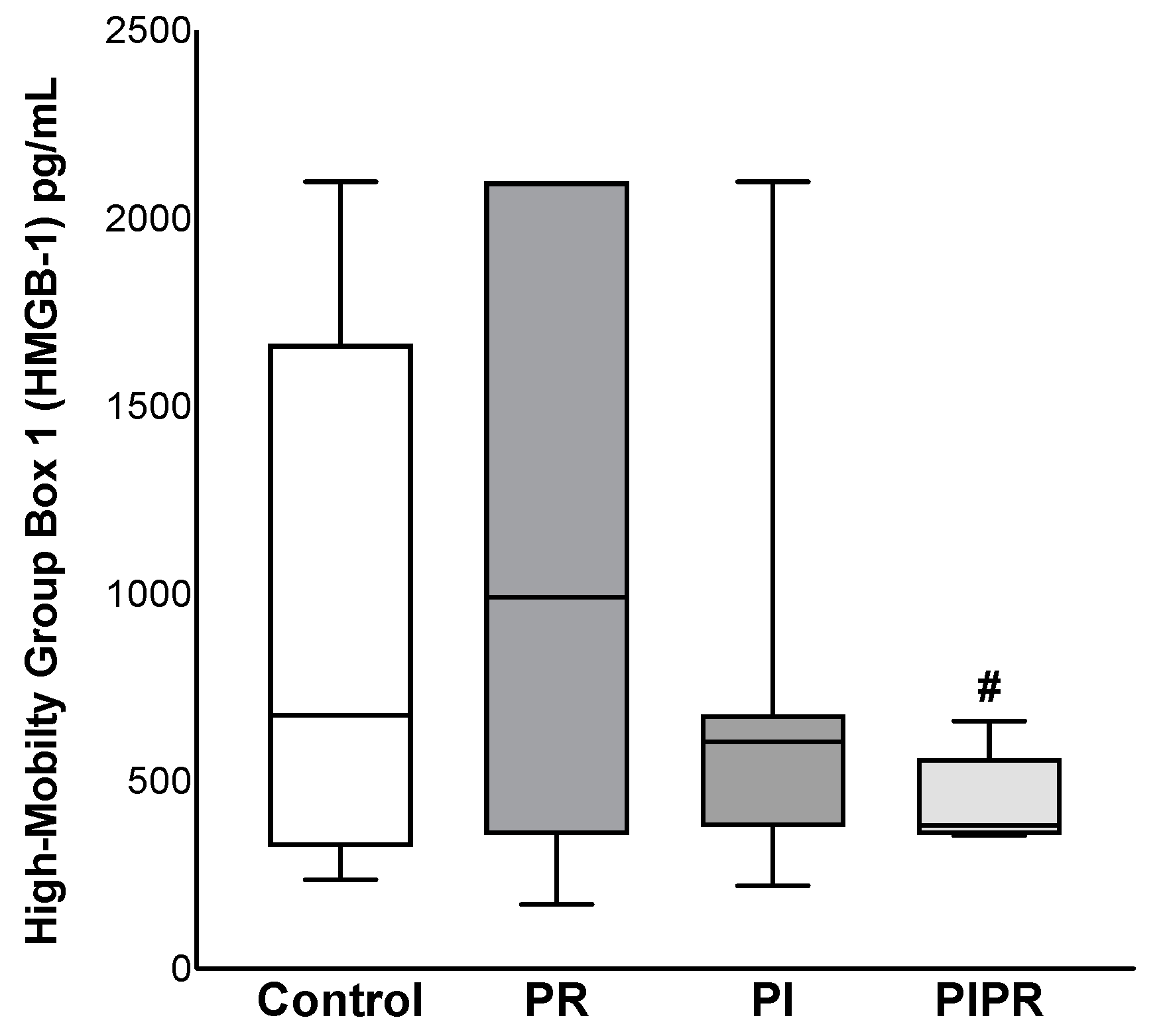
2.1. Biochemical Results
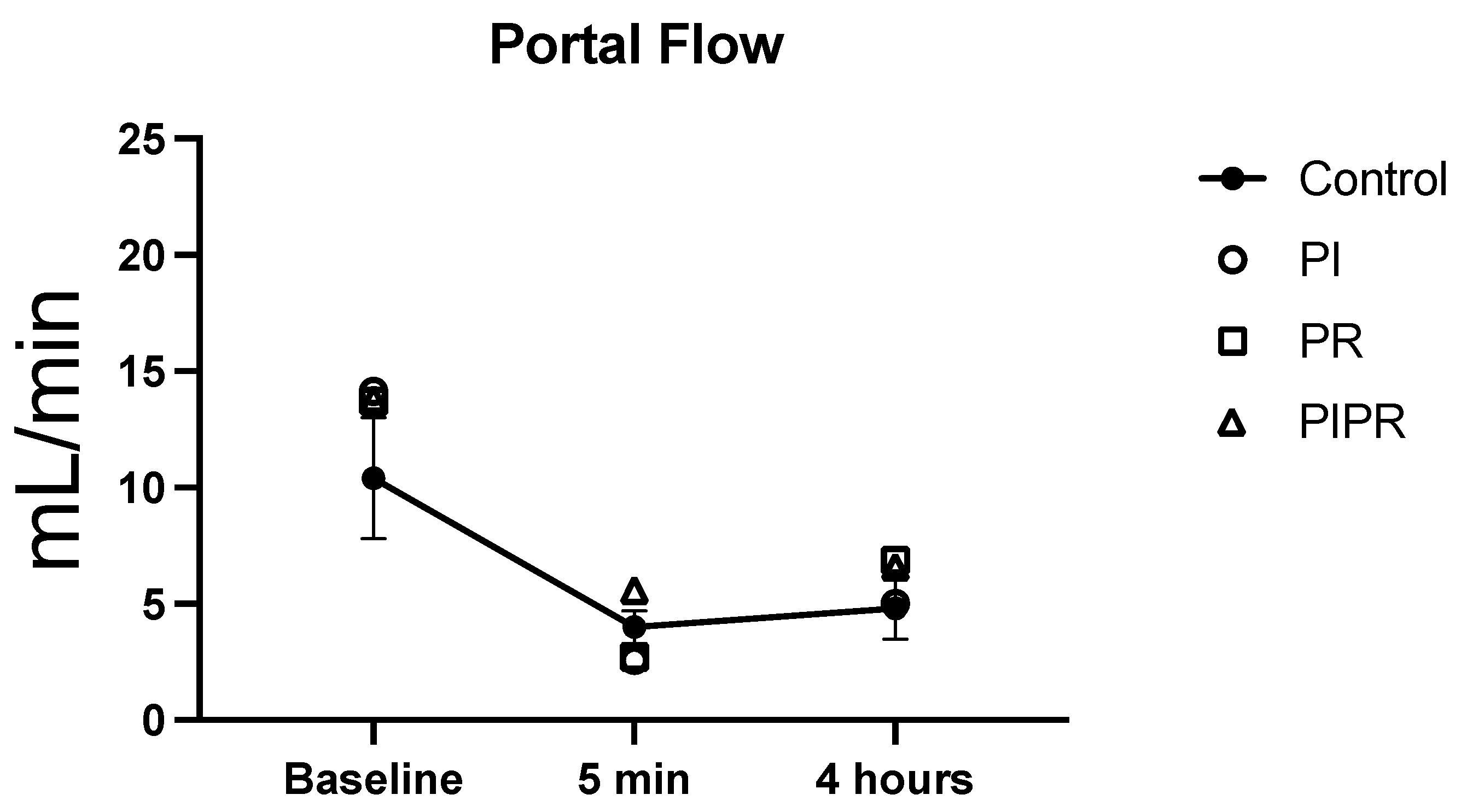
2.2. Portal Venous Flow
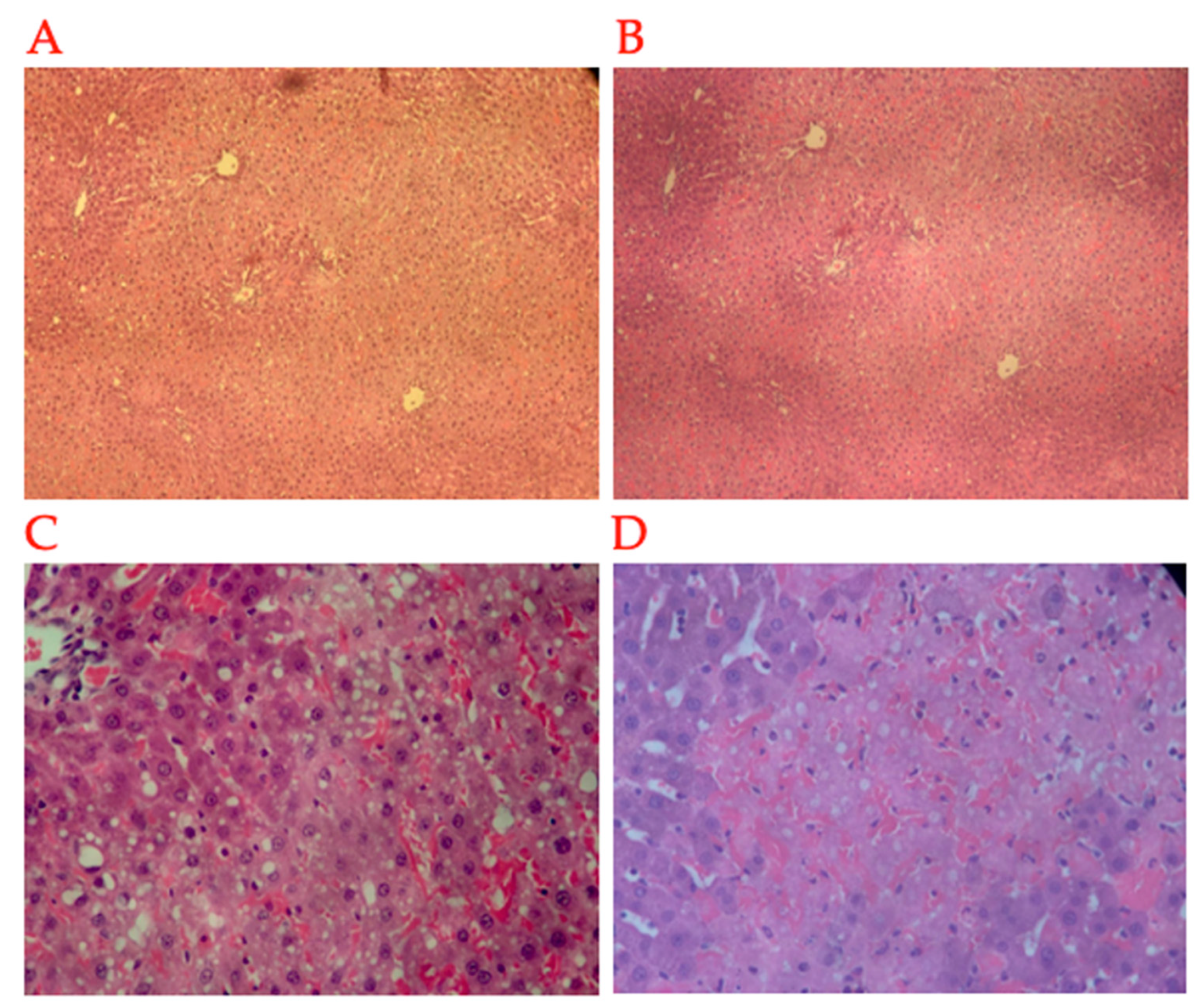
2.3. Histopathology
3. Discussion
4. Materials and Methods
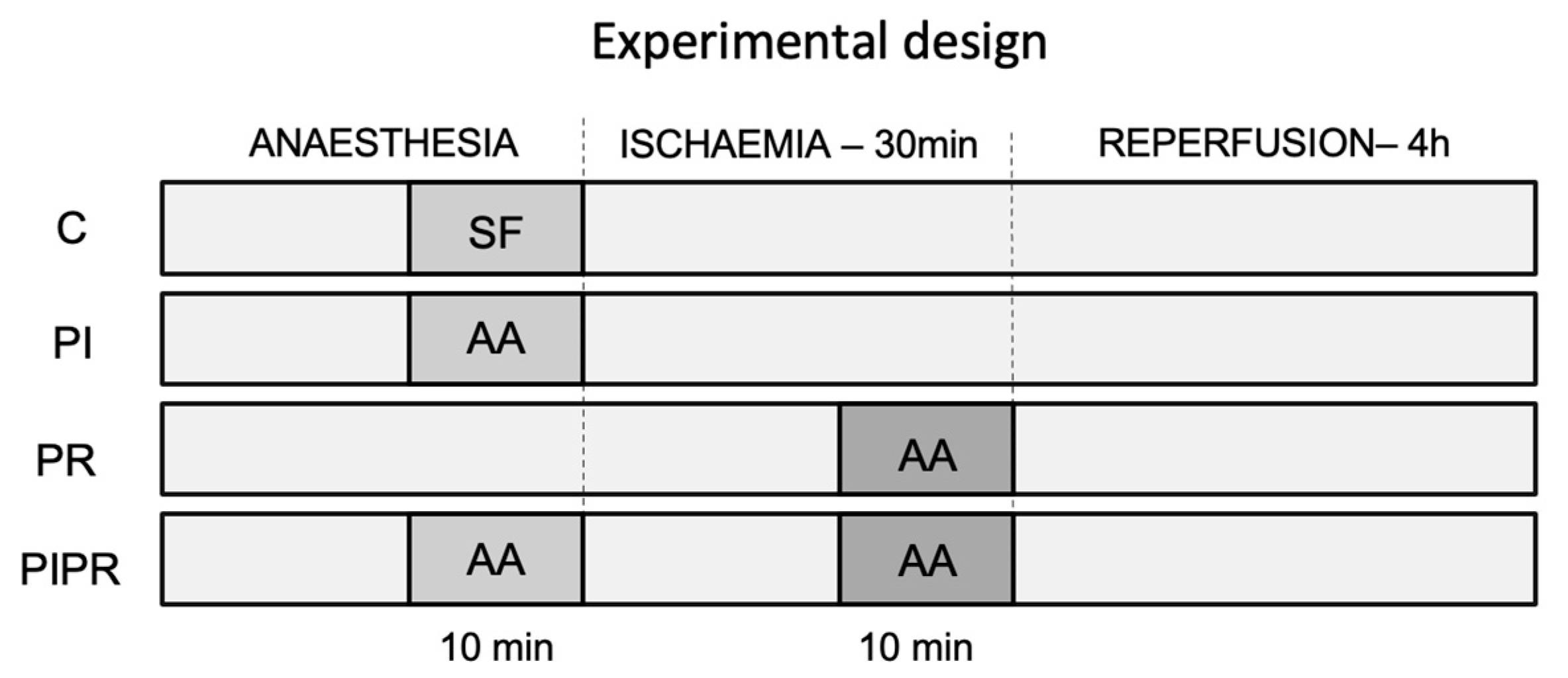
4.1. Animals and Study Design
4.2. Anaesthesia and Surgical Procedure
4.3. Portal Venous Flow
4.4. Laboratory Analyses (Liver Enzymes, Blood Gas, Interleukins, and High-Mobility Group Box-1)
4.5. Histopathological Data Collection and Analyses
4.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serracino-Inglott, F.; Habib, N.A.; Mathie, R.T. Hepatic ischemia-reperfusion injury. Am. J. Surg. 2001, 181, 160–166. [Google Scholar] [CrossRef]
- Anaya-Prado, R.; Toledo-Pereyra, L.H. The molecular events underlying ischemia/reperfusion injury. Transplant. Proc. 2002, 34, 2518–2519. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Prado, R.; Toledo-Pereyra, L.H.; Lentsch, A.B.; Ward, P.A. Ischemia/reperfusion injury. J. Surg. Res. 2002, 105, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Naini, B.V.; Markovic, D.; Aziz, A.; Younan, S.; Lu, M.; Hirao, H.; Kadono, K.; Kojima, H.; DiNorcia, J., III; et al. Ischemia-reperfusion injury and its relationship with early allograft dysfunction in liver transplant patients. Am. J. Transplant. 2021, 21, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Cerwenka, H.; Khoschsorur, G.; Bacher, H.; Werkgartner, G.; El-Shabrawi, A.; Quehenberger, F.; Rabl, H.; Mischinger, H. Normothermic liver ischemia and antioxidant treatment during hepatic resections. Free Radic. Res. 1999, 30, 463–469. [Google Scholar] [CrossRef]
- Powner, D.J. Factors during donor care that may affect liver transplantation outcome. Prog. Transplant. Aliso Viejo Calif 2004, 14, 241–249. [Google Scholar] [CrossRef]
- Montalvo-Jave, E.E.; Escalante-Tattersfield, T.; Ortega-Salgado, J.A.; Pina, E.; Geller, D.A. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J. Surg. Res. 2008, 147, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M. Liver transplantation and rejection: An overview. Hepato-Gastroenterol. 1999, 46, 1482–1484. [Google Scholar]
- Pringle, J.H. Notes on the arrest of hepatic hemorrhage due to trauma. Ann. Surg. 1908, 48, 541–549. [Google Scholar] [CrossRef]
- Shiratori, Y.; Kiriyama, H.; Fukushi, Y.; Nagura, T.; Takada, H.; Hai, K.J.; Kamii, K. Modulation of ischemia-reperfusion-induced hepatic-injury by kupffer cells. Dig. Dis. Sci. 1994, 39, 1265–1272. [Google Scholar] [CrossRef]
- Elias-Miro, M.; Jimenez-Castro, M.B.; Rodes, J.; Peralta, C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic. Res. 2013, 47, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Cannistra, M.; Ruggiero, M.; Zullo, A.; Gallelli, G.; Serafini, S.; Maria, M.; Naso, A.; Grande, R.; Serra, R.; Nardo, B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int. J. Surg. 2016, 33, S57–S70. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Lentsch, A.B. Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration. Gene Expr. 2017, 17, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A.; Fernandez, V. Biochemical aspects of cellular oxidative stress. Arch. Biol. Med. Exp. 1988, 21, 85–92. [Google Scholar] [PubMed]
- Dossi, C.G.; Vargas, R.G.; Valenzuela, R.; Videla, L.A. Beneficial effects of natural compounds on experimental liver ischemia-reperfusion injury. Food Funct. 2021, 12, 3787–3798. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.Y.; Lee, S.M. Protective effect of low dose of ascorbic acid on hepatobiliary function in hepatic ischemia/reperfusion in rats. J. Hepatol. 2002, 36, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Lee, J.S.; Lee, S.M. Protective effects of combined ischemic preconditioning and ascorbic acid on mitochondrial injury in hepatic ischemia/reperfusion. J. Surg. Res. 2007, 142, 45–52. [Google Scholar] [CrossRef]
- Passoni, C.; Coelho, C.A.R. Ascorbic acid supplementation has a cytoprotective effect on secondary biliary cirrhosis: Experimental study in young rats. J. Pediatr. 2008, 84, 522–528. [Google Scholar] [CrossRef]
- Bonatsos, V.; Kappas, I.; Birbas, K.; Vlachodimitropoulos, D.; Toutouzas, K.; Karampela, E.; Syrmos, N.; Bonatsos, G.; Papalois, A.E. Effects of U-74389G (21-Lazaroid) and Ascorbic Acid on Liver Recovery After Acute Ischemia and Reperfusion in Rats. In Vivo 2015, 29, 585–594. [Google Scholar]
- Huseyin, S.; Guclu, O.; Yuksel, V.; Erkul, G.S.A.; Can, N.; Turan, F.N.; Canbaz, S. Avoiding Liver Injury with Papaverine and Ascorbic Acid Due to Infrarenal Cross-Clamping: An Experimental Study. Braz. J. Cardiovasc. Surg. 2017, 32, 197–201. [Google Scholar] [CrossRef][Green Version]
- Zaccaria, A.; Weinzweig, N.; Yoshitake, M.; Matsuda, T.; Cohen, M. Vitamin-C reduces ischemia-reperfusion injury in a rat epigastric island skin flap model. Ann. Plast. Surg. 1994, 33, 620–623. [Google Scholar] [CrossRef]
- Zhu, Y.B.; Zhang, Y.P.; Zhang, J.; Zhang, Y.B. Evaluation of Vitamin C Supplementation on Kidney Function and Vascular Reactivity Following Renal Ischemic Injury in Mice. Kidney Blood Press. Res. 2016, 41, 460–470. [Google Scholar] [CrossRef]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-van Straaten, H.M. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit. Care 2018, 22, 125–139. [Google Scholar] [CrossRef]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-Van Straaten, H.M. Vitamin C: Should we supplement? Curr. Opin. Crit. Care 2018, 24, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Action of ascorbic-acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54, S1119–S1124. [Google Scholar] [CrossRef]
- Matsukawa, H.; Yagi, T.; Matsuda, H.; Kawahara, H.; Yamamoto, I.; Matsuoka, J.; Tanaka, N. Ascorbic acid 2-glucoside prevents sinusoidal endothelial cell apoptosis in supercooled preserved grafts in rat liver transplantation. Transplant. Proc. 2000, 32, 313–317. [Google Scholar] [CrossRef]
- Liu, J.; Yagi, T.; Sadamori, H.; Matsukawa, H.; Sun, D.S.; Mitsuoka, N.; Yamamura, M.; Matsuoka, J.; Jin, Z.; Yamamoto, I.; et al. Annexin V assay-proven anti-apoptotic effect of ascorbic acid 2-glucoside after cold ischemia/reperfusion injury in rat liver transplantation. Acta Medica Okayama 2003, 57, 209–216. [Google Scholar]
- Park, S.W.; Lee, S.M. Antioxidant and prooxidant properties of ascorbic acid on hepatic dysfunction induced by cold ischemia/reperfusion. Eur. J. Pharmacol. 2008, 580, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Washino, Y.; Koga, E.; Ito, A.; Kawagoe, M.; Nakazaki, C.; Kiso, K.; Ichi, I.; Matsura, T.; Kojo, S. Oxidative Stress in the Ischemic and Non-Ischemic Parts of the Rat Liver after Two-Thirds Ischemia/Reperfusion. Biosci. Biotechnol. Biochem. 2010, 74, 979–983. [Google Scholar] [CrossRef]
- Tang, D.L.; Kang, R.; Zeh, H.J.; Lotze, M.T. High-Mobility Group Box 1, Oxidative Stress, and Disease. Antioxid. Redox Signal. 2011, 14, 1315–1335. [Google Scholar] [CrossRef]
- Zhai, Y.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Liver Ischemia and Reperfusion Injury: New Insights into Mechanisms of Innate-Adaptive Immune-Mediated Tissue Inflammation. Am. J. Transplant. 2011, 11, 1563–1569. [Google Scholar] [CrossRef]
- Collard, C.D.; Gelman, S. Pathophysiology clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 2001, 94, 1133–1138. [Google Scholar] [CrossRef]
- Jaeschke, H.; Woolbright, B.L. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant. Rev. 2012, 26, 103–114. [Google Scholar] [CrossRef]
- Pan, E.T.; Yoeli, D.; Galvan, N.T.N.; Kueht, M.L.; Cotton, R.T.; O’Mahony, C.A.; Goss, J.A.; Rana, A. Cold ischemia time is an important risk factor for post-liver transplant prolonged length of stay. Liver Transplant. 2018, 24, 762–768. [Google Scholar] [CrossRef]
- Figueira, E.R.R.; Rocha, J.A.; Lanchotte, C.; Coelho, A.M.M.; Nakatani, M.; Tatebe, E.R.; Lima, J.A.V.; Mendes, C.O.; de Araujo, B.C.R.P.; Abdo, E.E.; et al. Sevoflurane Preconditioning plus Postconditioning Decreases Inflammatory Response with Hemodynamic Recovery in Experimental Liver Ischemia Reperfusion. Gastroenterol. Res. Pract. 2019, 2019, 5758984. [Google Scholar] [CrossRef]
- Moscoso, A.V.; Figueira, E.R.R.; Rocha, J.A.; Urner, M.; Lanchotte, C.; Jukemura, J.; Ximenes, J.L.S.; Nahas, S.C.; D’Albuquerque, L.A.C.; Galvao, F.H.F. Hexafluoroisopropanol decreases liver ischemia-reperfusion injury by downregulation of high mobility group box-1 protein. Pharmacol. Res. Perspect. 2022, 10, e01027. [Google Scholar] [CrossRef]
- Rocha-Santos, V.; Figueira, E.R.R.; Rocha, J.A.; Coelho, A.M.M.; Pinheiro, R.S.; Bacchella, T.; Machado, M.C.C.; D’Albuquerque, L.A.C. Pentoxifylline enhances the protective effects of hypertonic saline solution on liver ischemia reperfusion injury through inhibition of oxidative stress. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 194–200. [Google Scholar] [CrossRef]
- Vasques, E.R.; Figueira, E.R.R.; Rocha, J.A.; Lanchotte, C.; Ximenes, J.L.S.; Nader, H.B.; Tersariol, I.L.; A Lima, M.; Rodrigues, T.; Cunha, J.E.; et al. A new heparin fragment decreases liver ischemia-reperfusion injury. Hepatobiliary Pancreat. Dis. Int. 2022, 21, 190–192. [Google Scholar] [CrossRef]
- de Grooth, H.J.S.; Spoelstra-de Man, A.M.E.; Oudemans-van Straaten, H.M. Early plasma vitamin c concentration, organ dysfunction and icu mortality. Intensive Care Med. 2014, 40, S199. [Google Scholar]
- de Grooth, H.J.; Manubulu-Choo, W.P.; Zandvliet, A.S.; Spoelstra-de Man, A.M.E.; Girbes, A.R.; Swart, E.L.; Oudemans-van Straaten, H.M. Vitamin C Pharmacokinetics in Critically Ill Patients A Randomized Trial of Four IV Regimens. Chest 2018, 153, 1368–1377. [Google Scholar] [CrossRef]
- Rozemeijer, S.; Spoelstra-de Man, A.M.E.; Coenen, S.; Smit, B.; Elbers, P.W.G.; de Grooth, H.J.; Girbes, A.R.J.; Straaten, H.M.O.-V. Estimating Vitamin C Status in Critically Ill Patients with a Novel Point-of-Care Oxidation-Reduction Potential Measurement. Nutrients 2019, 11, 1031. [Google Scholar] [CrossRef]
- Layton, M.E.; Wood, J.G.; Yan, Z.Y.; Forster, J. Ischemia/reperfusion alters uric acid and ascorbic acid levels in liver. J. Surg. Res. 1996, 64, 1–5. [Google Scholar] [CrossRef]
- Figueira, E.R.R.; Bacchella, T.; Coelho, A.M.M.; Sampietre, S.N.; Molan, N.A.T.; Leitao, R.M.C.; Machado, M.C.C. Timing-dependent protection of hypertonic saline solution administration in experimental liver ischemia/reperfusion injury. Surgery 2010, 147, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Nagel, E.; Vilsendorf, A.M.Z.; Bartels, M.; Pichlmayr, R. Antioxidative vitamins in prevention of ischemia/reperfusion injury. Int. J. Vitam. Nutr. Res. 1997, 67, 298–306. [Google Scholar]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.E.; de Waard, M.C. Vitamin C revisited. Crit. Care 2014, 18, 460. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.T.; Probert, M.R. Vitamin C Revisited. Acta Crystallogr. a-Found. Adv. 2016, 72, S310. [Google Scholar] [CrossRef]
- Shen, X.D.; Ke, B.; Zhai, Y.; Gao, F.; Busuttil, R.W.; Cheng, G.H.; Kupiec-Weglinski, J.W. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am. J. Transplant. 2005, 5, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Joshi, D.; Xu, S.W.; Hamilton, G.; Abraham, D.; Baker, D.; Shaw, S.; Tsui, J. The Toll-like Receptor 2 Ligand HMGB-1 Contributes to Skeletal Muscle Damage in Critical Limb Ischemia. J. Vasc. Surg. 2011, 53, 82S–83S. [Google Scholar] [CrossRef][Green Version]
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005, 201, 1135–1143. [Google Scholar] [CrossRef]
- Sachdev, U.; Tzeng, E. HMGB-1 induces pro-angiogenic effects in endothelial cells: Implications for lower extremity ischemia. J. Am. Coll. Surg. 2007, 205, S108–S109. [Google Scholar] [CrossRef]
- Sachdev, U.; Seung, N.; Tzeng, E. HMGB-1 promotes pro-angiogenic effects in cultured endothelial cells, and may mediate revascularization of ischemic tissue in a murine model of hind-limb ischemia. J. Leukoc. Biol. 2008, 84, A37. [Google Scholar]
- Kim, W.K.; Kwon, Y.; Park, M.; Yun, S.; Kwon, J.Y.; Kim, H. Identification of specifically activated angiogenic molecules in HMGB-1-induced angiogenesis. Bmb Rep. 2017, 50, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Tsung, A.; Hoffman, R.A.; Izuishi, K.; Critchlow, N.D.; Nakao, A.; Chan, M.H.; Lotze, M.T.; Geller, D.A.; Billiar, T.R. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J. Immunol. 2005, 175, 7661–7668. [Google Scholar] [CrossRef]
- Zhai, Y.; Shen, X.D.; O’Connell, R.; Gao, T.; Lassman, C.; Busuttil, R.W.; Cheng, G.; Kupiec-Weglinski, J.W. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J. Immunol. 2004, 173, 7115–7119. [Google Scholar] [CrossRef] [PubMed]
- Hadi, N.R.; Al-Amran, F.; Tweij, T.A.R.; Al-Mudhafar, D.H. Dimethyl Fumarate Treatment Mitigates Ischemia Reperfusion-Induced Renal Damage by Suppressing HMGB-1-TLR Signaling Pathway. Lat. Am. J. Pharm. 2020, 39, 1176–1186. [Google Scholar]
- Watanabe, T.; Kubota, S.; Nagaya, M.; Ozaki, S.; Nagafuchi, H.; Akashi, K.; Taira, Y.; Tsukikawa, S.; Oowada, S.; Nakano, S. The role of HMGB-1 on the development of necrosis during hepatic ischemia and hepatic ischemia/reperfusion injury in mice. J. Surg. Res. 2005, 124, 59–66. [Google Scholar] [CrossRef]
- Qiu, J.; Nishimura, M.; Wang, Y.; Sims, J.R.; Qiu, S.; Savitz, S.I.; Salomone, S.; Moskowitz, M.A. Early release of HMGB-1 from neurons after the onset of brain ischemia. J. Cereb. Blood Flow Metab. 2008, 28, 927–938. [Google Scholar]
- Chen, G.; Li, J.H.; Gong, Q.; Zhong, S.; Ruan, Y.L.; Chen, S.; Xiang, Y.; Gong, F.; Chen, S. Neutralization of Extracellular HMGB-1 Significantly Protects Mice Against Renal Ischemia Reperfusion Injury. Am. J. Transplant. 2009, 9, 340. [Google Scholar]
- Jin, H.; Liu, A.D.; Huang, H.; Dirsch, O.; Deng, M.H. Prolonged cold ischemia leads to HMGB-1 release, but does not interfere with spontaneous liver graft acceptance. Transpl. Int. 2009, 22, 364. [Google Scholar]
- Ueno, T.; Ikeda, T.; Ikeda, K.; Taniuchi, H.; Suda, S.; Yeung, M.Y.; Matsuno, N. HMGB-1 as a Useful Prognostic Biomarker in Sepsis-Induced Organ Failure in Patients Undergoing PMX-DHP. J. Surg. Res. 2011, 171, 183–190. [Google Scholar]
- Houben, P.; Hohenberger, R.; Yamanaka, K.; Buchler, M.W.; Schemmer, P. Evaluation of Graft Effluent High Mobility Group Box-1 (HMGB-1) for Prediction of Outcome After Liver Transplantation. Ann. Transplant. 2018, 23, 475–480. [Google Scholar] [CrossRef]
- Nunes, G.; Figueira, E.R.R.; Rocha, J.A.; Lanchotte, C.; Nacif, L.S.; Ferreira, D.M.; Romano, V.C.; Abdo, E.E.; D’Albuquerque, L.A.C.; Galvão, F.H.F. Hypertonic saline solution decreases oxidative stress in liver hypothermic ischemia. Surgery 2021, 169, 1512–1518. [Google Scholar] [CrossRef]
- Quireze, C.; Montero, E.F.D.; Leitao, R.M.C.; Juliano, Y.; Fagundes, D.J.; Poli-de-Figueiredo, L.F. Ischemic preconditioning prevents apoptotic cell death and necrosis in early and intermediate phases of liver ischemia-reperfusion injury in rats. J. Investig. Surg. 2006, 19, 229–236. [Google Scholar] [CrossRef]
| Control | PI | PR | PIPR | |
|---|---|---|---|---|
| pH | 7.08 ± 0.07 | 7.19 ± 0.1 | 7.11 ± 0.13 | 7.11 ± 0.08 |
| Base excess | −7.98 ± 2.39 | −2.85 ± 6.18 | −8.29 ± 4.88 | −7.31 ± 5.21 |
| HCO3 | 20.56 ± 1.75 | 24.40 ± 5.19 | 19.81 ± 3.46 | 19.81 ± 4.44 |
| Haemoglobin (g/dL) | 19.28 ± 0.59 | 17.73 ± 1.48 | 17.71 ± 1.40 | 17.71 ± 1.19 |
| Potassium (mEq/L) | 5.40 ± 0.76 | 6.14 ± 0.71 | 6.15 ± 0.69 | 6.15 ± 0.66 |
| Ionised calcium (mg/dL) | 3.93 ± 0.72 | 4.17 ± 0.83 | 3.48 ± 0.72 | 3.48 ± 0.91 |
| Lactate (mg/dL) | 31.00 ± 20.22 | 25.75 ± 10.73 | 34.50 ± 15.05 | 34.50 ± 9.50 |
| Glucose (mg/dL) | 327 ± 156 | 311 ± 105 | 340 ± 91 | 340 ± 212 |
| Control | PI | PR | PIPR | |
|---|---|---|---|---|
| IL-1beta | 412.46 ± 373.47 | 192.82 ± 136.06 | 711.10 ± 1085.13 | 414.39 ± 277.03 |
| IL-6 | 544.12 ± 992.88 | 688.00 ± 1212.27 | 1225.11 ± 1314.19 | 903.42 ± 1262.83 |
| IL-10 | 699.08 ± 647.46 | 401.97 ± 178.27 | 763.92 ± 513.45 | 601.60 ± 241.56 |
| IL-12p70 | 165.93 ± 242.07 | 84.79 ± 86.25 | 85.20 ± 142.43 | 162.94 ± 180.11 |
| TNF alfa | 12.81 ± 14.47 | 12.63 ± 5.64 | 11.81 ± 7.40 | 12.82 ± 9.10 |
| HMGB-1 | 930.61 ± 752.51 | 712.16 ± 591.13 | 1151.83 ± 849.92 | 444.19 ± 124.11 |
| Variable | Total, n = 27 | Control, n = 5 | PI, n = 8 | PR, n = 8 | PIPR, n = 6 |
|---|---|---|---|---|---|
| Congestion | |||||
| Absent | 2 (7.4%) | 1 (20%) | 1 (12%) | 0 (0%) | 0 (0%) |
| Mild | 16 (59%) | 4 (80%) | 3 (38%) | 5 (62%) | 4 (67%) |
| Moderate | 7 (26%) | 0 (0%) | 4 (50%) | 1 (12%) | 2 (33%) |
| Severe | 2 (7.4%) | 0 (0%) | 0 (0%) | 2 (25%) | 0 (0%) |
| Portal inflammatory infiltrate | |||||
| No | 26 (96%) | 5 (100%) | 7 (88%) | 8 (100%) | 6 (100%) |
| Yes | 1 (3.7%) | 0 (0%) | 1 (12%) | 0 (0%) | 0 (0%) |
| Lobular inflammatory infiltrate | |||||
| No | 23 (85%) | 5 (100%) | 6 (75%) | 6 (75%) | 6 (100%) |
| Yes | 4 (15%) | 0 (0%) | 2 (25%) | 2 (25%) | 0 (0%) |
| Detrabeculation | |||||
| No | 19 (70%) | 4 (80%) | 6 (75%) | 5 (62%) | 4 (67%) |
| Yes | 8 (30%) | 1 (20%) | 2 (25%) | 3 (38%) | 2 (33%) |
| Necrosis | |||||
| No | 13 (48%) | 3 (60%) | 3 (38%) | 4 (50%) | 3 (50%) |
| Mild | 5 (19%) | 2 (40%) | 1 (12%) | 0 (0%) | 2 (33%) |
| Moderate | 7 (26%) | 0 (0%) | 4 (50%) | 2 (25%) | 1 (17%) |
| Severe | 2 (7.4%) | 0 (0%) | 0 (0%) | 2 (25%) | 0 (0%) |
| Steatosis | |||||
| No | 8 (30%) | 2 (40%) | 2 (25%) | 1 (12%) | 3 (50%) |
| Mild | 14 (52%) | 2 (40%) | 6 (75%) | 4 (50%) | 2 (33%) |
| Moderate | 5 (19%) | 1 (20%) | 0 (0%) | 3 (38%) | 1 (17%) |
| Sinusoidal Cells | |||||
| Mild | 26 (96%) | 5 (100%) | 7 (88%) | 8 (100%) | 6 (100%) |
| Moderate | 1 (3.7%) | 0 (0%) | 1 (12%) | 0 (0%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ximenes, J.L.S.; Rocha-Filho, J.A.; Galvão, F.H.F.; Lanchotte, C.; Kubrusly, M.S.; Leitão, R.M.C.; Jukemura, J.; Moscoso, A.V.; Abdo, E.E.; D’Albuquerque, L.A.C.; et al. The Effect of Ascorbic Acid on Hepatic Ischaemia–Reperfusion Injury in Wistar Rats: An Experimental Study. Int. J. Mol. Sci. 2024, 25, 8833. https://doi.org/10.3390/ijms25168833
Ximenes JLS, Rocha-Filho JA, Galvão FHF, Lanchotte C, Kubrusly MS, Leitão RMC, Jukemura J, Moscoso AV, Abdo EE, D’Albuquerque LAC, et al. The Effect of Ascorbic Acid on Hepatic Ischaemia–Reperfusion Injury in Wistar Rats: An Experimental Study. International Journal of Molecular Sciences. 2024; 25(16):8833. https://doi.org/10.3390/ijms25168833
Chicago/Turabian StyleXimenes, Jorge Luiz Saraiva, Joel Avancini Rocha-Filho, Flavio Henrique Ferreira Galvão, Cinthia Lanchotte, Marcia Saldanha Kubrusly, Regina Maria Cubero Leitão, Jose Jukemura, Agustin Vintimilla Moscoso, Emilio Elias Abdo, Luiz Augusto Carneiro D’Albuquerque, and et al. 2024. "The Effect of Ascorbic Acid on Hepatic Ischaemia–Reperfusion Injury in Wistar Rats: An Experimental Study" International Journal of Molecular Sciences 25, no. 16: 8833. https://doi.org/10.3390/ijms25168833
APA StyleXimenes, J. L. S., Rocha-Filho, J. A., Galvão, F. H. F., Lanchotte, C., Kubrusly, M. S., Leitão, R. M. C., Jukemura, J., Moscoso, A. V., Abdo, E. E., D’Albuquerque, L. A. C., & Figueira, E. R. R. (2024). The Effect of Ascorbic Acid on Hepatic Ischaemia–Reperfusion Injury in Wistar Rats: An Experimental Study. International Journal of Molecular Sciences, 25(16), 8833. https://doi.org/10.3390/ijms25168833








