A New Tailored Nanodroplet Carrier of Astaxanthin Can Improve Its Pharmacokinetic Profile and Antioxidant and Anti-Inflammatory Efficacies
Abstract
:1. Introduction
2. Results
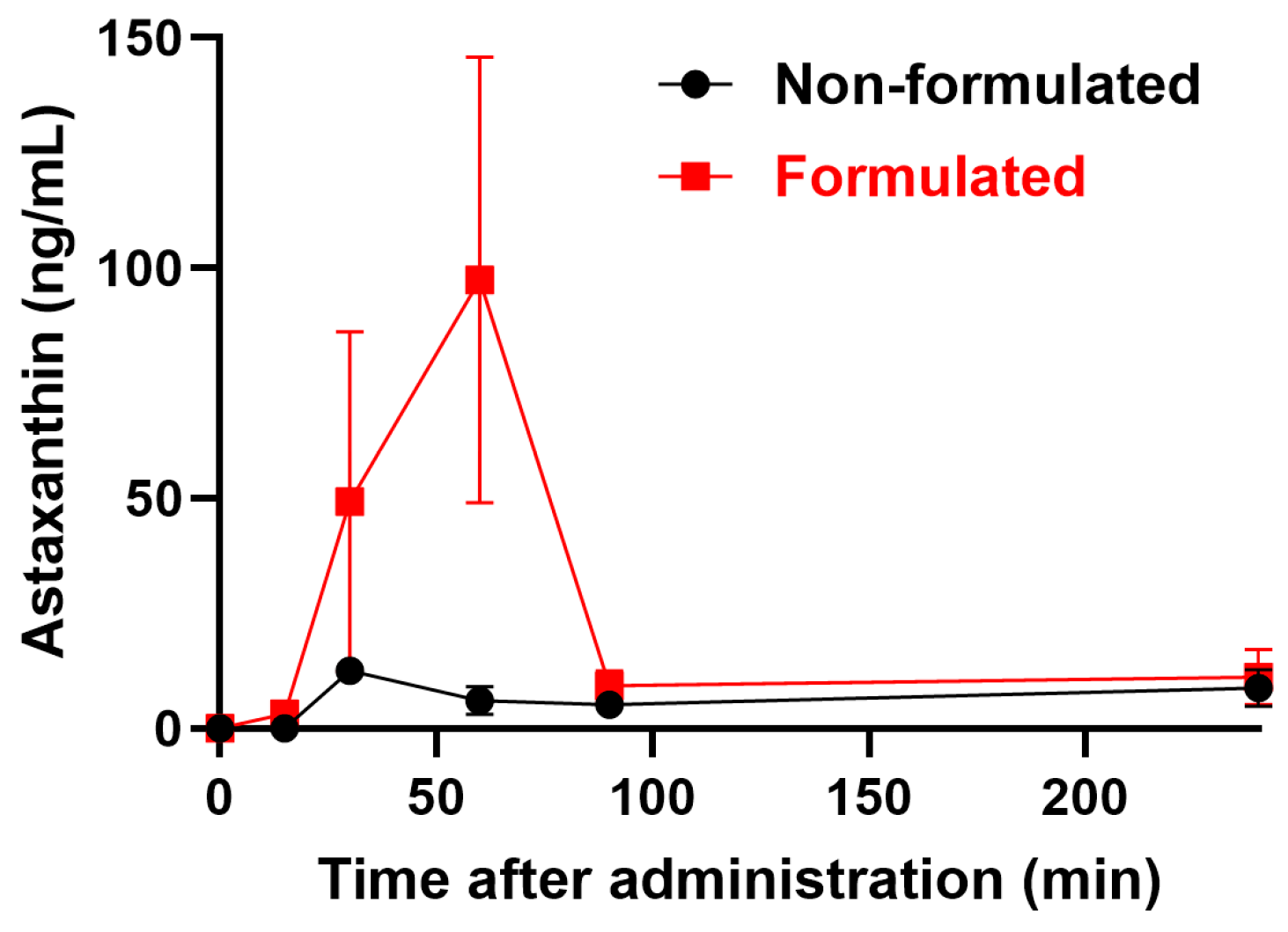
2.1. Pharmacokinetics of LDS-ATX vs. ATX
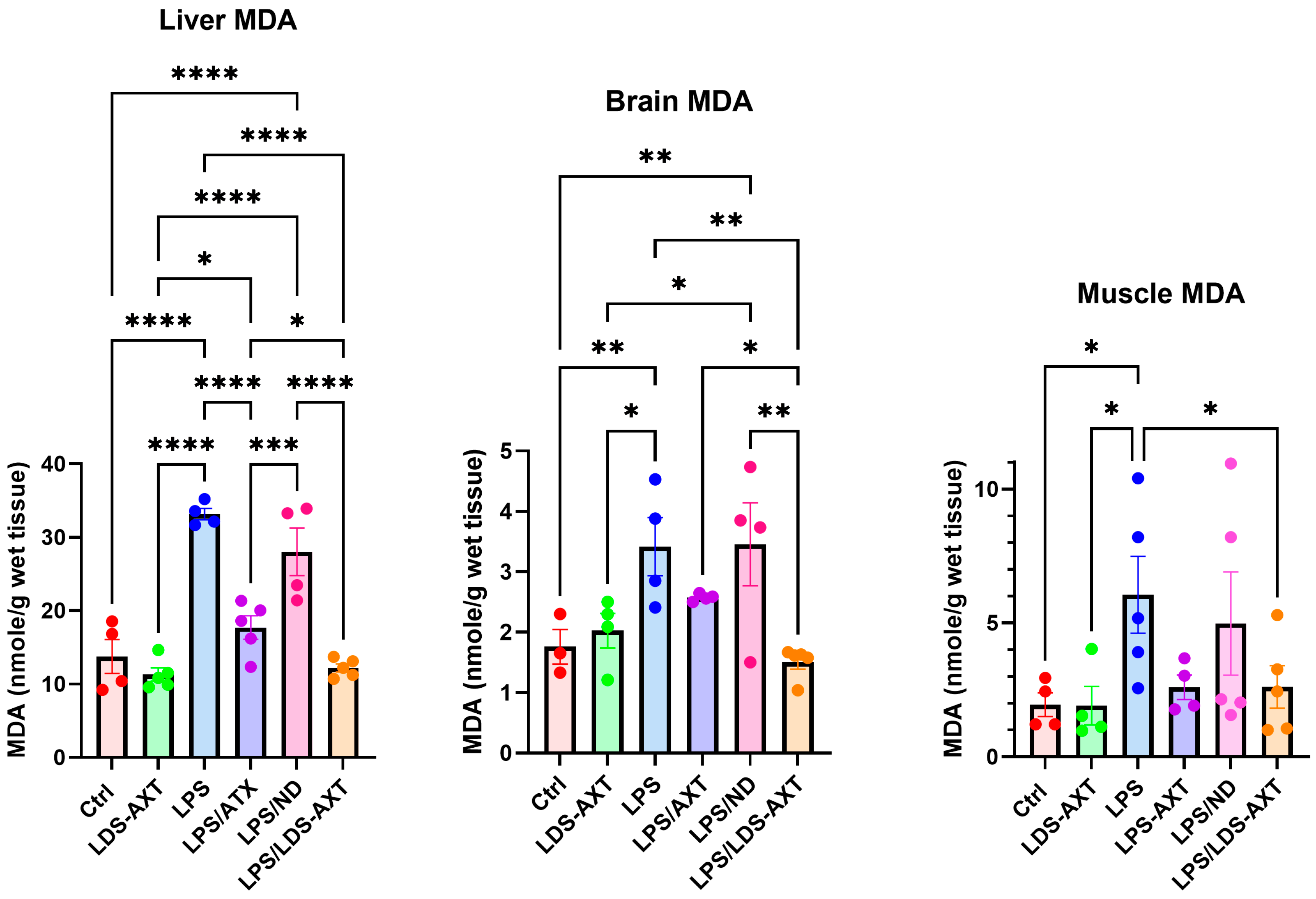
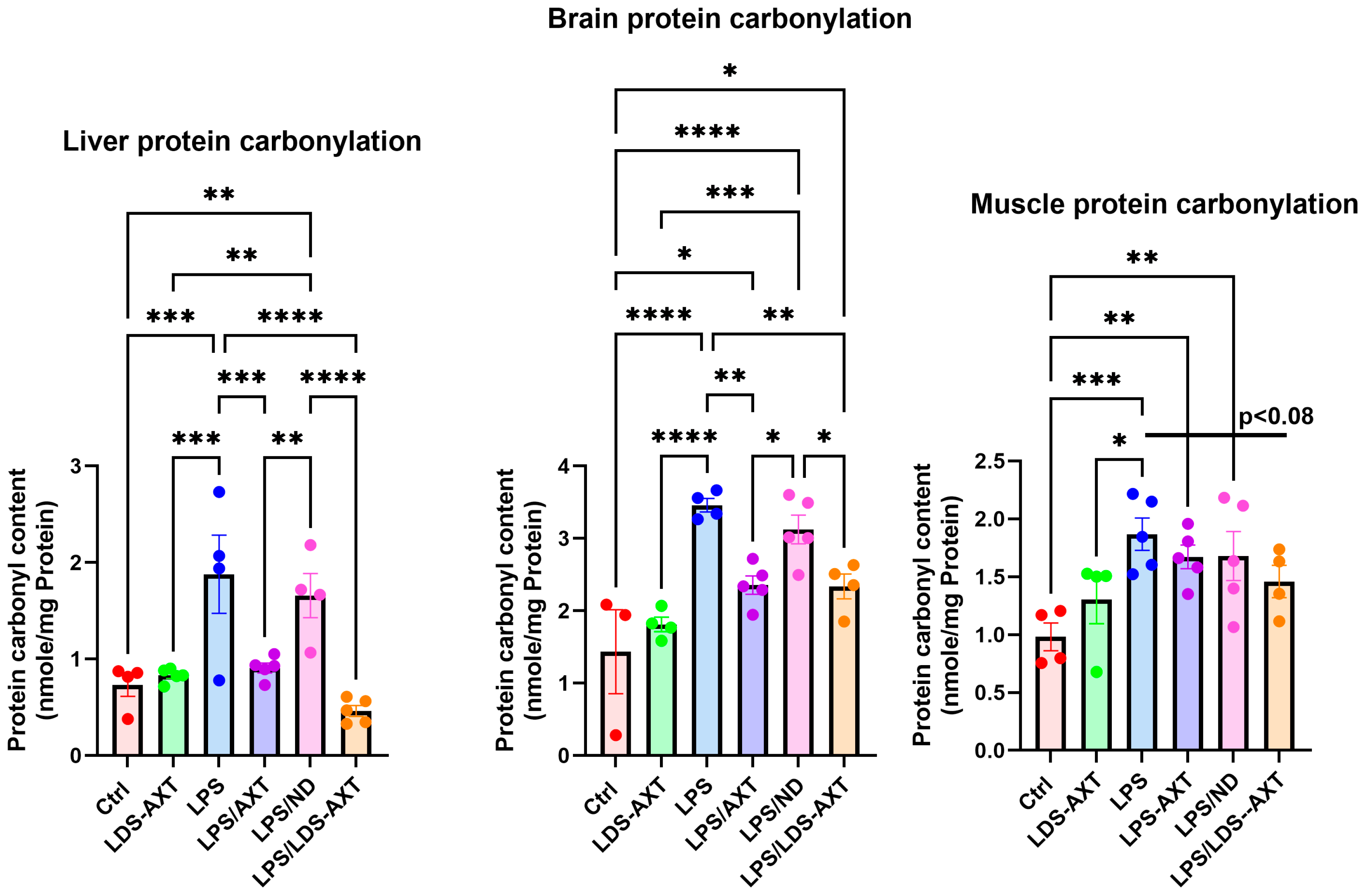
2.2. Antioxidant Efficacy of LDS-ATX vs. ATX
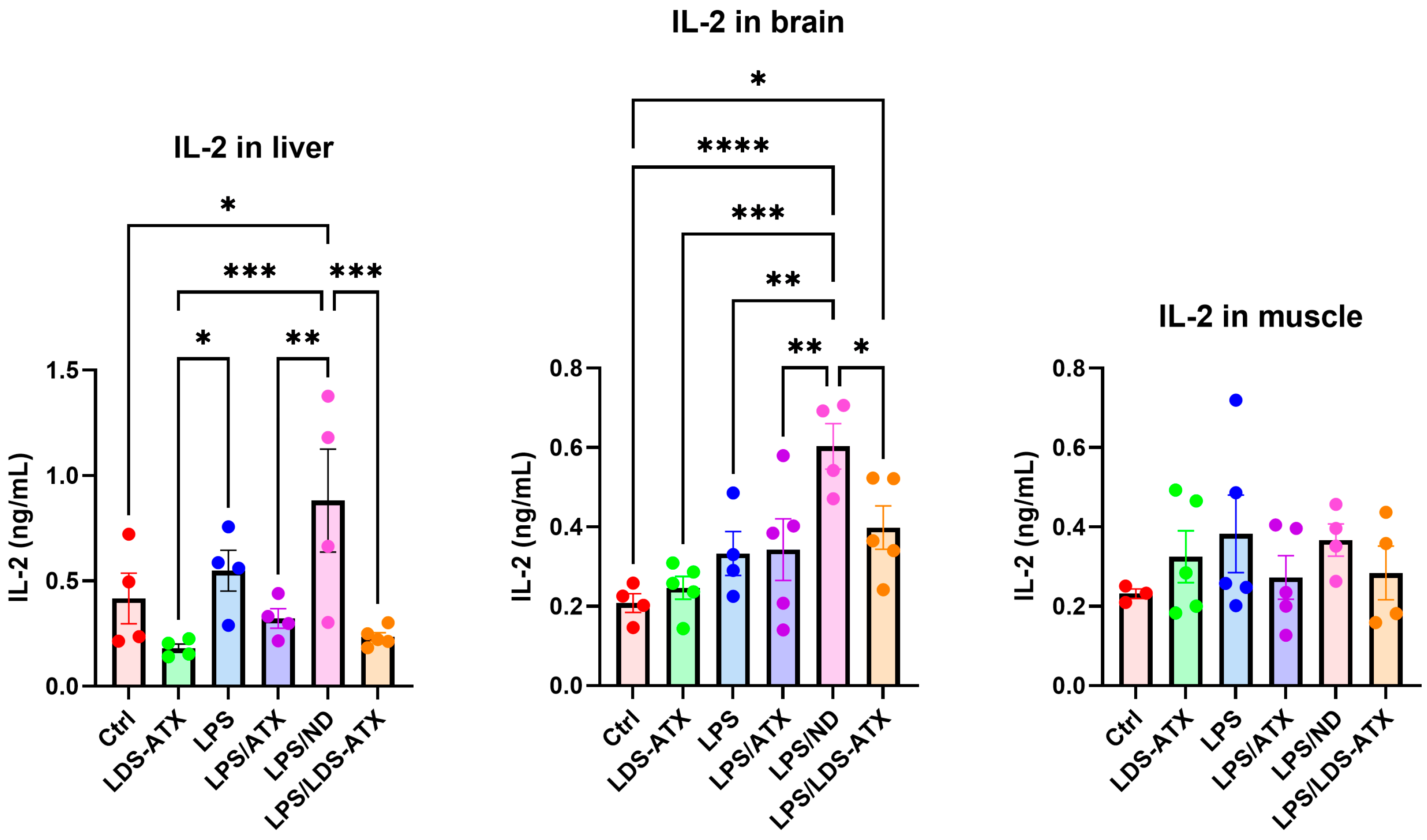
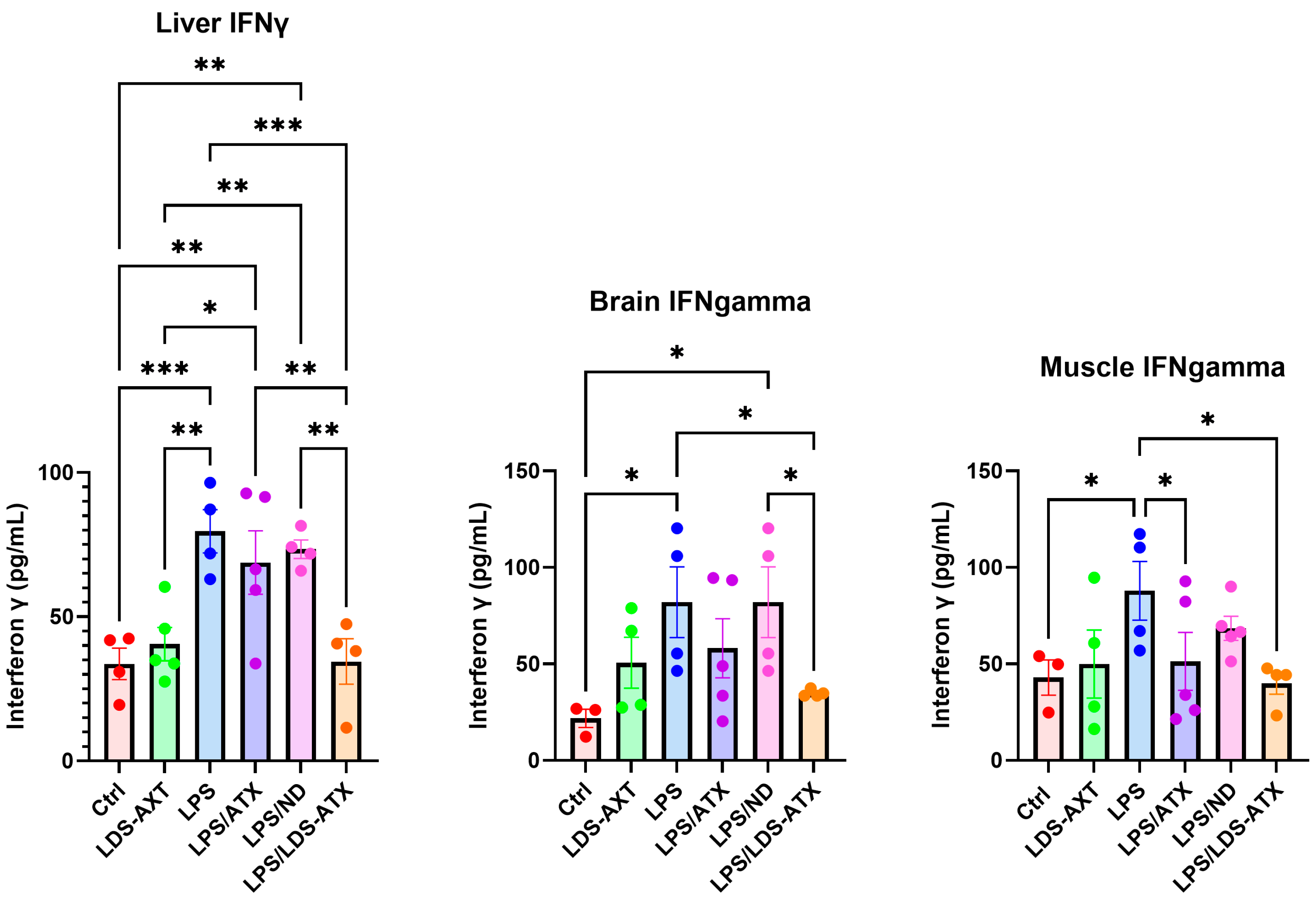
2.3. Anti-Inflammatory Efficacy of LDS-ATX vs. ATX
2.4. Organ Specificity of the LDS-ATX and ATX Effects
3. Discussion
4. Materials and Methods
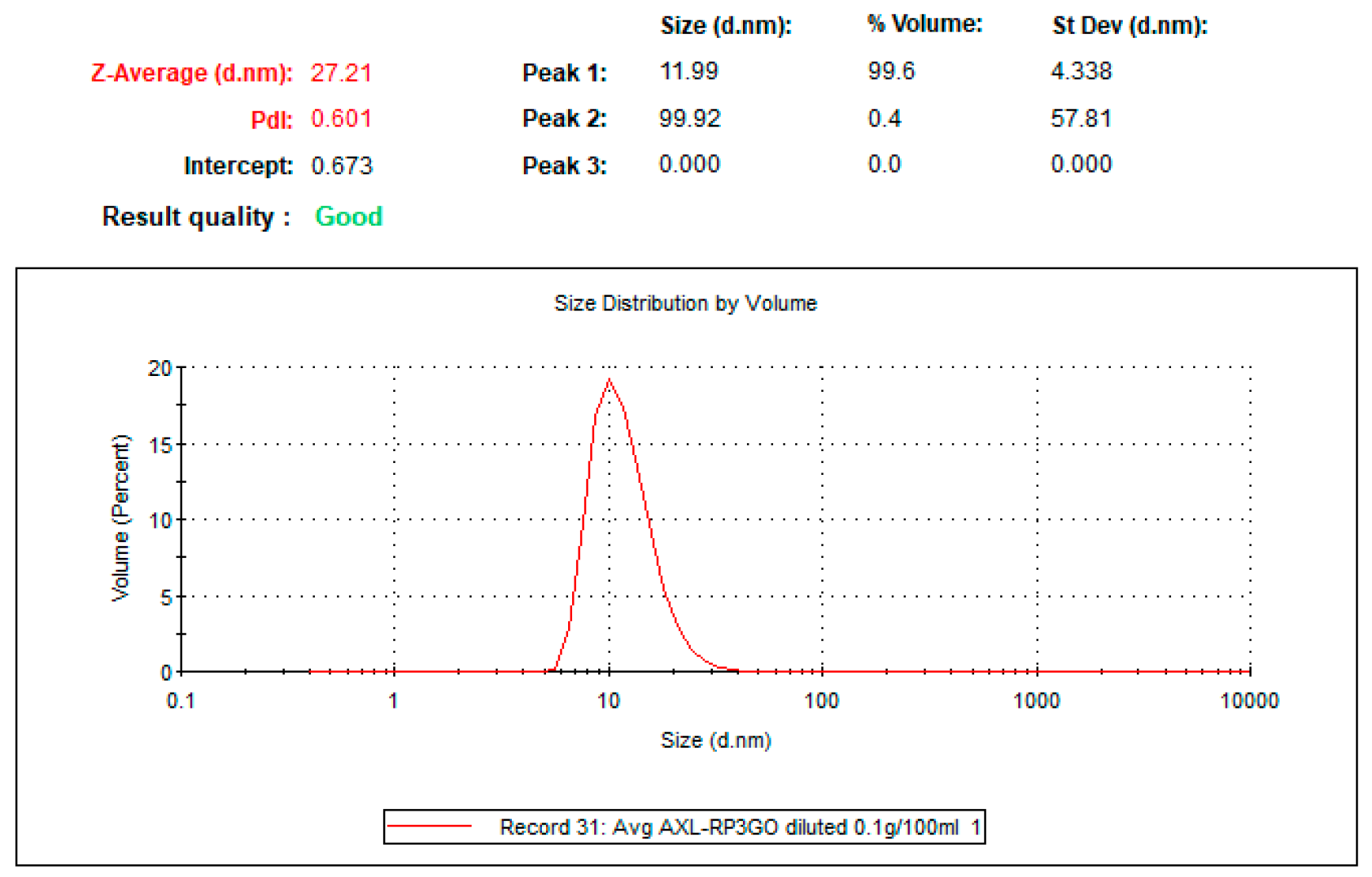
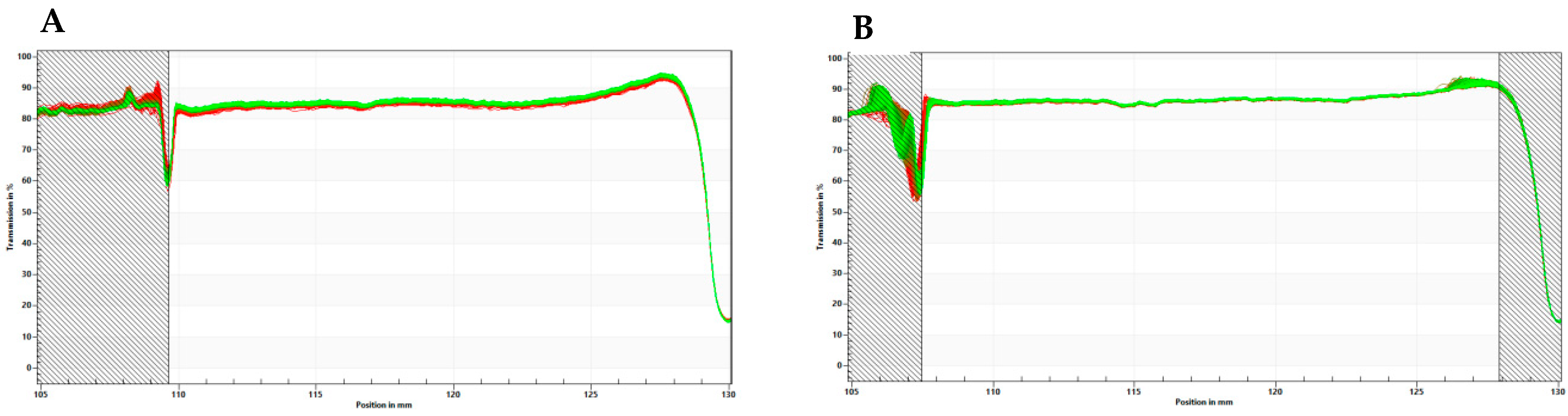
4.1. Preparation of Tailored Nanodroplets for Astaxanthin Delivery (LDS-ATX)
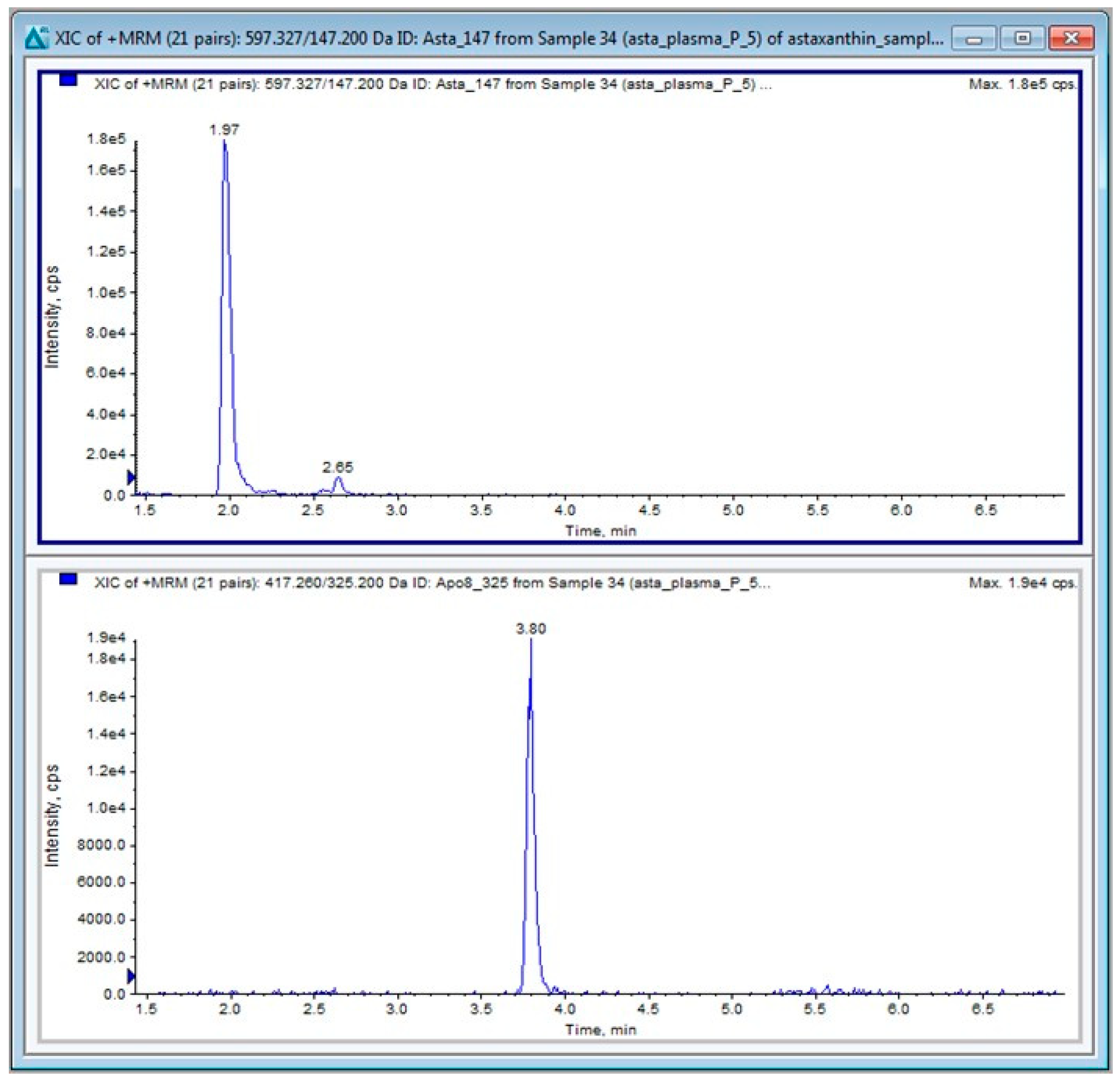
4.2. Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
4.2.1. LC-MS/MS Materials
4.2.2. Ultra-High Performance Liquid Chromatography (UHPLC)
4.2.3. MS/MS Conditions
4.3. Pharmacokinetics
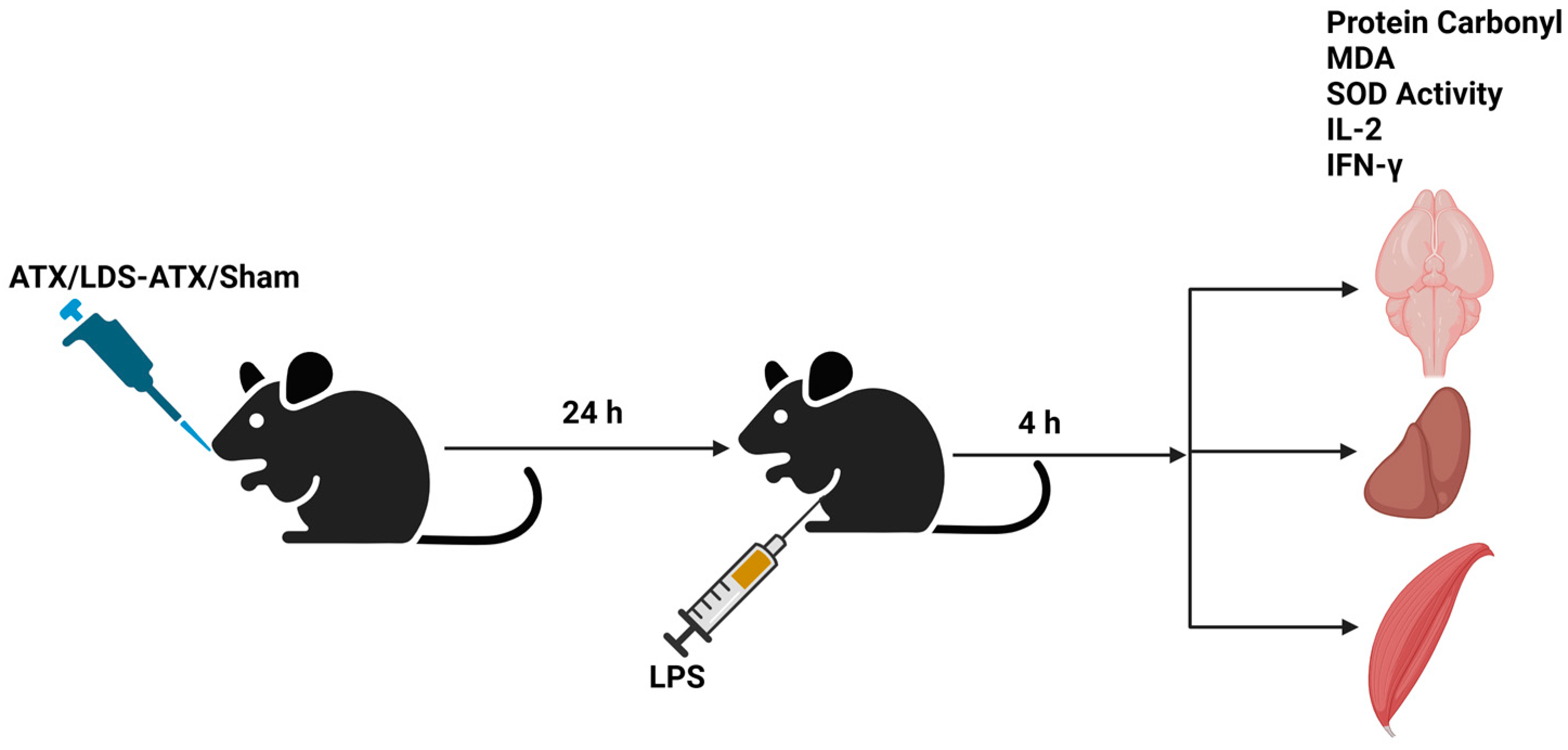
4.4. In Vivo and Ex Vivo Studies
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morilla, M.J.; Ghosal, K.; Romero, E.L. More Than Pigments: The Potential of Astaxanthin and Bacterioruberin-Based Nanomedicines. Pharmaceutics 2023, 15, 1828. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Sun, X.-B.; Xu, Y.-X.; Zhao, H.; Zhu, Q.-Y.; Zhu, C.-Q. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010, 1360, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, X.; Liu, H.; Chen, X.; Shi, H.; Fan, Y.; Hou, D.; Zhang, X. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol. Vis. 2013, 19, 1656–1666. [Google Scholar]
- Ma, H.; Chen, S.; Xiong, H.; Wang, M.; Hang, W.; Zhu, X.; Zheng, Y.; Ge, B.; Li, R.; Cui, H. Astaxanthin from Haematococcus pluvialis ameliorates the chemotherapeutic drug (doxorubicin) induced liver injury through the Keap1/Nrf2/HO-1 pathway in mice. Food Funct. 2020, 11, 4659–4671. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Jiao, D.; Yang, S.; Li, L.; Li, P. Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway. Molecules 2020, 25, 1386. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tao, J.; Xie, X. Astaxanthin Promotes Nrf2/ARE Signaling to Inhibit HG-Induced Renal Fibrosis in GMCs. Mar. Drugs 2018, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Petyaev, I.M.; Klochkov, V.A.; Chalyk, N.E.; Pristensky, D.V.; Chernyshova, M.P.; Kyle, N.; Bashmakov, Y.K. Markers of Hypoxia and Oxidative Stress in Aging Volunteers Ingesting Lycosomal Formulation of Dark Chocolate Containing Astaxanthin. J. Nutr. Health Aging 2018, 22, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ma, X.; Zhang, B.; Zhang, B.; Ma, N.; Ma, N.; Liu, C.; Liu, C.; Miao, Y.; Miao, Y.; et al. Unveiling the Mechanism of Alleviating Ischemia Reperfusion Injury via a Layered Double Hydroxide-Based Nanozyme. ACS Appl. Mater. Interfaces 2023, 15, 13869–13878. [Google Scholar] [CrossRef]
- Diao, L.; Ding, M.; Sun, H.; Xu, Y.; Yin, R.; Chen, H. Micro-algal astaxanthin ameliorates polystyrene microplastics-triggered necroptosis and inflammation by mediating mitochondrial Ca2+ homeostasis in carp’s head kidney lymphocytes (Cyprinus carpio L.). Fish Shellfish Immunol. 2023, 143, 109205. [Google Scholar] [CrossRef]
- Medoro, A.; Davinelli, S.; Milella, L.; Willcox, B.J.; Allsopp, R.C.; Scapagnini, G.; Willcox, D.C. Dietary Astaxanthin: A Promising Antioxidant and Anti-Inflammatory Agent for Brain Aging and Adult Neurogenesis. Mar. Drugs 2023, 21, 643. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-H.; Chang, C.-C.; Lin, S.-T.; Chyau, C.-C.; Peng, R.Y. Improved Hepatoprotective Effect of Liposome-Encapsulated Astaxanthin in Lipopolysaccharide-Induced Acute Hepatotoxicity. Int. J. Mol. Sci. 2016, 17, 1128. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Yamashita, E.; Miki, W. Quenching Activities of Common Hydrophilic and Lipophilic Antioxidants against Singlet Oxygen Using Chemiluminescence Detection System. Carotenoid Sci. 2007, 11, 16–20. [Google Scholar]
- Eren, B.; Tanrıverdi, S.T.; Köse, F.A.; Özer, Ö. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2018, 18, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, D.; Kagan, D.; Seshadri, S. A Study on the Bioavailability of a Proprietary, Sustained-release Formulation of Astaxanthin. Integr. Med. 2018, 17, 38–42. [Google Scholar]
- Garti, N. Delivery of microparticulated liquid systems in food. In Handbook of Nonmedical Applications of Liposomes; CRC Press: Boca Raton, FL, USA, 2018; Volume 3, pp. 143–198. [Google Scholar]
- Prigat, Y.; Fattori, A.; Shames, A.I.; Ottaviani, M.F.; Garti, N. Micro-characterization of modified microemulsions loaded with gossypol, pure and extracted from cottonseed. Colloids Surfaces B Biointerfaces 2019, 180, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Meneses, G.; Rosetti, M.; Espinosa, A.; Florentino, A.; Bautista, M.; Díaz, G.; Olvera, G.; Bárcena, B.; Fleury, A.; Adalid-Peralta, L.; et al. Recovery from an acute systemic and central LPS-inflammation challenge is affected by mouse sex and genetic background. PLoS ONE 2018, 13, e0201375. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, R.; Xia, F.; Zou, T.; Huang, A.; Xiong, S.; Zhang, J. LPS converts Gr-1+CD115+ myeloid-derived suppressor cells from M2 to M1 via P38 MAPK. Exp. Cell Res. 2013, 319, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Chen, D.-Y.; Lou, Q.-Q.; Cao, P.; Hu, R.; Jin, Y.; Wang, D.; Hu, S.-S. Angiotensin Type 2 Receptor Pharmacological Agonist Relieves Neurocognitive Deficits via Reducing Neuroinflammation and Microglial Engulfment of Dendritic Spines. J. Neuroimmune Pharmacol. 2022, 18, 41–57. [Google Scholar] [CrossRef]
- Babu, G.; Mohanty, B. Neurotensin modulation of lipopolysaccharide induced inflammation of gut-liver axis: Evaluation using neurotensin receptor agonist and antagonist. Neuropeptides 2023, 97, 102297. [Google Scholar] [CrossRef]
- Kilic, G.A.; Alsafi, M. β-Glucan Regulates Lipopolysaccharide Induced Genotoxic Damage to The Liver through The Induction of BRCA1 Protein Expression. Cell J. 2023, 25, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Dansou, D.M.; Wang, H.; Nugroho, R.D.; He, W.; Zhao, Q.; Zhang, J. Assessment of Response to Moderate and High Dose Supplementation of Astaxanthin in Laying Hens. Animals 2021, 11, 1138. [Google Scholar] [CrossRef] [PubMed]
- Costalonga, M.; Zell, T. Lipopolysaccharide enhances in vivo interleukin-2 production and proliferation by naive antigen-specific CD4 T cells via a Toll-like receptor 4-dependent mechanism. Immunology 2007, 122, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Lin, K.-C.; Lu, W.-J.; Thomas, P.-A.; Jayakumar, T.; Sheu, J.-R. Astaxanthin, a Carotenoid, Stimulates Immune Responses by Enhancing IFN-γ and IL-2 Secretion in Primary Cultured Lymphocytes in Vitro and ex Vivo. Int. J. Mol. Sci. 2015, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Komai-Koma, M.; Gilchrist, D.S.; Xu, D. Direct recognition of LPS by human but not murine CD8+ T cells via TLR4 complex. Eur. J. Immunol. 2009, 39, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Luo, H.; Li, Z.; Zhong, T.; Tang, J.; Jiang, Y. Screening cytokine/chemokine profiles in serum and organs from an endotoxic shock mouse model by LiquiChip. Sci. China Life Sci. 2017, 60, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Gan, M.; Regenstein, J.M.; Luan, Q. Improving the biological activities of astaxanthin using targeted delivery systems. Crit. Rev. Food Sci. Nutr. 2023, 64, 6902–6923. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.J.; Jeong, Y.-I.; Lee, K.-J.; Yu, Y.-B.; Ohk, S.-H.; Lee, S.-Y. Anticancer Activity of Astaxanthin-Incorporated Chitosan Nanoparticles. Molecules 2024, 29, 529. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, H.; Li, S.; Xue, Y.; Chen, Y.; Adu-Frimpong, M.; Xu, Y.; Yu, J.; Xu, X.; Smyth, H.D.; et al. Preparation of astaxanthin-loaded composite micelles with coaxial electrospray technology for enhanced oral bioavailability and improved antioxidation capability. J. Sci. Food Agric. 2023, 104, 1408–1419. [Google Scholar] [CrossRef]
- Yu, F.; Chen, J.; Wei, Z.; Zhu, P.; Qing, Q.; Li, B.; Chen, H.; Lin, W.; Yang, H.; Qi, Z.; et al. Preparation of carrier-free astaxanthin nanoparticles with improved antioxidant capacity. Front. Nutr. 2022, 9, 1022323. [Google Scholar] [CrossRef]
- Wisniewska, A.; Subczynski, W.K. Effects of polar carotenoids on the shape of the hydrophobic barrier of phospholipid bilayers. Biochim. Biophys. Acta (BBA)-Biomembr. 1998, 1368, 235–246. [Google Scholar] [CrossRef]
- Fukuta, T.; Hirai, S.; Yoshida, T.; Maoka, T.; Kogure, K. Enhancement of antioxidative activity of astaxanthin by combination with an antioxidant capable of forming intermolecular interactions. Free Radic. Res. 2020, 54, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2006, 10, 389–406. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Carotenoids: Nutrition and health. In Carotenoids against Disease: Part C: The Immune System and Disease; Britton, G., Liaanen-Jensen, S., Pfander, H., Eds.; Birkhauser Press: Berlin, Germany, 2009; pp. 363–382. [Google Scholar]
- English, K.; Kwan, R.; Holz, L.E.; McGuffog, C.; Krol, J.M.M.; Kempe, D.; Kaisho, T.; Heath, W.R.; Lisowski, L.; Biro, M.; et al. A hepatic network of dendritic cells mediates CD4 T cell help outside lymphoid organs. Nat. Commun. 2024, 15, 1261. [Google Scholar] [CrossRef]



| Time (min) | Solvent A (%) | Solvent B (%) |
|---|---|---|
| 0.0 | 23 | 77 |
| 0.5 | 23 | 77 |
| 2.5 | 5 | 95 |
| 4.5 | 5 | 95 |
| 5.0 | 23 | 77 |
| 7.0 | 23 | 77 |
| Name | Precursor (m/z) | Product (m/z) | DP (V) | CE (eV) | CXP (V) | Rt (min) |
|---|---|---|---|---|---|---|
| ATX | 597.3 | Quantifier 147.2 | 6 | 29 | 16 | 2.0 |
| Qualifier 173.1 | 11 | 23 | 10 | |||
| APO | 417.2 | Quantifier 325.2 | 16 | 13 | 12 | 3.8 |
| Qualifier 159.2 | 21 | 27 | 16 |
| LDS-ATX | ATX | Unloaded Nanodroplet | LPS | No. of Animals |
|---|---|---|---|---|
| + | − | − | + | 5 |
| − | − | + | + | 5 |
| − | + | − | + | 5 |
| − | − | − | + | 5 |
| + | − | − | − | 5 |
| − | − | − | − | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, K.; Khatib, N.; Barasch, D.; Kumar, P.; Garti, S.; Garti, N.; Kakhlon, O. A New Tailored Nanodroplet Carrier of Astaxanthin Can Improve Its Pharmacokinetic Profile and Antioxidant and Anti-Inflammatory Efficacies. Int. J. Mol. Sci. 2024, 25, 7861. https://doi.org/10.3390/ijms25147861
Mishra K, Khatib N, Barasch D, Kumar P, Garti S, Garti N, Kakhlon O. A New Tailored Nanodroplet Carrier of Astaxanthin Can Improve Its Pharmacokinetic Profile and Antioxidant and Anti-Inflammatory Efficacies. International Journal of Molecular Sciences. 2024; 25(14):7861. https://doi.org/10.3390/ijms25147861
Chicago/Turabian StyleMishra, Kumudesh, Nadin Khatib, Dinorah Barasch, Pradeep Kumar, Sharon Garti, Nissim Garti, and Or Kakhlon. 2024. "A New Tailored Nanodroplet Carrier of Astaxanthin Can Improve Its Pharmacokinetic Profile and Antioxidant and Anti-Inflammatory Efficacies" International Journal of Molecular Sciences 25, no. 14: 7861. https://doi.org/10.3390/ijms25147861
APA StyleMishra, K., Khatib, N., Barasch, D., Kumar, P., Garti, S., Garti, N., & Kakhlon, O. (2024). A New Tailored Nanodroplet Carrier of Astaxanthin Can Improve Its Pharmacokinetic Profile and Antioxidant and Anti-Inflammatory Efficacies. International Journal of Molecular Sciences, 25(14), 7861. https://doi.org/10.3390/ijms25147861







