Ruthenium(II) Complex-Based Tetradentate Schiff Bases: Synthesis, Spectroscopic, Antioxidant, and Antibacterial Investigations
Abstract
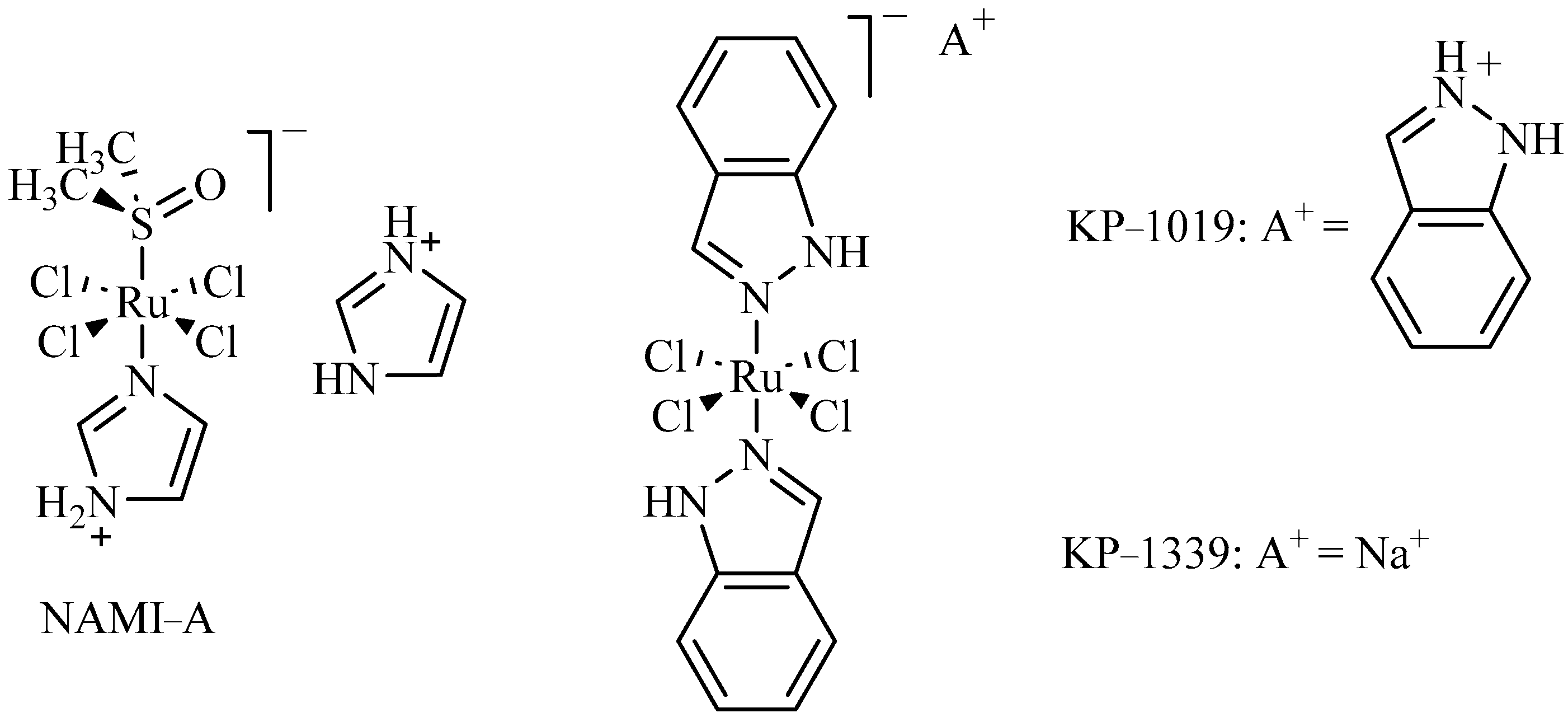
:1. Introduction
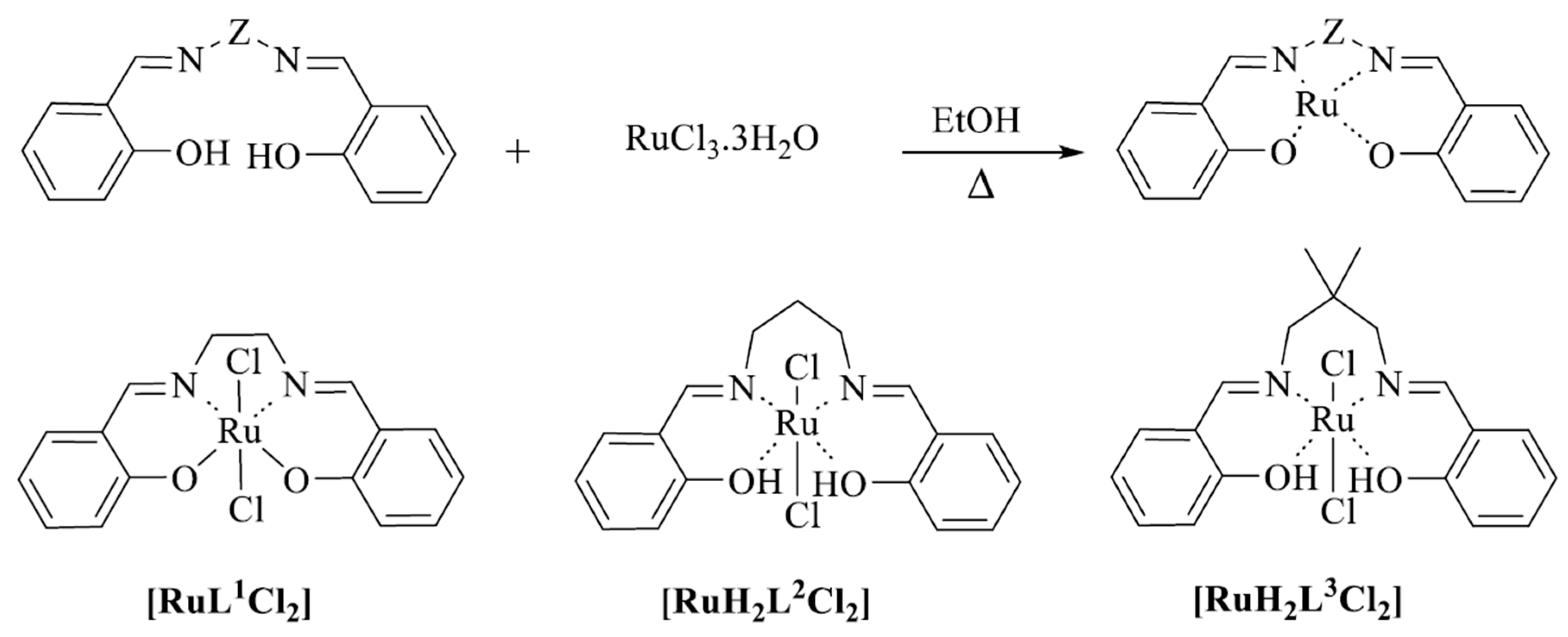
2. Results and Discussion
2.1. Spectroscopic Studies
2.2. UV-Vis Spectra
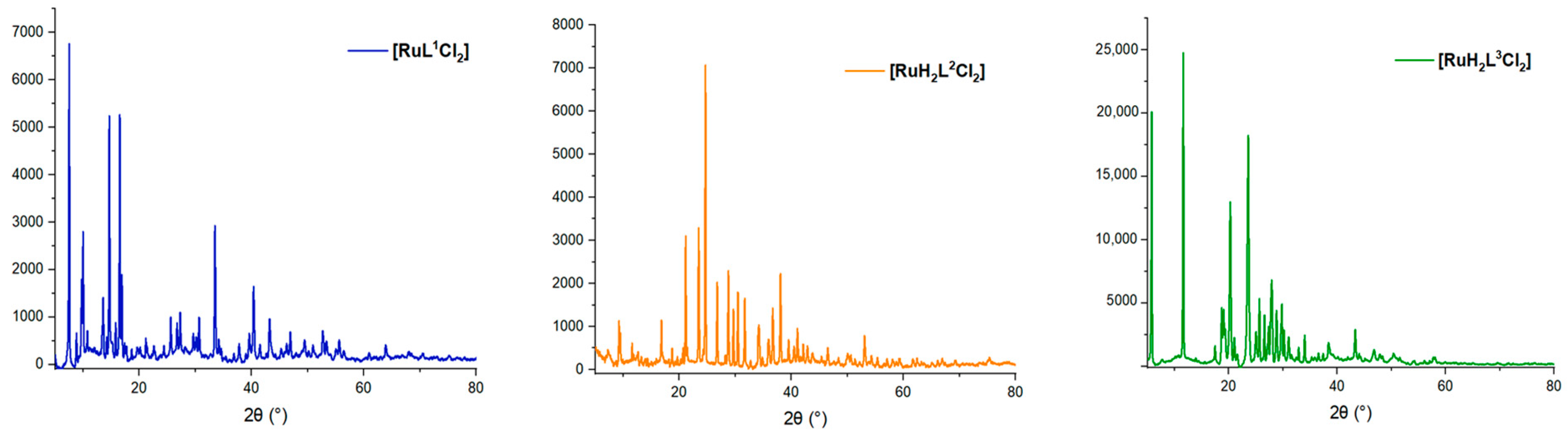
2.3. X-ray Powder
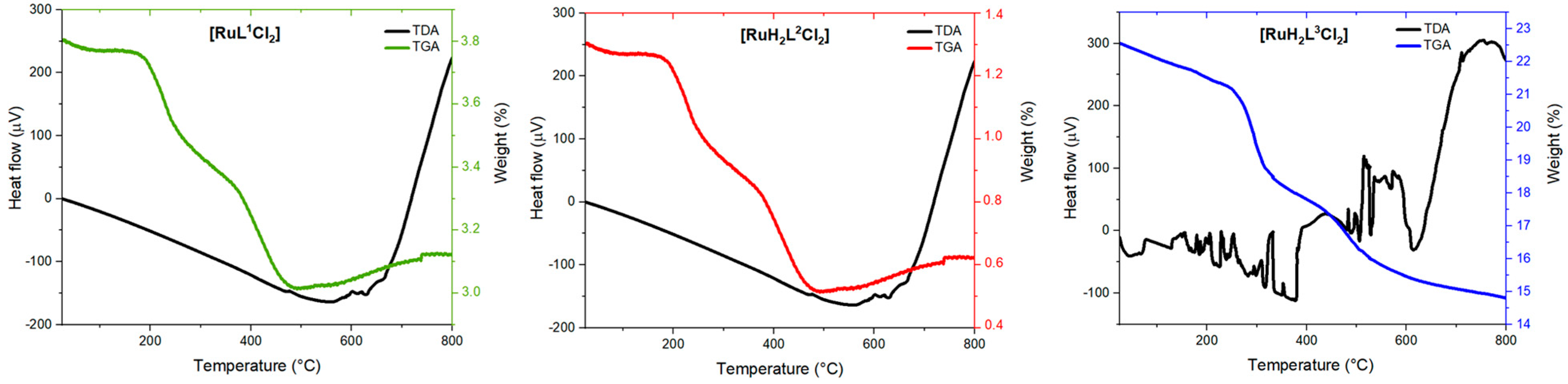
2.4. Thermal Analysis
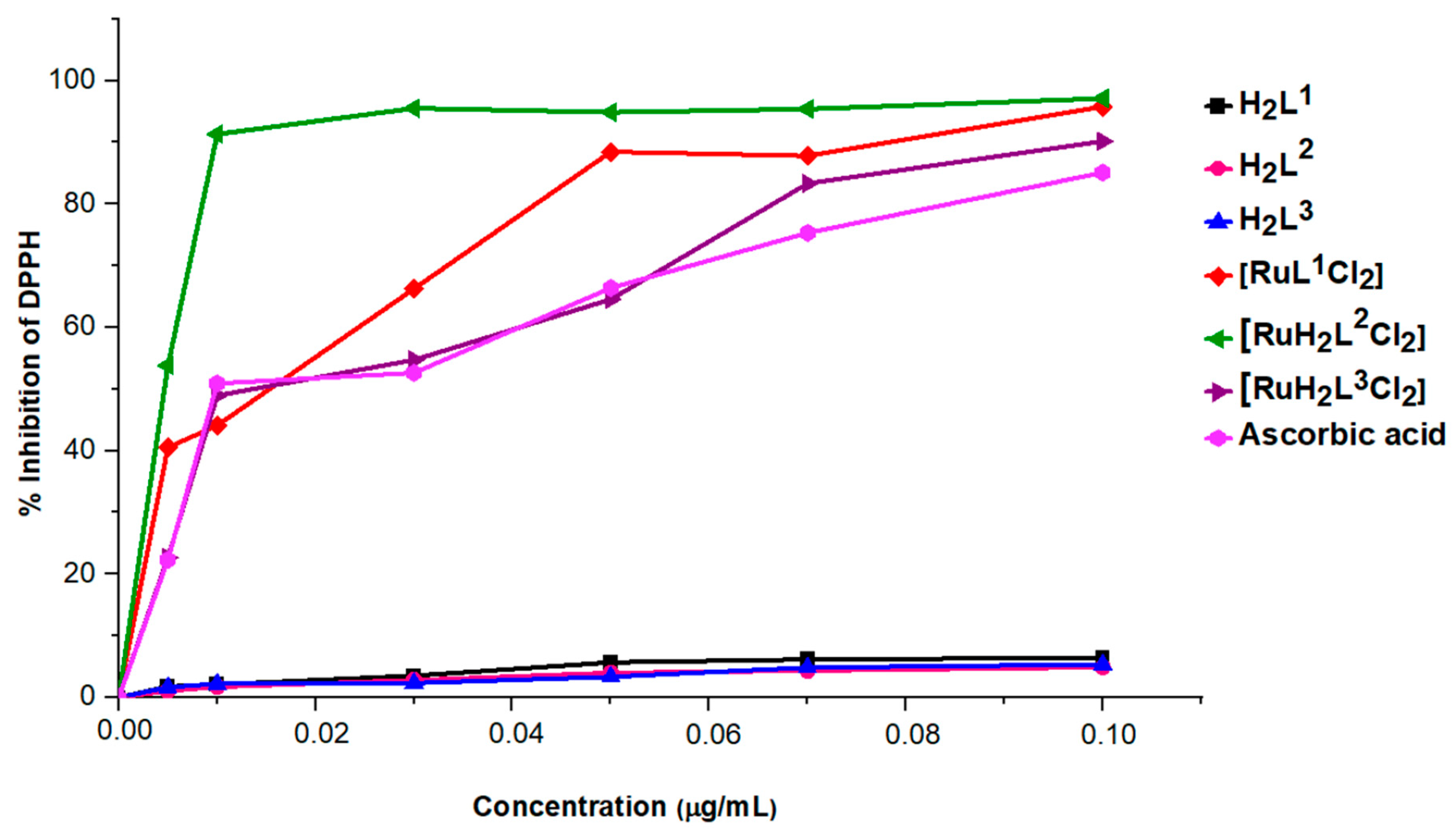
2.5. Antioxidant Activities Essays
2.6. Antibacterial Activity
3. Materials and Methods
3.1. Experimental
3.2. General Procedure for the Preparation of Ligands
3.3. In Vitro Antioxidant Activity Assays
3.3.1. DPPH Radical Scavenging Method
3.3.2. FRAP Method
3.3.3. Total Antioxidant Capacity (TAC)
3.4. In Vitro Antibacterial Activity
3.4.1. Pathogen Bacteria and Growth Conditions
3.4.2. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.Y.; Kim, C.Y.; Nam, T.-G. Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Des. Dev. Ther. 2020, 14, 5375–5392. [Google Scholar] [CrossRef]
- Tomar, N.; Agrawal, A.; Dhaka, V.S.; Surolia, P.K. Ruthenium Complexes Based Dye Sensitized Solar Cells: Fundamentals and Research Trends. Solar Energy 2020, 207, 59–76. [Google Scholar] [CrossRef]
- Aghazada, S.; Nazeeruddin, M.K. Ruthenium Complexes as Sensitizers in Dye-Sensitized Solar Cells. Inorganics 2018, 6, 52. [Google Scholar] [CrossRef]
- Gawin, R.; Tracz, A.; Chwalba, M.; Kozakiewicz, A.; Trzaskowski, B.; Skowerski, K. Cyclic Alkyl Amino Ruthenium Complexes—Efficient Catalysts for Macrocyclization and Acrylonitrile cross Metathesis. ACS Catal. 2017, 7, 5443–5449. [Google Scholar] [CrossRef]
- Li, K.; Niu, J.-L.; Yang, M.-Z.; Li, Z.; Wu, L.-Y.; Hao, X.-Q.; Song, M.-P. New Type of 2,6-Bis(Imidazo[1,2-a]Pyridin-2-Yl)Pyridine-Based Ruthenium Complexes: Active Catalysts for Transfer Hydrogenation of Ketones. Organometallics 2015, 34, 1170–1176. [Google Scholar] [CrossRef]
- Liu, J.; Lai, H.; Xiong, Z.; Chen, B.; Chen, T. Functionalization and Cancer-Targeting Design of Ruthenium Complexes for Precise Cancer Therapy. Chem. Commun. 2019, 55, 9904–9914. [Google Scholar] [CrossRef] [PubMed]
- Golbaghi, G.; Castonguay, A. Rationally Designed Ruthenium Complexes for Breast Cancer Therapy. Molecules 2020, 25, 265. [Google Scholar] [CrossRef]
- Lin, K.; Zhao, Z.-Z.; Bo, H.-B.; Hao, X.-J.; Wang, J.-Q. Applications of Ruthenium Complex in Tumor Diagnosis and Therapy. Front. Pharmacol. 2018, 9, 1323. [Google Scholar] [CrossRef]
- Munteanu, A.-C.; Uivarosi, V. Ruthenium Complexes in the Fight against Pathogenic Microorganisms. An Extensive Review. Pharmaceutics 2021, 13, 874. [Google Scholar] [CrossRef]
- Sasahara, G.L.; Gouveia Júnior, F.S.; Rodrigues, R.d.O.; Zampieri, D.S.; Fonseca, S.G.d.C.; Gonçalves, R.d.C.R.; Athaydes, B.R.; Kitagawa, R.R.; Santos, F.A.; Sousa, E.H.S.; et al. Nitro-Imidazole-Based Ruthenium Complexes with Antioxidant and Anti-Inflammatory Activities. J. Inorg. Biochem. 2020, 206, 111048. [Google Scholar] [CrossRef]
- İnan, A.; İkiz, M.; Tayhan, S.E.; Bilgin, S.; Genç, N.; Sayın, K.; Ceyhan, G.; Köse, M.; Dağ, A.; İspir, E. Antiproliferative, Antioxidant, Computational and Electrochemical Studies of New Azo-Containing Schiff Base Ruthenium(II) Complexes. New J. Chem. 2018, 42, 2952–2963. [Google Scholar] [CrossRef]
- Sun, W.; Yu, B.; Kühn, F.E. Ruthenium(II)–Salen Complexes-Catalyzed Olefination of Aldehydes with Ethyl Diazoacetate. Tetrahedron Lett. 2006, 47, 1993–1996. [Google Scholar] [CrossRef]
- Gill, C.S.; Venkatasubbaiah, K.; Jones, C.W. Recyclable Polymer- and Silica-Supported Ruthenium(II)-Salen Bis-Pyridine Catalysts for the Asymmetric Cyclopropanation of Olefins. Adv. Synth. Catal. 2009, 351, 1344–1354. [Google Scholar] [CrossRef]
- Singh, A.; Barman, P. Recent Advances in Schiff Base Ruthenium Metal Complexes: Synthesis and Applications. Top. Curr. Chem. (Z) 2021, 379, 29. [Google Scholar] [CrossRef] [PubMed]
- Yasbin, R.E.; Matthews, C.R.; Clarke, M.J. Mutagenic and Toxic Effects of Ruthenium. Chem.-Biol. Interact. 1980, 31, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Kapitza, S.; Jakupec, M.A.; Uhl, M.; Keppler, B.K.; Marian, B. The Heterocyclic Ruthenium(III) Complex KP1019 (FFC14A) Causes DNA Damage and Oxidative Stress in Colorectal Tumor Cells. Cancer Lett. 2005, 226, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Heffeter, P.; Riabtseva, A.; Senkiv, Y.; Kowol, C.R.; Körner, W.; Jungwith, U.; Mitina, N.; Keppler, B.K.; Konstantinova, T.; Yanchuk, I.; et al. Nanoformulation Improves Activity of the (Pre)Clinical Anticancer Ruthenium Complex KP1019. J. Biomed. Nanotechnol. 2014, 10, 877–884. [Google Scholar] [CrossRef]
- Coluccia, M.; Sava, G.; Loseto, F.; Nassi, A.; Boccarelli, A.; Giordano, D.; Alessio, E.; Mestroni, G. Anti-Leukaemic Action of RuCl2(DMSO)4 Isomers and Prevention of Brain Involvement on P388 Leukaemia and on P388DDP Subline. Eur. J. Cancer 1993, 29, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, R.A.; Wani, M.Y.; Shreaz, S.; Hashmi, A.A. Synthesis, Characterization and Biological Screening of Some Schiff Base Macrocyclic Ligand Based Transition Metal Complexes as Antifungal Agents. Arab. J. Chem. 2016, 9, S743–S751. [Google Scholar] [CrossRef]
- Murray, K.S.; Van den Bergen, A.M.; West, B.O. Ruthenium Complexes with a Tetradentate Salicylaldimine Schiff Base. Aust. J. Chem. 1978, 31, 203–207. [Google Scholar] [CrossRef]
- Sampath, K.; Jayabalakrishnan, C. Ruthenium(II) Tetradentate Schiff Base Complexes: Synthesis, Characterization, DNA Binding, and Antioxidant Studies. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2015, 45, 1145–1153. [Google Scholar] [CrossRef]
- Buldurun, K.; Turan, N.; Aras, A.; Mantarcı, A.; Turkan, F.; Bursal, E. Spectroscopic and Structural Characterization, Enzyme Inhibitions, and Antioxidant Effects of New Ru(II) and Ni(II) Complexes of Schiff Base. Chem. Biodivers. 2019, 16, e1900243. [Google Scholar] [CrossRef]
- Ramadan, R.M.; Abu Al-Nasr, A.K.; Ali, O.A.M. Synthesis, Spectroscopic, DFT Studies and Biological Activity of Some Ruthenium Carbonyl Derivatives of Bis-(Salicylaldehyde)Phenylenediimine Schiff Base Ligand. J. Mol. Struct. 2018, 1161, 100–107. [Google Scholar] [CrossRef]
- Devagi, G.; Dallemer, F.; Kalaivani, P.; Prabhakaran, R. Organometallic Ruthenium(II) Complexes Containing NS Donor Schiff Bases: Synthesis, Structure, Electrochemistry, DNA/BSA Binding, DNA Cleavage, Radical Scavenging and Antibacterial Activities. J. Organomet. Chem. 2018, 854, 1–14. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Roy, S.; Chakraborty, J.; Chattopadhyay, S. Two New Hetero-Dinuclear Nickel(II)/Zinc(II) Complexes with Compartmental Schiff Bases: Synthesis, Characterization and Self Assembly. Polyhedron 2016, 112, 109–117. [Google Scholar] [CrossRef]
- El Deeb, S.; Ma, B.N.; Baecker, D.; Gust, R. Studies on the Stability of the Anticancer-Active [N,N′-Bis(Salicylidene)-1,2-Phenylenediamine]Chloridoiron(III) Complex under Pharmacological-like Conditions. Inorganica Chim. Acta 2019, 487, 76–80. [Google Scholar] [CrossRef]
- Martin, C.S.; Gouveia-Caridade, C.; Crespilho, F.N.; Constantino, C.J.L.; Brett, C.M.A. Nickel-N,N’–Bis(Salicylidene)-1,3-Propanediamine (Ni-Salpn) Film-Modified Electrodes. Influence of Electrodeposition Conditions and of Electrode Material on Electrochemical Behaviour in Aqueous Solution. Electrochim. Acta 2015, 178, 80–91. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Dieng, S.I.M.; Fall, A.D.; Diatta-Badji, K.; Sarr, A.; Sene, M.; Sene, M.; Mbaye, A.; Diatta, W.; Bassene, E. Evaluation de l’activité Antioxydante Des Extraits Hydro-Ethanoliques Des Feuilles et Écorces de Piliostigma Thonningii Schumach. Int. J. Biol. Chem. Sci. 2017, 11, 768–776. [Google Scholar] [CrossRef]
- Alonso-Calleja, C.; Martínez-Fernández, B.; Prieto, M.; Capita, R. Microbiological Quality of Vacuum-Packed Retail Ostrich Meat in Spain. Food Microbiol. 2004, 21, 241–246. [Google Scholar] [CrossRef]
- Benali, T.; Bouyahya, A.; Habbadi, K.; Zengin, G.; Khabbach, A.; Achbani, E.H.; Hammani, K. Chemical Composition and Antibacterial Activity of the Essential Oil and Extracts of Cistus Ladaniferus Subsp. Ladanifer and Mentha Suaveolens against Phytopathogenic Bacteria and Their Ecofriendly Management of Phytopathogenic Bacteria. Biocatal. Agric. Biotechnol. 2020, 28, 101696. [Google Scholar] [CrossRef]
- Benali, T.; Habbadi, K.; Khabbach, A.; Marmouzi, I.; Zengin, G.; Bouyahya, A.; Chamkhi, I.; Chtibi, H.; Aanniz, T.; Achbani, E.H.; et al. GC–MS Analysis, Antioxidant and Antimicrobial Activities of Achillea Odorata subsp. Pectinata and Ruta Montana Essential Oils and Their Potential Use as Food Preservatives. Foods 2020, 9, 668. [Google Scholar] [CrossRef]




| Complexes | [RuL1Cl2] | [RuH2L2Cl2] | [RuH2L3Cl2] |
|---|---|---|---|
| Formula | C16H14Cl2RuN2O2 | C17H18Cl2RuN2O2 | C19H22Cl2RuN2O2 |
| MW | 437.95 | 453.97 | 482.01 |
| System | Monoclinic | Monoclinic | Monoclinic |
| Space Group | C 1 2/c 1 | P 1 21/c 1 | P 1 21 1 |
| T(K) | 296 | 296 | 296 |
| λ (Å) | 1.54060 | 1.54060 | 1.54060 |
| a/ | 13.4890 | 16.0430 | 7.1520 |
| b/ | 9.6910 | 12.9140 | 10.7120 |
| c/ | 16.6850 | 16.5030 | 9.9590 |
| α/o | 90.0000 | 90.0000 | 90.0000 |
| β/o | 112.3650 | 114.7750 | 91.2000 |
| γ/o | 90.0000 | 90.0000 | 90.0000 |
| V (Å3) | 762.81 | 3104.39 | 762.81 |
| Density (g/cm3) | 1.71 | 1.35 | 1.89 |
| Radiation | CuKα | CuKα | CuKα |
| Scan range 2θ (°) | 10–80 | 10–80 | 10–80 |
| Compounds | H2L1 | H2L2 | H2L3 | [RuL1Cl2] | [RuH2L2Cl2] | [RuH2L3Cl2] | Ascorbic Acid |
|---|---|---|---|---|---|---|---|
| IC50 ± SD (μg/mL) | - | 0.99 ± 0.01 | - | 0.09 ± 0.03 | 0.15 ± 0.03 | 0.04 ± 0.01 | 0.14 ± 0.01 |
| [RuL1Cl2] | [RuH2L2Cl2] | [RuH2L3Cl2] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DIZ * | MIC+ | MBC− | DIZ * | MIC | MBC | DIZ * | MIC | MBC | |
| S. aureus | na | nt | nt | na | nt | nt | na | nt | nt |
| B. subtilis | na | nt | nt | 21 ± 1.41 | 0.97 | 0.97 | 17 ± 1.41 | 0.39 | 0.39 |
| P. aeruginosa | na | nt | nt | na | nt | nt | na | nt | nt |
| P. mirabilis | na | nt | nt | 25.5 ± 0.70 | 0.97 | 0.97 | 16.50 ± 0.70 | 0.78 | 0.78 |
| E. coli | na | nt | nt | na | nt | nt | na | nt | nt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Es-Sounni, B.; Harboul, K.; Mouhib, A.; Alanazi, A.S.; Hefnawy, M.; Bakhouch, M.; Benali, T.; Hammani, K.; Mazoir, N.; El Yazidi, M.; et al. Ruthenium(II) Complex-Based Tetradentate Schiff Bases: Synthesis, Spectroscopic, Antioxidant, and Antibacterial Investigations. Int. J. Mol. Sci. 2024, 25, 7879. https://doi.org/10.3390/ijms25147879
Es-Sounni B, Harboul K, Mouhib A, Alanazi AS, Hefnawy M, Bakhouch M, Benali T, Hammani K, Mazoir N, El Yazidi M, et al. Ruthenium(II) Complex-Based Tetradentate Schiff Bases: Synthesis, Spectroscopic, Antioxidant, and Antibacterial Investigations. International Journal of Molecular Sciences. 2024; 25(14):7879. https://doi.org/10.3390/ijms25147879
Chicago/Turabian StyleEs-Sounni, Bouchra, Kaoutar Harboul, Ayoub Mouhib, Ashwag S. Alanazi, Mohamed Hefnawy, Mohamed Bakhouch, Taoufiq Benali, Khalil Hammani, Noureddine Mazoir, Mohamed El Yazidi, and et al. 2024. "Ruthenium(II) Complex-Based Tetradentate Schiff Bases: Synthesis, Spectroscopic, Antioxidant, and Antibacterial Investigations" International Journal of Molecular Sciences 25, no. 14: 7879. https://doi.org/10.3390/ijms25147879





