Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing
Abstract
1. Introduction
2. The Impact of PRP in PR-PRP
2.1. Rationale for PRP Therapies
2.2. PRP Classification
2.3. Platelet Structures and Biological Content
2.3.1. α-Granules
Platelet-Derived Exosomes
2.3.2. Dense Granules
2.3.3. Lysosomes
2.4. Variability in Leukocyte Presence in PRP and PR-PRP
2.5. Tissue Repair and Regeneration Provoked by PRP
2.5.1. Anti-Inflammatory Effects
2.5.2. Immunomodulation
2.5.3. Angiogenesis
2.5.4. Analgesic Effects
3. The Contribution of PPP in PR-PRP
3.1. Platelet-Poor Plasma Composition
3.1.1. Plasma-Based Growth Factors
3.1.2. Human Albumin
3.1.3. Alpha-2-Macroglobulin
3.1.4. Fibrinogen
4. PR-PRP Characteristics
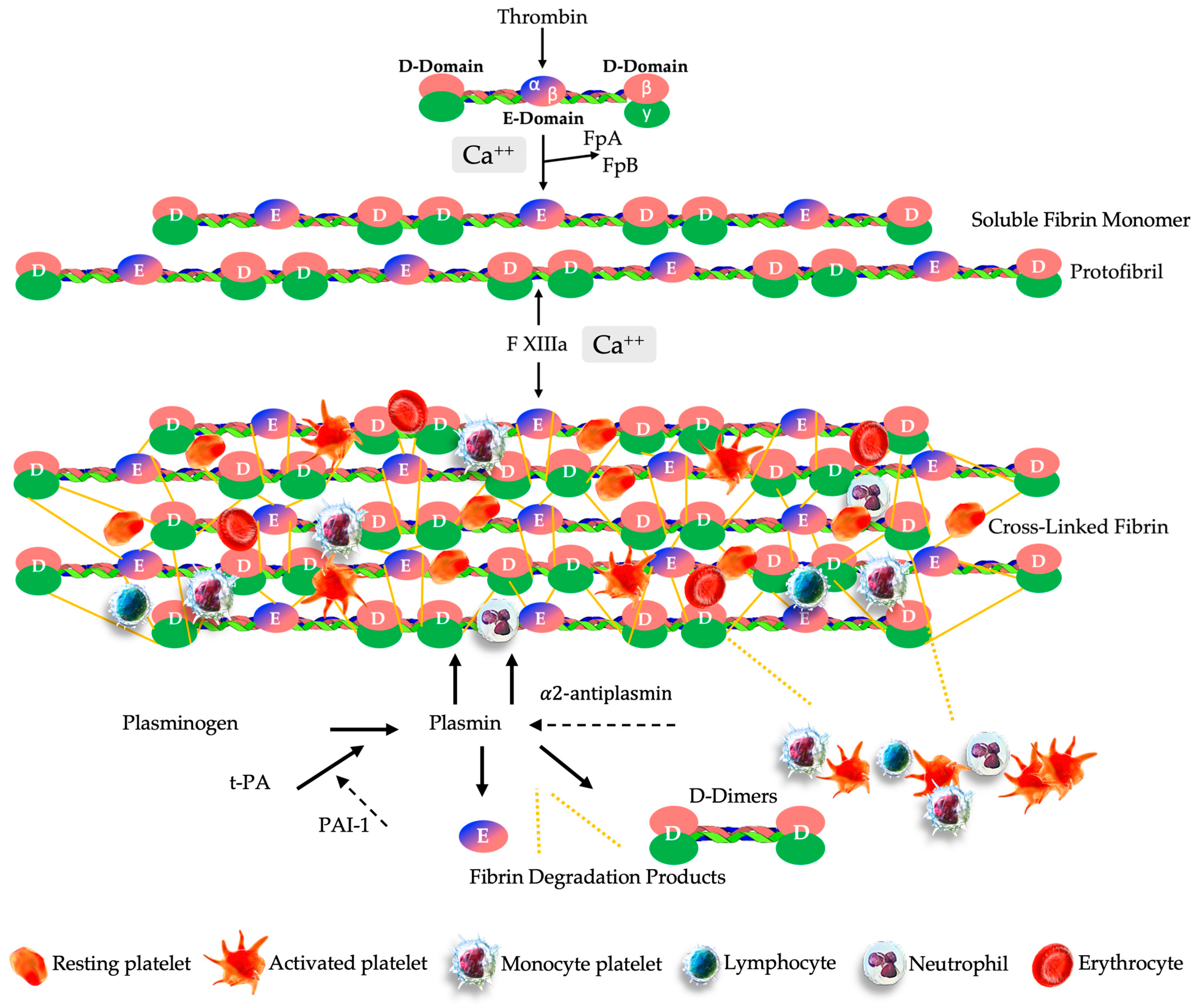
4.1. PR-PRP Matrix Formation
4.2. PR-PRP Matrix Fibrinolysis
4.3. Sustained Cellular Matrix Release
5. PR-PRP Matrix Biological Properties
5.1. Fibrin, Fibrinogen, and Macrophage Responses
5.2. Fibrin to Support in Immune Responses and Inflammation
PR-PRP’s Clinical Application Potential
5.3. Endothelial Cell Interactions with Fibrin Matrix
5.4. Role of Fibrin Matrix in Angiogenesis
5.5. Antimicrobial Activities of Fibrin(Ogen)
5.6. Matrix Similarities and Differences
6. Limitations and Future Directions
7. Conclusions
Funding
Conflicts of Interest
References
- Vallet-Regí, M. Evolution of Biomaterials. Front. Mater 2022, 9, 864016. [Google Scholar] [CrossRef]
- Todros, S.; Todesco, M.; Bagno, A. Biomaterials and Their Biomedical Applications: From Replacement to Regeneration. Processes 2021, 9, 1949. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Bioceramics: From bone substitutes to nanoparticles for drug delivery. Pure Appl. Chem. 2019, 91, 687–706. [Google Scholar] [CrossRef] [PubMed]
- Van Belleghem, S.M.; Mahadik, B.; Snodderly, K.L.; Fisher, J.P. Overview of tissue engineering concepts and applications. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1289–1316. [Google Scholar]
- Van Blitterswijk, C.; De Boer, J. Tissue Engineering; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. New biotechnologies for musculoskeletal injuries. Surgeon 2019, 17, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Su, C.A.; Jildeh, T.R.; Vopat, M.L.; Waltz, R.A.; Millett, P.J.; Provencher, M.T.; Philippon, M.J.; Huard, J. Current state of platelet-rich plasma and cell-based therapies for the treatment of osteoarthritis and tendon and ligament injuries. J. Bone Jt. Surg. 2022, 104, 1406–1414. [Google Scholar] [CrossRef]
- Kingsley, C.S. Blood Coagulation: Evidence of an antagonist to factor VI in platelet-rich human plasma. Nature 1954, 173, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Parayaruthottam, P.; Roshan, G.; Menon, V.; Fidha, M.; Fernandes, A.K. Platelets and their pathways in dentistry: Systematic review. J. Int. Soc. Prev. Community Dent. 2017, 7 (Suppl. 2), S55–S60. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.R.; Egbert, P.R.; Harbury, C.; Hopkins, J.L.; Rubenstein, E. Use of platelet-fibrinogen-thrombin mixture to seal experimental penetrating corneal wounds. Albrecht Von Graefes Arch. Für Klin. Und Exp. Ophthalmol. 1978, 207, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Knighton, D.R.; Ciresi, K.F.; Fiegel, V.D.; Austin, L.L.; Butler, E.L. Classification and treatment of chronic nonhealing wounds. Ann. Surg. 1986, 204, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Whitman, D.H.; Berry, R.L.; Green, D.M. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 1997, 55, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-Rich Plasma (PRP): What Is PRP and What Is Not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, M.; Vatanmakanian, M.; Arami, M.K.; Shirazi, F.S.; Esmaeili, N.; Hydarporian, S.; Jafari, S. A comparative study between platelet-rich plasma and platelet-poor plasma effects on angiogenesis. Med Mol. Morphol. 2018, 51, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; A Fortier, L.; Giuffrida, A.; Jo, C.H.; et al. Platelet-rich plasma for the treatment of knee osteoarthritis: An expert opinion and proposal for a novel classification and coding system. Expert Opin. Biol. Ther. 2020, 20, 1447–1460. [Google Scholar] [CrossRef]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; LaPrade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. J. Bone Jt. Surg. 2017, 99, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.R.; Robinson, P.G.; West, C.C.; Goudie, E.B.; Yong, L.Y.; White, T.O.; LaPrade, R.F. Reporting Standards in Clinical Studies Evaluating Bone Marrow Aspirate Concentrate: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Mishra, A.; Borzini, P.; Inchingolo, F.; Sammartino, G.; Rasmusson, L.; Everts, P.A. In Search of a Consensus Terminology in the Field of Platelet Concentrates for Surgical Use: Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), Fibrin Gel Polymerization and Leukocytes. Curr. Pharm. Biotechnol. 2012, 13, 1131–1137. [Google Scholar] [CrossRef]
- Rodeo, S.A.; Delos, D.; Williams, R.J.; Adler, R.S.; Pearle, A.; Warren, R.F. The Effect of Platelet-Rich Fibrin Matrix on Rotator Cuff Tendon Healing: A Prospective, Randomized Clinical Study. Am. J. Sports Med. 2012, 40, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Castricini, R.; De Benedetto, M.; Panfoli, N.; Pirani, P.; Zini, R.; Maffulli, N.; Denaro, V. Paper # 117: Platelet-Rich Fibrin Matrix Augmentation for Arthroscopic Rotator Cuff Repair: A Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, e145–e146. [Google Scholar] [CrossRef]
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Duong, V.; Eyles, J.; Kasza, J.; Wang, Y.; Cicuttini, F.; Buchbinder, R.; Forbes, A.; et al. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients with Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA 2021, 326, 2021. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.; Oneisi, A.; Ghieh, F. Platelet-Rich Plasma Facial Rejuvenation: Myth or Reality? Aesthetic Plast. Surg. 2021, 45, 2928–2938. [Google Scholar] [CrossRef] [PubMed]
- Beitia, M.; Delgado, D.; Mercader, J.; Sánchez, P.; López De Dicastillo, L.; Sánchez, M. Action of Platelet-Rich Plasma on In Vitro Cellular Bioactivity: More than Platelets. Int. J. Mol. Sci. 2023, 24, 5367. [Google Scholar] [CrossRef] [PubMed]
- Acebes-Huerta, A.; Arias-Fernández, T.; Bernardo, Á.; Muñoz-Turrillas, M.C.; Fernández-Fuertes, J.; Seghatchian, J.; Gutiérrez, L. Platelet-derived bio-products: Classification update, applications, concerns and new perspectives. Transfus. Apher. Sci. 2020, 59, 102716. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.M.; Beitia, M.; Delgado, D.; Sánchez, P.; Guadilla, J.; de Arrilucea, C.P.; Benito-Lopez, F.; Basabe-Desmonts, L.; Sánchez, M. Method Based on Ultrafiltration to Obtain a Plasma Rich in Platelet and Plasma Growth Factors. J. Clin. Med. 2023, 12, 5941. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.; Garate, A.; Sánchez, P.; Bilbao, A.M.; Garcia Del Cano, G.; Salles, J.; Sánchez, M. Biological and structural effects after intraosseous infiltrations of age-dependent platelet-rich plasma: An in vivo study. J. Orthop. Res. 2020, 38, 1931–1941. [Google Scholar] [CrossRef]
- Muir, S.M.; Reisbig, N.; Baria, M.; Kaeding, C.; Bertone, A.L. The Concentration of Plasma Provides Additional Bioactive Proteins in Platelet and Autologous Protein Solutions. Am. J. Sports Med. 2019, 47, 1955–1963. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L.; Yang, W.; Cao, Y.; Shi, Y.; Li, X.; Zhang, Q. The effects of different doses of IGF-1 on cartilage and subchondral bone during the repair of full-thickness articular cartilage defects in rabbits. Osteoarthr. Cartil. 2017, 25, 309–320. [Google Scholar] [CrossRef]
- Longobardi, L.; O’Rear, L.; Aakula, S.; Johnstone, B.; Shimer, K.; Chytil, A.; A Horton, W.; Moses, H.L.; Spagnoli, A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-β signaling. J. Bone Miner. Res. 2006, 21, 626–636. [Google Scholar] [CrossRef]
- Yamada, S.; Behfar, A.; Terzic, A. Regenerative medicine clinical readiness. Regen. Med. 2021, 16, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.M.; Devilee, R.J.J.; Oosterbos, C.J.M.; Mahoney, C.B.; Schattenkerk, M.E.; Knape, J.T.A.; van Zundert, A. Autologous platelet gel and fibrin sealant enhance the efficacy of total knee arthroplasty: Improved range of motion, decreased length of stay and a reduced incidence of arthrofibrosis. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Devilee, R.J.J.; Brown Mahoney, C.; Van Erp, A.; Oosterbos, C.J.M.; Stellenboom, M.; Knape, J.T.A.; Van Zundert, A. Exogenous Application of Platelet-Leukocyte Gel during Open Subacromial Decompression Contributes to Improved Patient Outcome. Eur. Surg. Res. 2008, 40, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Mazzola, T.; Mautner, K.; Randelli, P.S.; Podesta, L. Modifying Orthobiological PRP Therapies Are Imperative for the Advancement of Treatment Outcomes in Musculoskeletal Pathologies. Biomedicines 2022, 10, 2933. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S. Systematic Review—The Potential Implications of Different Platelet-Rich Plasma (PRP) Concentrations in Regenerative Medicine for Tissue Repair. Int. J. Mol. Sci. 2020, 21, 5702. [Google Scholar] [CrossRef] [PubMed]
- Miroshnychenko, O.; Chalkley, R.J.; Leib, R.D.; Everts, P.A.; Dragoo, J.L. Proteomic analysis of platelet-rich and platelet-poor plasma. Regen. Ther. 2020, 15, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Piao, Y.; Liu, Q.; Yang, X. Platelet-rich plasma-derived extracellular vesicles: A superior alternative in regenerative medicine? Cell Prolif. 2021, 54, e13123. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Lana, J.F.; Onishi, K.; Buford, D.; Peng, J.; Mahmood, A.; Fonseca, L.F.; van Zundert, A.; Podesta, L. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. Biomedicines 2023, 11, 1922. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. A contemporary view of platelet-rich plasma therapies: Moving toward refined clinical protocols and precise indications. Regen. Med. 2018, 13, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Kunicki, T.J. Platelet membrane glycoproteins and their function: An overview. Blut 1989, 59, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, N.P. Types of Glycoprotein Receptors and Signal Transduction Pathways. J. Cell Signal. 2023, 8, 1000340. [Google Scholar]
- Polasek, J. Platelet secretory granules or secretory lysosomes? Platelets 2005, 16, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Sharda, A.; Flaumenhaft, R. The life cycle of platelet granules. F1000Research 2018, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Gleissner, C.A.; Von Hundelshausen, P.; Ley, K. Platelet chemokines in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Maynard, D.M.; Heijnen, H.F.G.; Horne, M.K.; White, J.G.; Gahl, W.A. Proteomic analysis of platelet α-granules using mass spectrometry. J. Thromb. Haemost. 2007, 5, 1945–1955. [Google Scholar] [CrossRef]
- Guo, S.C.; Tao, S.C.; Yin, W.J.; Qi, X.; Yuan, T.; Zhang, C.Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Rui, S.; Yuan, Y.; Du, C.; Song, P.; Chen, Y.; Wang, H.; Fan, Y.; Armstrong, D.G.; Deng, W.; Li, L. Comparison and Investigation of Exosomes Derived from Platelet-Rich Plasma Activated by Different Agonists. Cell Transplant. 2021, 30, 096368972110178. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Bajracharya, S.D.; Yuen, P.S.T.; Zhou, H.; Star, R.A.; Illei, G.G.; Alevizos, I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int. Orthop. 2018, 42, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.; Kolhe, R.; Hunter, M.; Isales, C.; Hamrick, M.; Fulzele, S. Stem cell-derived exosomes: A potential alternative therapeutic agent in orthopaedics. Stem Cells Int. 2016, 2016, 5802529. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Dong, P.Y.; Yang, G.M.; Gurunathan, S. A comprehensive review on the composition, biogenesis, purification, and multifunctional role of exosome as delivery vehicles for cancer therapy. Biomed. Pharmacother. 2023, 165, 115087. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Gopal, S.K.; Xu, R.; Simpson, R.J.; Chen, W. Exosomes and Their Roles in Immune Regulation and Cancer; Elsevier: Amsterdam, The Netherlands, 2015; pp. 72–81. [Google Scholar]
- Beit-Yannai, E.; Tabak, S.; Stamer, W.D. Physical exosome: Exosome interactions. J. Cell. Mol. Med. 2018, 22, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Huber, H.J.; Holvoet, P. Exosomes: Emerging roles in communication between blood cells and vascular tissues during atherosclerosis. Curr. Opin. Lipidol. 2015, 26, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, H.F.G.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived from Exocytosis of Multivesicular Bodies and α-Granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef] [PubMed]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef]
- Torreggiani, E.; Perut, F.; Roncuzzi, L.; Zini, N.; Baglìo, S.R.; Baldini, N. Exosomes: Novel effectors of human platelet lysate activity. Eur. Cells Mater. 2014, 28, 137–151. [Google Scholar] [CrossRef]
- Burgess, D.J. Vesicle vehicles of genetic information. Nat. Rev. Genet. 2014, 15, 514. [Google Scholar] [CrossRef]
- Mistry, D.S.; Chen, Y.; Sen, G.L. Progenitor function in self-renewing human epidermis is maintained by the exosome. Cell Stem Cell 2012, 11, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Kazlauskas, A. Axl is essential for VEGF-A-dependent activation of PI3K/Akt. EMBO J. 2012, 31, 1692–1703. [Google Scholar] [CrossRef]
- Iberg, C.A.; Hawiger, D. Natural and Induced Tolerogenic Dendritic Cells. J. Immunol. 2020, 204, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Ganor, Y.; Besser, M.; Ben-Zakay, N.; Unger, T.; Levite, M. Human T cells express a functional ionotropic glutamate receptor GluR3, and glutamate by itself triggers integrin-mediated adhesion to laminin and fibronectin and chemotactic migration. J. Immunol. 2003, 170, 4362–4372. [Google Scholar] [CrossRef]
- Łukasik, Z.M.; Makowski, M.; Makowska, J.S. From blood coagulation to innate and adaptive immunity: The role of platelets in the physiology and pathology of autoimmune disorders. Rheumatol. Int. 2018, 38, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Cloez-Tayarani, I. Differential effect of serotonin on cytokine production in lipopolysaccharide-stimulated human peripheral blood mononuclear cells: Involvement of 5-hydroxytryptamine2A receptors. Int. Immunol. 2003, 15, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Velasco-Velázquez, M.A.; Garcés-Alvarez, M.E.; Hurtado-Alvarado, G.; Quintero-Fabian, S.; Pavón, L. Immunomodulatory Effects Mediated by Serotonin. J. Immunol. Res. 2015, 2015, 354957. [Google Scholar] [CrossRef]
- Ciferri, S.; Emiliani, C.; Guglielmini, G.; Orlacchio, A.; Nenci, G.G.; Gresele, P. Platelets release their lysosomal content in vivo in humans upon activation. Thromb. Haemost. 2000, 83, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M.; Hausman, R. A molecular approach. In The Cell, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Heijnen, H.; van der Sluijs, P. Platelet secretory behaviour: As diverse as the granules … or not? J. Thromb. Haemost. 2015, 13, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Wunderli, S.L.; Blache, U.; Piccoli, A.B.; Niederöst, B.; Holenstein, C.N.; Passini, F.S.; Silván, U.; Bundgaard, L.; Keller, U.A.D.; Snedeker, J.G. Tendon response to matrix unloading is determined by the patho-physiological niche. Matrix Biol. 2020, 89, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: Granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.S.D.; Purita, J.; Paulus, C.; Huber, S.C.; Rodrigues, B.L.; Rodrigues, A.A.; Santana, M.H.; Madureira, J.L.; Luzo, C.M.; Belangero, W.D.; et al. Contributions for classification of platelet rich plasma—proposal of a new classification: MARSPILL. Regen. Med. 2017, 12, 565–574. [Google Scholar] [CrossRef]
- Everts, P.A.; Flanagan, G.; Podesta, L. Autologous Orthobiologics. In Clinical Guide to Musculoskeletal Medicine: A Multidisciplinary Approach; Mostoufi, S.A., George, T.K., Tria, A.J., Jr., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 651–679. [Google Scholar] [CrossRef]
- Bielecki, T.; Dohan Ehrenfest, D.A.M.; Everts, P.; Wiczkowski, A. The Role of Leukocytes from L-PRP/L-PRF in Wound Healing and Immune Defense: New Perspectives. Curr. Pharm. Biotechnol. 2012, 13, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; Huber, S.C.; Purita, J.; Tambeli, C.H.; Santos, G.S.; Paulus, C.; Annichino-Bizzacchi, J.M. Leukocyte-rich PRP versus leukocyte-poor PRP—The role of monocyte/macrophage function in the healing cascade. Clin. Orthop. Trauma 2019, 10, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Dejnek, M.; Moreira, H.; Płaczkowska, S.; Barg, E.; Reichert, P.; Królikowska, A. Leukocyte-Rich Platelet-Rich Plasma as an Effective Source of Molecules That Modulate Local Immune and Inflammatory Cell Responses. Oxidative Med. Cell. Longev. 2022, 2022, 8059622. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Jiang, L.; Zhang, C.; Liu, M.; Luo, Y.; Hu, Z.; Mou, X.; Zhu, Y. Biologic Mechanisms of Macrophage Phenotypes Responding to Infection and the Novel Therapies to Moderate Inflammation. Int. J. Mol. Sci. 2023, 24, 8358. [Google Scholar] [CrossRef] [PubMed]
- Margraf, A.; Zarbock, A. Platelets in Inflammation and Resolution. J. Immunol. 2019, 203, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Kapur, R.; Zufferey, A.; Boilard, E.; Semple, J.W. Nouvelle Cuisine: Platelets Served with Inflammation. J. Immunol. 2015, 194, 5579–5587. [Google Scholar] [CrossRef]
- Scheuerer, B.; Ernst, M.; Dürrbaum-Landmann, I.; Fleischer, J.; Grage-Griebenow, E.; Brandt, E.; Flad, H.-D.; Petersen, F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 2000, 95, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Muiños-López, E.; Delgado, D.; Sánchez, P.; Paiva, B.; Anitua, E.; Fiz, N.; Aizpurua, B.; Guadilla, J.; Padilla, S.; Granero-Moltó, F.; et al. Modulation of Synovial Fluid-Derived Mesenchymal Stem Cells by Intra-Articular and Intraosseous Platelet Rich Plasma Administration. Stem Cells Int. 2016, 2016, 1247950. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.; Clemetson, J.; Proudfoot, A.; Power, C.; Baggiolini, M.; Wells, T. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood 2000, 96, 4046–4054. [Google Scholar] [CrossRef] [PubMed]
- Woodell-May, J.E.; Sommerfeld, S.D. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. 2020, 38, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Dib, P.R.B.; Quirino-Teixeira, A.C.; Merij, L.B.; Pinheiro, M.B.M.; Rozini, S.V.; Andrade, F.B.; Hottz, E.D. Innate immune receptors in platelets and platelet-leukocyte interactions. J. Leucoc. Biol. 2020, 108, 1157–1182. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Li, Y.; Lang, S.; Yougbare, I.; Zhu, G.; Chen, P.; Ni, H. Crosstalk between Platelets and the Immune System: Old Systems with New Discoveries. Adv. Hematol. 2012, 2012, 384685. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, F.; Cattani, C.; Dimartino, V.; Mirisola, C.; Cavani, A. Platelet Derivatives and the Immunomodulation of Wound Healing. Int. J. Mol. Sci. 2022, 23, 8370. [Google Scholar] [CrossRef]
- Rayes, J.; Bourne, J.H.; Brill, A.; Watson, S.P. The dual role of platelet-innate immune cell interactions in thrombo-inflammation. Res. Pract. Thromb. Haemost. 2020, 4, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, Y.; Sun, R.; Hu, H.; Liu, Y.; Herrmann, M.; Zhao, Y.; Muñoz, L.E. Receptor-mediated NETosis on neutrophils. Front. Immunol. 2021, 12, 775267. [Google Scholar] [CrossRef]
- Patel, A.A.; Ginhoux, F.; Yona, S. Monocytes, macrophages, dendritic cells and neutrophils: An update on lifespan kinetics in health and disease. Immunology 2021, 163, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.S.; Migliari Branco, L.; Franklin, B.S. Regulation of innate immune responses by platelets. Front. Immunol. 2019, 10, 460217. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Crivellato, E. “Sprouting angiogenesis”, a reappraisal. Dev. Biol. 2012, 372, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Brill, A. Differential role of platelet granular mediators in angiogenesis. Cardiovasc. Res. 2004, 63, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Bir, S.C.; Esaki, J.; Marui, A.; Sakaguchi, H.; Kevil, C.G.; Ikeda, T.; Komeda, M.; Tabata, Y.; Sakata, R. Therapeutic Treatment with Sustained-Release Platelet-Rich Plasma Restores Blood Perfusion by Augmenting Ischemia-Induced Angiogenesis and Arteriogenesis in Diabetic Mice. J. Vasc. Res. 2011, 48, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Rughetti, A.; D’Ascenzo, S.; Millimaggi, D.; Pavan, A.; Dell’Orso, L.; Dolo, V. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion 2009, 49, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Łanocha-Arendarczyk, N.; Baranowska-Bosiacka, I.; Gutowska, I.; Kolasa-Wołosiuk, A.; Kot, K.; Łanocha, A.; Metryka, E.; Wiszniewska, B.; Chlubek, D.; Kosik-Bogacka, D. The activity of matrix metalloproteinases (MMP-2, MMP-9) and their tissue inhibitors (TIMP-1, TIMP-3) in the cerebral cortex and hippocampus in experimental acanthamoebiasis. Int. J. Mol. Sci. 2018, 19, 4128. [Google Scholar] [CrossRef] [PubMed]
- Jaipersad, A.S.; Lip, G.Y.H.; Silverman, S.; Shantsila, E. The Role of Monocytes in Angiogenesis and Atherosclerosis. J. Am. Coll. Cardiol. 2014, 63, A22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, Y.; Li, Y.; Lin, C.; Wang, S.; Wang, J.; Ma, C.; Wu, S. Comparison of Leukocyte-Rich and Leukocyte-Poor Platelet-Rich Plasma on Pressure Ulcer in a Rat Model. J. Burn. Care Res. 2023, 44, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Nasiri, S.; Mohammadi, M.H.; Mohammadi, A.M.; Nikbakht, M.; Panah, M.Z.; Safar, H.; Mostafaei, S.; Norooznezhad, A.H.; Soroosh, A.R.; et al. Evaluation of platelet-rich plasma gel potential in acceleration of wound healing duration in patients underwent pilonidal sinus surgery: A randomized controlled parallel clinical trial. Transfus. Apher. Sci. 2017, 56, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Bansal, H.; Leon, J.; Pont, J.L.; Wilson, D.A.; ST, S.M.; Bansal, A.; Preoteasa, I. Platelet Rich Plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci. Rep. 2021, 11, 397. [Google Scholar] [CrossRef]
- Jain, D.; Goyal, T.; Verma, N.; Paswan, A.K.; Dubey, R.K. Intradiscal Platelet-Rich Plasma Injection for Discogenic Low Back Pain and Correlation with Platelet Concentration: A Prospective Clinical Trial. Pain Med. 2020, 21, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Johal, H.; Khan, M.; Yung, S.-H.P.; Dhillon, M.S.; Fu, F.H.; Bedi, A.; Bhandari, M. Impact of Platelet-Rich Plasma Use on Pain in Orthopaedic Surgery: A Systematic Review and Meta-analysis. Sports Health 2019, 11, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Lutz, C.; Cheng, J.; Prysak, M.; Zukofsky, T.; Rothman, R.; Lutz, G. Clinical outcomes following intradiscal injections of higher-concentration platelet-rich plasma in patients with chronic lumbar discogenic pain. Int. Orthop. 2022, 46, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Funasaki, H.; Marumo, K. Efficacy of autologous leukocyte-reduced platelet-rich plasma therapy for patellar tendinopathy in a rat treadmill model. Muscle Ligaments Tendons J. 2019, 6, 205–215. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Sprott, H.; Franke, S.; Kluge, H.; Hein, G. Pain treatment of fibromyalgia by acupuncture. Rheumatol. Int. 1998, 18, 35–36. [Google Scholar] [CrossRef]
- Kuffler, D. Variables affecting the potential efficacy of PRP in providing chronic pain relief. J. Pain Res. 2018, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Leeman, M.; Choi, J.; Hansson, S.; Storm, M.U.; Nilsson, L. Proteins and antibodies in serum, plasma, and whole blood—Size characterization using asymmetrical flow field-flow fractionation (AF4). Anal. Bioanal. Chem. 2018, 410, 4867–4873. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Aso, H.; Watanabe, K.; Nara, H.; Rose, M.T.; Ohwada, S.; Yamaguchi, T. Sequence of IGF-I, IGF-II, and HGF expression in regenerating skeletal muscle. Histochem. Cell Biol. 2000, 122, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.L.; Anderson, N.G. The Human Plasma Proteome: History, Character, and Diagnostic Prospects. Mol. Cell. Proteom. 2003, 2, 50. [Google Scholar] [CrossRef]
- Gerszten, R.E.; Accurso, F.; Bernard, G.R.; Caprioli, R.M.; Klee, E.W.; Klee, G.G.; Kullo, I.; Laguna, T.A.; Roth, F.P.; Sabatine, M.; et al. Challenges in translating plasma proteomics from bench to bedside: Update from the NHLBI Clinical Proteomics Programs. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 295, L16–L22. [Google Scholar] [CrossRef] [PubMed]
- Monastero, R.N.; Pentyala, S. Cytokines as biomarkers and their respective clinical cutoff levels. Int. J. Inflamm. 2017, 2017, 4309485. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quintela, A.; Alende, R.; Gude, F.; Campos-Franco, J.; Rey, J.; Meijide, L.M.; Fernandez-Merino, C.; Vidal, C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 2008, 151, 42–50. [Google Scholar] [CrossRef]
- Perrin, A. Identification and Characterisation of Host-Pathogen Protein-Protein Interactions in the Blood Stages of Malaria. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2015. [Google Scholar]
- Mungunsukh, O.; McCart, E.; Day, R. Hepatocyte Growth Factor Isoforms in Tissue Repair, Cancer, and Fibrotic Remodeling. Biomedicines 2014, 2, 301–326. [Google Scholar] [CrossRef] [PubMed]
- Garoufalia, Z.; Papadopetraki, A.; Karatza, E.; Vardakostas, D.; Philippou, A.; Kouraklis, G.; Mantas, D. Insulin-like growth factor-I and wound healing, a potential answer to non-healing wounds: A systematic review of the literature and future perspectives. Biomed. Rep. 2021, 15, 1442. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Nakamura, T. Hepatocyte growth factor: Molecular structure, roles in liver regeneration, and other biological functions. Crit. Rev. Oncog. 1992, 3, 27–54. [Google Scholar] [PubMed]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B 2010, 86, 588–610. [Google Scholar] [CrossRef] [PubMed]
- Huh, C.G.; Factor, V.M.; Sánchez, A.; Uchida, K.; Conner, E.A.; Thorgeirsson, S.S. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc. Natl. Acad. Sci. USA 2004, 101, 4477–4482. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, M.; Xu, L.; Chang, Z.; Shu, X.; Zhou, L. Therapeutic role of human hepatocyte growth factor (HGF) in treating hair loss. PeerJ 2016, 4, e2624. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Lee, J.; Lee, J.; Lee, S.H.; Kim, S. Hepatocyte Growth Factor Regulates Macrophage Transition to the M2 Phenotype and Promotes Murine Skeletal Muscle Regeneration. Front. Physiol. 2019, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Goto, Y.; Sumida, H.; Noguchi, T.; Baba, T.; Miyazaki, S.; Nonogi, H. Angiotensin-converting enzyme inhibition restores hepatocyte growth factor production in patients with congestive heart failure. Hypertension 1999, 33, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Sisson, T.H.; Nguyen, M.H.; Yu, B.; Novak, M.L.; Simon, R.H.; Koh, T.J. Urokinase-type plasminogen activator increases hepatocyte growth factor activity required for skeletal muscle regeneration. Blood J. Am. Soc. Hematol. 2009, 114, 5052–5061. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Yang, S.; Ingle, G.; Zlot, C.; Rangell, L.; Kowalski, J.; Schwall, R.; Ferrara, N.; Gerritsen, M.E. Hepatocyte Growth Factor Enhances Vascular Endothelial Growth Factor-Induced Angiogenesis in Vitro and in Vivo. Am. J. Pathol. 2001, 158, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Zhang, T.; Shi, M.; Song, X.; Yang, S.; Liu, H.; Zhang, M.; Cui, Q.; Li, Z. Hepatocyte Growth Factor-Induced Tendon Stem Cell Conditioned Medium Promotes Healing of Injured Achilles Tendon. Front. Cell Dev. Biol. 2021, 9, 654084. [Google Scholar] [CrossRef] [PubMed]
- Bailes, J.; Soloviev, M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C. Insulin-like growth factor (IGF)-binding proteins: Interactions with IGFs and intrinsic bioactivities. Am. J. Physiol.-Endocrinol. Metab. 2000, 278, E967–E976. [Google Scholar] [CrossRef] [PubMed]
- Miescher, I.; Rieber, J.; Calcagni, M.; Buschmann, J. In Vitro and In Vivo Effects of IGF-1 Delivery Strategies on Tendon Healing: A Review. Int. J. Mol. Sci. 2023, 24, 2370. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Weinstein, D.; Bentov, I. Similarities and differences between insulin and IGF-I: Structures, receptors, and signalling pathways. Arch. Physiol. Biochem. 2008, 114, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Philippou, A.; Halapas, A.; Maridaki, M.; Koutsilieris, M. Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy. J. Musculoskelet Neuronal Interact. 2007, 7, 208–218. [Google Scholar] [PubMed]
- Wang, T.; Thien, C.; Wang, C.; Ni, M.; Gao, J.; Wang, A.; Jiang, Q.; Tuan, R.S.; Zheng, Q.; Zheng, M.H. 3D uniaxial mechanical stimulation induces tenogenic differentiation of tendon-derived stem cells through a PI3K/AKT signaling pathway. FASEB J. 2018, 32, 4804–4814. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.C.X.; Kihara, A.H.; Goulart, V.A.; Tonelli, F.M.; Gomes, K.N.; Ulrich, H.; Resende, R.R. Calcium signaling and cell proliferation. Cell. Signal. 2015, 27, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Disser, N.P.; Sugg, K.B.; Talarek, J.R.; Sarver, D.C.; Rourke, B.J.; Mendias, C.L. Insulin-like growth factor 1 signaling in tenocytes is required for adult tendon growth. FASEB J. 2019, 33, 12680. [Google Scholar] [CrossRef]
- Lavoie, H.; Therrien, M. Regulation of RAF protein kinases in ERK signalling. Nat. Rev. Mol. Cell Biol. 2015, 16, 281–298. [Google Scholar] [CrossRef]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, F.; Yeung, E.W.; Li, Y. IGF-IEc expression, regulation and biological function in different tissues. Growth Horm. IGF Res. 2010, 20, 275–281. [Google Scholar] [CrossRef]
- Luo, Q.; Wu, K.; Zhang, B.; Song, G. Mechano growth factor E peptide promotes rat bone marrow-derived mesenchymal stem cell migration through CXCR4-ERK1/2. Growth Factors 2015, 33, 210–219. [Google Scholar]
- Ashraf, S.; Cha, B.H.; Kim, J.S.; Ahn, J.; Han, I.; Park, H.; Lee, S.H. Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthr. Cartil. 2016, 24, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Asparuhova, M.B.; Riedwyl, D.; Aizawa, R.; Raabe, C.; Couso-Queiruga, E.; Chappuis, V. Local concentrations of TGF-β1 and IGF-1 appear determinant in regulating bone regeneration in human postextraction tooth sockets. Int. J. Mol. Sci. 2023, 24, 8239. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.Y.; Kim, H.T. Chondrocyte apoptosis induced by collagen degradation: Inhibition by caspase inhibitors and IGF-1. J. Orthop. Res. 2004, 22, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Rowan, A.; Cawston, T. Insulin-like growth factor 1 blocks collagen release and down regulates matrix metalloproteinase-1,-3,-8, and-13 mRNA expression in bovine nasal cartilage stimulated with oncostatin M in combination with interleukin 1α. Ann. Rheum. Dis. 2001, 60, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef] [PubMed]
- Nakao-Hayashi, J.; Ito, H.; Kanayasu, T.; Morita, I.; Murota, S.-I. Stimulatory effects of insulin and insulin-like growth factor I on migration and tube formation by vascular endothelial cells. Atherosclerosis 1992, 92, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.H.; Shen, W.; Perruzzi, C.; Soker, S.; Kinose, F.; Xu, X.; Robinson, G.; Driver, S.; Bischoff, J.; Zhang, B.; et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat. Med. 1999, 5, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Wasterlain, A.S.; Braun, H.J.; Harris, A.H.S.; Kim, H.J.; Dragoo, J.L. The Systemic Effects of Platelet-Rich Plasma Injection. Am. J. Sports Med. 2013, 41, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ionita, C.R.; Troillet, A.R.; Vahlenkamp, T.W.; Winter, K.; Brehm, W.; Ionita, J.C. Comparison of humoral insulin-like growth factor-1, platelet-derived growth factor-BB, transforming growth factor-β1, and interleukin-1 receptor antagonist concentrations among equine autologous blood-derived preparations. Am. J. Vet. Res. 2016, 77, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Gugjoo, M.B.; Amarpal; Abdelbaset-Ismail, A.; Aithal, H.P.; Kinjavdekar, P.; Pawde, A.M.; Kumar, G.S.; Sharma, G.T. Mesenchymal stem cells with IGF-1 and TGF-β1 in laminin gel for osteochondral defects in rabbits. Biomed. Pharmacother. 2017, 93, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Seifarth, C.; Csaki, C.; Shakibaei, M. Anabolic actions of IGF-I and TGF-B1 on Interleukin-1B-treated human articular chondrocytes: Evaluation in two and three dimensional cultures. Histol. Histopathol. 2009, 24, 1245–1262. [Google Scholar] [PubMed]
- Van de Wouw, J.; Joles, J.A. Albumin is an interface between blood plasma and cell membrane, and not just a sponge. Clin. Kidney J. 2022, 15, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Hui, J.H.P. The potential of stem cells in orthopaedic surgery. J. Bone Jt. Surg. 2006, 88, 11. [Google Scholar] [CrossRef] [PubMed]
- Horváthy, D.B.; Simon, M.; Schwarz, C.M.; Masteling, M.; Vácz, G.; Hornyák, I.; Lacza, Z. Serum albumin as a local therapeutic agent in cell therapy and tissue engineering. BioFactors 2017, 43, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Vaudaux, P.E.; Pittet, D.; Auckenthaler, R.; Lew, P.D.; Perdreau, F.S.; Peters, G.; Waldvogel, F.A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 1988, 158, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Sawada, N.; Yamaguchi, M. Expression of albumin in bone tissues and osteoblastic cells: Involvement of hormonal regulation. Int. J. Mol. Med. 2004, 14, 891–895. [Google Scholar] [CrossRef]
- Mijiritsky, E.; Assaf, H.D.; Peleg, O.; Shacham, M.; Cerroni, L.; Mangani, L. Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review. Biology 2021, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Itoh, Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Front. Immunol. 2021, 12, 803244. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wei, X.; Zhou, J.; Zhang, J.; Li, K.; Chen, Q.; Terek, R.; Fleming, B.C.; Goldring, M.B.; Ehrlich, M.G.; et al. Identification of α2 -Macroglobulin as a Master Inhibitor of Cartilage-Degrading Factors That Attenuates the Progression of Posttraumatic Osteoarthritis: α2 M Attentuates Posttraumatic OA Progression. Arthritis Rheumatol. 2014, 66, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.B.; Quigley, J.P. α2-macroglobulin: An evolutionarily conserved arm of the innate immune system. Dev. Comp. Immunol. 1999, 23, 375–390. [Google Scholar] [CrossRef]
- Marrero, A.; Duquerroy, S.; Trapani, S.; Goulas, T.; Guevara, T.; Andersen, G.R.; Navaza, J.; Sottrup-Jensen, L.; Gomis-Rüth, F.X. The crystal structure of human α2-macroglobulin reveals a unique molecular cage. Angew. Chem. Int. Ed. 2012, 51, 3340–3344. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.; Moestrup, S.K.; Gliemann, J.; Bendtsen, L.; Sand, O.; Sottrup-Jensen, L. Evidence that the newly cloned low-density-lipoprotein receptor related protein (LRP) is the α2-macroglobulin receptor. FEBS Lett. 1990, 276, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, G.S.; Barrett, A.J. Covalent binding of proteinases in their reaction with α2-macroglobulin. Biochem. J. 1980, 187, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Goulas, T.; Garcia-Ferrer, I.; Marrero, A.; Marino-Puertas, L.; Duquerroy, S.; Gomis-Rüth, F.X. Structural and functional insight into pan-endopeptidase inhibition by α2-macroglobulins. Biol. Chem. 2017, 398, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhao, B.; Wei, L.; Wang, S. Alpha-2-macroglobulin, a native and powerful proteinase inhibitor, prevents cartilage degeneration disease by inhibiting majority of catabolic enzymes and cytokines. Curr. Mol. Biol. Rep. 2021, 7, 1–7. [Google Scholar] [CrossRef]
- McDaniel, M.C.; Laudico, R.; Papermaster, B.W. Association of macrophage-activation factor from a human cultured lymphoid cell line with albumin and α2-macroglobulin. Clin. Immunol. Immunopathol. 1976, 5, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Dennis, P.; Saksela, O.; Harpel, P.; Rifkin, D. α2-Macroglobulin is a binding protein for basic fibroblast growth factor. J. Biol. Chem. 1989, 264, 7210–7216. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; O’Grady, P.; Huang, J. Human transforming growth factor beta. alpha 2-macroglobulin complex is a latent form of transforming growth factor beta. J. Biol. Chem. 1988, 263, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Danielpour, D.; Sporn, M.B. Differential inhibition of transforming growth factor beta 1 and beta 2 activity by alpha 2-macroglobulin. J. Biol. Chem. 1990, 265, 6973–6977. [Google Scholar] [CrossRef] [PubMed]
- Tchetverikov, I. Matrix metalloproteinases-3, -8, -9 as markers of disease activity and joint damage progression in early rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Hu, J.; Zhou, Q.; Wang, J.; Liu, P.; Wei, Y. Application of co-expressed genes to articular cartilage: New hope for the treatment of osteoarthritis. Mol. Med. Rep. 2012, 6, 16–18. [Google Scholar] [PubMed]
- Cuellar, J.M. Intradiscal Injection of an Autologous Alpha-2-Macroglobulin (A2M) Concentrate Alleviates Back Pain in FAC-Positive Patients. OROAJ 2017, 4, 555634. [Google Scholar] [CrossRef]
- Luan, Y.; Kong, L.; Howell, D.; Ilalov, K.; Fajardo, M.; Bai, X.-H.; Di Cesare, P.; Goldring, M.; Abramson, S.; Liu, C.-J. Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthr. Cartil. 2008, 16, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Eliasberg, C.D.; Rodeo, S.A. Cytokines, Chemokines, Alpha-2-Macroglobulin, Growth Factors. In Orthobiologics: Injectable Therapies for the Musculoskeletal System; Springer: Cham, Switzerland, 2022; pp. 123–132. [Google Scholar]
- Everts, P.A.; Podesta, L.; Lana, J.F.; Poovendran, G.; Santos, G.S.; Huber, S.C. Alpha-2-Macroglobulin Concentrate as Orthobiologic in Osteoarthritis. In Musculoskeletal Injections Manual: Basics, Techniques and Injectable Agents; Springer: Cham, Switzerland, 2024; pp. 133–140. [Google Scholar]
- Weisel, J.W. The mechanical properties of fibrin for basic scientists and clinicians. Biophys. Chem. 2004, 112, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, R.F. A detailed consideration of a principal domain of vertebrate fibrinogen and its relatives. Protein Sci. 1992, 1, 1563–1577. [Google Scholar] [CrossRef] [PubMed]
- Wolberg, A.S. Fibrinogen and fibrin: Synthesis, structure, and function in health and disease. J. Thromb. Haemost. 2023, 21, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B. Fibrinogen and factor XIII at the intersection of coagulation, fibrinolysis and inflammation. Thromb. Haemost. 2014, 112, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Pieters, M.; Wolberg, A.S. Fibrinogen and fibrin: An illustrated review. Res. Pract. Thromb. Haemost. 2019, 3, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Vilar, R.; Fish, R.J.; Casini, A.; Neerman-Arbez, M. Fibrin(ogen) in human disease: Both friend and foe. Haematologica 2020, 105, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.P.; Flick, M.J. Fibrinogen is at the interface of host defense and pathogen virulence in Staphylococcus aureus infection. Semin. Thromb. Hemost. 2016, 42, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Rubel, C.; Fernández, G.C.; Rosa, F.A.; Gómez, S.; Bompadre, M.B.; Coso, O.A.; Isturiz, M.A.; Palermo, M.S. Soluble fibrinogen modulates neutrophil functionality through the activation of an extracellular signal-regulated kinase-dependent pathway. J. Immunol. 2002, 168, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Solovjov, D.A.; Ugarova, T.P.; Plow, E.F. Integrin αMβ2-mediated cell migration to fibrinogen and its recognition peptides. J. Exp. Med. 2001, 193, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Bach, T.L.; Barsigian, C.; Chalupowicz, D.G.; Busler, D.; Yaen, C.H.; Grant, D.S.; Martinez, J. VE-cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels1. Exp. Cell Res. 1998, 238, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, Y.; Su, B.; Zhao, W.; Kizhakkedathu, J.N.; Zhao, C. Advances in enhancing hemocompatibility of hemodialysis hollow-fiber membranes. Adv. Fiber Mater. 2023, 5, 1198–1240. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.M.; Imre, S.; Vari, C.E.; Muntean, D.L.; Tero-Vescan, A. Ultrafiltration method for plasma protein binding studies and its limitations. Processes 2021, 9, 382. [Google Scholar] [CrossRef]
- Fadadu, P.P.; Mazzola, A.J.; Hunter, C.W.; Davis, T.T. Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: A call for PRP standardization. Reg. Anesth Pain Med. 2019, 44, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Magalon, J.; Brandin, T.; Francois, P.; Degioanni, C.; De Maria, L.; Grimaud, F.; Veran, J.; Dignat-George, F.; Sabatier, F. Technical and biological review of authorized medical devices for platelets-rich plasma preparation in the field of regenerative medicine. Platelets 2021, 32, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.; Bulsara, M.K.; McCrory, P.R.; Richardson, M.D.; Zheng, M.H. Analysis of Platelet-Rich Plasma Extraction: Variations in Platelet and Blood Components Between 4 Common Commercial Kits. Orthop. J. Sports Med. 2017, 5, 232596711667527. [Google Scholar] [CrossRef]
- Magalon, J.; Bausset, O.; Serratrice, N.; Giraudo, L.; Aboudou, H.; Veran, J.; Magalon, G.; Dignat-Georges, F.; Sabatier, F. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 629–638. [Google Scholar] [CrossRef]
- Castillo, T.N.; Pouliot, M.A.; Kim, H.J.; Dragoo, J.L. Comparison of Growth Factor and Platelet Concentration from Commercial Platelet-Rich Plasma Separation Systems. Am. J. Sports Med. 2011, 39, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Chai, J.; Zhang, P.; Li, Y.; Wang, Y.; Mourão, C.F.d.A.B.; Sculean, A.; Kobayashi, M.F.; Zhang, Y. A novel method for harvesting concentrated platelet-rich fibrin (C-PRF) with a 10-fold increase in platelet and leukocyte yields. Clin. Oral Investig. 2020, 24, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Medina, T.; Vaquette, C.; Ivanovski, S. Systematic Comparison of the Effect of Four Clinical-Grade Platelet Rich Hemoderivatives on Osteoblast Behaviour. Int. J. Mol. Sci. 2019, 20, 6243. [Google Scholar] [CrossRef] [PubMed]
- Prysak, M.H.; Lutz, C.G.; Zukofsky, T.A.; Katz, J.M.; Everts, P.A.; Lutz, G.E. Optimizing the safety of intradiscal platelet-rich plasma: An in vitro study with Cutibacterium acnes. Regen. Med. 2019, 14, 955–967. [Google Scholar] [CrossRef]
- Everts, P.A. A Single, Same-Day Procedure Using Multiple PRP Formulations to Treat Complex Knee Pathologies. A Case Report. Orthop. Res. Online J. 2023, 10, 1148–1152. [Google Scholar] [CrossRef]
- Degen, R.M.; Bernard, J.A.; Oliver, K.S.; Dines, J.S. Commercial Separation Systems Designed for Preparation of Platelet-Rich Plasma Yield Differences in Cellular Composition. HSS J. 2017, 13, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, P.; Mitchell, P.J.T.; Shybut, T.B.; Moseley, B.J.; Lee, B. Leukocyte-Rich Platelet-Rich Plasma Is Predominantly Anti-inflammatory Compared with Leukocyte-Poor Platelet-Rich Plasma in Patients with Mild-Moderate Knee Osteoarthritis: A Prospective, Descriptive Laboratory Study. Am. J. Sports Med. 2023, 51, 2133–2140. [Google Scholar] [CrossRef]
- Yesudasan, S.; Averett, R.D. Recent advances in computational modeling of fibrin clot formation: A review. Comput. Biol. Chem. 2019, 83, 107148. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Sproul, E.P.; Hannan, R.T.; Brown, A.C. Controlling fibrin network morphology, polymerization, and degradation dynamics in fibrin gels for promoting tissue repair. In Biomaterials for Tissue Engineering: Methods and Protocols; Humana Press: New York, NY, USA, 2018; pp. 85–99. [Google Scholar]
- Singh, S.; Dodt, J.; Volkers, P.; Hethershaw, E.; Philippou, H.; Ivaskevicius, V.; Imhof, D.; Oldenburg, J.; Biswas, A. Structure functional insights into calcium binding during the activation of coagulation factor XIII A. Sci. Rep. 2019, 9, 11324. [Google Scholar] [CrossRef]
- Mosesson, M.W.; Siebenlist, K.R.; Meh, D.A. The Structure and Biological Features of Fibrinogen and Fibrin. Ann. N. Y. Acad. Sci. 2006, 936, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, G.; Ciccarelli, G.; Golino, P. Role of tissue factor in the coagulation network. Semin. Thromb. Hemost. 2015, 41, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.B.; Hvas, A.M. Fibrin Clot Formation and Lysis in Plasma. Methods Protoc. 2020, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Nurden, P.; Prado, R.; Nurden, A.T.; Padilla, S. Autologous fibrin scaffolds: When platelet- and plasma-derived biomolecules meet fibrin. Biomaterials 2019, 192, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.d.M.; Sharmin, S.; Kim, H.J.; Hong, S.T. Identification and Characterization of Plasmin-Independent Thrombolytic Enzymes. Circ. Res. 2021, 128, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Castellino, F.J.; Ploplis, V.A. Structure and function of the plasminogen/plasmin system. Thromb. Haemost. 2005, 93, 647–654. [Google Scholar]
- Schaller, J.; Gerber, S.S. The plasmin–antiplasmin system: Structural and functional aspects. Cell. Mol. Life Sci. 2011, 68, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, M.; Zakrzewska, E.; Kiciński, P.; Przybylska-Kuć, S.; Dybała, A.; Myśliński, W.; Pastryk, J.; Tomaszewski, T.; Mosiewicz, J. Evaluation of fibrinolytic inhibitors: Alpha-2-antiplasmin and plasminogen activator inhibitor 1 in patients with obstructive sleep apnoea. PLoS ONE 2016, 11, e0166725. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Park, D.; Lesty, C.; Soria, J.; Soria, C.; Montalescot, G.; Weisel, J.W. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: Dynamic and structural approaches by confocal microscopy. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.A.; Muga, K.; Boothroyd, E.M. The effect of fibrin structure on fibrinolysis. J. Biol. Chem. 1992, 267, 24259–24263. [Google Scholar] [CrossRef] [PubMed]
- Risman, R.A.; Paynter, B.; Percoco, V.; Shroff, M.; Bannish, B.E.; Tutwiler, V. Internal fibrinolysis of fibrin clots is driven by pore expansion. Sci. Rep. 2024, 14, 2623. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. In Fibrous Proteins: Structures and Mechanisms; Subcellular Biochemistry; Parry, D.A.D., Squire, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 82, pp. 405–456. [Google Scholar]
- Wahl, E.A.; Fierro, F.A.; Peavy, T.R.; Hopfner, U.; Dye, J.F.; Machens, H.-G.; Egaña, J.T.; Schenck, T.L. In vitro evaluation of scaffolds for the delivery of mesenchymal stem cells to wounds. BioMed Res. Int. 2015, 2015, 108571. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Jimbo, R.; Barbé, G.; Del Corso, M.; Inchingolo, F.; Sammartino, G. Do the Fibrin Architecture and Leukocyte Content Influence the Growth Factor Release of Platelet Concentrates? An Evidence-based Answer Comparing a Pure Platelet-Rich Plasma (P-PRP) Gel and a Leukocyte- and Platelet-Rich Fibrin (L-PRF). Curr. Pharm. Biotechnol. 2012, 13, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Robson, S.; Shephard, E.; Kirsch, R. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1β, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br. J. Haematol. 1994, 86, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Petzelbauer, P.; A Zacharowski, P.; Miyazaki, Y.; Friedl, P.; Wickenhauser, G.; Castellino, F.J.; Gröger, M.; Wolff, K.; Zacharowski, K. The fibrin-derived peptide Bβ15–42 protects the myocardium against ischemia-reperfusion injury. Nat. Med. 2005, 11, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, X.; Liu, Y.; Qiao, J. Cytokine release kinetics of concentrated growth factors in different scaffolds. Clin. Oral Investig. 2019, 23, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.H.; Hess, K.; Price, J.F.; Strachan, M.; Baxter, P.D.; Cubbon, R.; Phoenix, F.; Gamlen, T.; Ariëns, R.A.S.; Grant, P.J.; et al. Gender-specific alterations in fibrin structure function in type 2 diabetes: Associations with cardiometabolic and vascular markers. J. Clin. Endocrinol. Metab. 2012, 97, E2282–E2287. [Google Scholar] [CrossRef] [PubMed]
- Crisci, A.; Crescenzo, U.D.; Crisci, M. Platelet-rich concentrates (L-PRF, PRP) in tissue regeneration: Control of apoptosis and interactions with regenerative cells. J. Clin. Mol. Med. 2018, 1, 5. [Google Scholar] [CrossRef]
- Ratajczak, J.; Vangansewinkel, T.; Gervois, P.; Merckx, G.; Hilkens, P.; Quirynen, M.; Lambrichts, I.; Bronckaers, A. Angiogenic Properties of ‘Leukocyte- and Platelet-Rich Fibrin’. Sci. Rep. 2018, 8, 14632. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Shin, J.; Bhang, S.H.; Shin, J.-Y.; Park, J.; Im, G.-I.; Kim, C.-S.; Kim, B.-S. Enhanced skin wound healing by a sustained release of growth factors contained in platelet-rich plasma. Exp. Mol. Med. 2011, 43, 622. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Matsushita, T.; Nagai, K.; Araki, D.; Hoshino, Y.; Kuroda, R. Fibrin clot and Leukocyte-rich platelet-rich fibrin show similar release kinetics and amount of growth factors: A pilot study. J. Orthop. Surg. Res. 2023, 18, 238. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; Purita, J.; Everts, P.A.; Neto, P.A.T.D.M.; Jorge, D.d.M.F.; Mosaner, T.; Huber, S.C.; Azzini, G.O.M.; da Fonseca, L.F.; Jeyaraman, M.; et al. Platelet-Rich Plasma Power-Mix Gel (ppm)—An Orthobiologic Optimization Protocol Rich in Growth Factors and Fibrin. Gels 2023, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Laurens, N.; Koolwijk, P.; De Maat, M.P.M. Fibrin structure and wound healing. J. Thromb. Haemost. 2006, 4, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Ciano, P.; Colvin, R.; Dvorak, A.; McDonagh, J.; Dvorak, H. Macrophage migration in fibrin gel matrices. Lab. Investig. A J. Tech. Methods Pathol. 1986, 54, 62–70. [Google Scholar]
- Delgado, D.; Beitia, M.; Mercader Ruiz, J.; Sánchez, P.; Montoya-Alzola, M.; Fiz, N.; Sánchez, M. A Novel Fibrin Matrix Derived from Platelet-Rich Plasma: Protocol and Characterization. Int. J. Mol. Sci. 2024, 25, 4069. [Google Scholar] [CrossRef] [PubMed]
- Dickneite, G.; Metzner, H.; Pfeifer, T.; Kroez, M.; Witzke, G. A comparison of fibrin sealants in relation to their in vitro and in vivo properties. Thromb. Res. 2003, 112, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhong, W.; Li, J.; Luo, J. Mechanical Properties and Cushioning Effectiveness of FPUF-EPS Combination Materials. Materials 2023, 16, 6886. [Google Scholar] [CrossRef]
- Padilla, S.; Sánchez, M.; Orive, G.; Anitua, E. Human-based biological and biomimetic autologous therapies for musculoskeletal tissue regeneration. Trends Biotechnol. 2017, 35, 192–202. [Google Scholar] [CrossRef] [PubMed]
- BalagBalagholi, S.; Kanavi, M.R.; Alizadeh, S.; Dabbaghi, R.; Karami, S.; Kheiri, B.; Daftarian, N. Effects of fibrin glue as a three-dimensional scaffold in cultivated adult human retinal pigment epithelial cells. J. Biomater. Appl. 2018, 33, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y. M1 and M2 polarization of macrophages: A mini-review. Med. Biol. Sci. Eng. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Flick, M.J.; Du, X.; Degen, J.L. Fibrin (ogen)-αMβ2 interactions regulate leukocyte function and innate immunity in vivo. Exp. Biol. Med. 2004, 229, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.Y.; Smith, T.D.; Meli, V.S.; Tran, T.N.; Botvinick, E.L.; Liu, W.F. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater. 2017, 47, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Whyte, C.S. All tangled up: Interactions of the fibrinolytic and innate immune systems. Front. Med. 2023, 10, 1212201. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Rosenkranz, A.; Assmann, K.J.; Goodman, M.J.; Gutierrez-Ramos, J.C.; Carroll, M.C.; Cotran, R.S.; Mayadas, T.N. A role for Mac-1 (CDIIb/CD18) in immune complex–stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcγ receptor–dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J. Exp. Med. 1997, 186, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- 246. Vasconcelos, D.M.; Gonçalves, R.M.; Almeida, C.R.; Pereira, I.O.; Oliveira, M.I.; Neves, N.; Silva, A.M.; Ribeiro, A.C.; Cunha, C.; Almeida, A.R.; et al. Fibrinogen scaffolds with immunomodulatory properties promote in vivo bone regeneration. Biomaterials 2016, 111, 163–178. [Google Scholar] [CrossRef]
- Almeida, C.R.; Vasconcelos, D.P.; Gonçalves, R.M.; Barbosa, M.A. Enhanced mesenchymal stromal cell recruitment via natural killer cells by incorporation of inflammatory signals in biomaterials. J. R. Soc. Interface 2012, 9, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Eken, C.; Sadallah, S.; Martin, P.J.; Treves, S.; Schifferli, J.A. Ectosomes of polymorphonuclear neutrophils activate multiple signaling pathways in macrophages. Immunobiology 2013, 218, 382–392. [Google Scholar] [CrossRef]
- Dinkla, S.; van Cranenbroek, B.; van der Heijden, W.A.; He, X.; Wallbrecher, R.; Dumitriu, I.E.; van der Ven, A.J.; Bosman, G.J.C.G.M.; Koenen, H.J.P.M.; Joosten, I. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood J. Am. Soc. Hematol. 2016, 127, 1976–1986. [Google Scholar] [CrossRef]
- Laffont, B.; Corduan, A.; Rousseau, M.; Duchez, A.C.; Lee, C.H.C.; Boilard, E.; Provost, P. Platelet microparticles reprogram macrophage gene expression and function. Thromb. Haemost. 2016, 115, 311–323. [Google Scholar]
- Kim, O.H.; Park, J.H.; Son, J.I.; Yoon, O.J.; Lee, H.J. Bone marrow mesenchymal stromal cells on silk fibroin scaffolds to attenuate polymicrobial sepsis induced by cecal ligation and puncture. Polymers 2021, 13, 1433. [Google Scholar] [CrossRef] [PubMed]
- Heher, P.; Mühleder, S.; Mittermayr, R.; Redl, H.; Slezak, P. Fibrin-based delivery strategies for acute and chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.V.; Bukhary, D.; Valdivieso, C.Y.L.; Tirelli, N. Fibrin Matrices as (Injectable) Biomaterials: Formation, Clinical Use, and Molecular Engineering. Macromol. Biosci. 2020, 20, 1900283. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, S.M.; Impeduglia, T.; Hessler, K.; Wang, X.; Carroll, R.J.; Dardik, H. Autologous platelet-rich fibrin matrix as cell therapy in the healing of chronic lower-extremity ulcers. Wound Repair Regen. 2008, 16, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.M.; Devilee, R.J.J.; Mahoney, C.B.; Eeftinck-Schattenkerk, M.; Box, H.A.M.; Knape, J.T.A.; Van Zundert, A. Platelet gel and fibrin sealant reduce allogeneic blood transfusions in total knee arthroplasty. Acta Anaesthesiol Scand 2006, 50, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Micovic, S.; Everts, P.; Calija, B.; Strugarevic, E.; Grubor, N.; Boricic, M.; Lesanovic, J.; Box, H.; Abazovic, D. Novel autologous, high concentrated fibrin as advanced hemostatic agent for coronary surgery. Transfus. Apher. Sci. 2021, 60, 103171. [Google Scholar] [CrossRef] [PubMed]
- Koolwijk, P.; van Erck, M.G.; de Vree, W.J.; A Vermeer, M.; A Weich, H.; Hanemaaijer, R.; van Hinsbergh, V.W. Cooperative effect of TNFalpha, bFGF, and VEGF on the formation of tubular structures of human microvascular endothelial cells in a fibrin matrix. Role of urokinase activity. J. Cell Biol. 1996, 132, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Clark, R.A.; Galanakis, D.; Tonnesen, M.G. Fibrin and collagen differentially regulate human dermal microvascular endothelial cell integrins: Stabilization of αv/β3 mRNA by fibrin. J. Investig. Dermatol. 1999, 113, 913–919. [Google Scholar] [CrossRef]
- Yakovlev, S.; Medved, L. Interaction of fibrin (ogen) with the endothelial cell receptor VE-cadherin: Localization of the fibrin-binding site within the third extracellular VE-cadherin domain. Biochemistry 2009, 48, 5171–5179. [Google Scholar] [CrossRef]
- Gorodetsky, R.; Vexler, A.; Levdansky, L.; Marx, G. Fibrin microbeads (FMB) as biodegradable carriers for culturing cells and for accelerating wound healing. In Biopolymer Methods in Tissue Engineering; Humana Press: Totowa, NJ, USA, 2004; pp. 11–24. [Google Scholar]
- Oliver, J.J.; Webb, D.J.; Newby, D.E. Stimulated Tissue Plasminogen Activator Release as a Marker of Endothelial Function in Humans. Arter. Thromb. Vasc. Biol. 2005, 25, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Fukao, H.; Ueshima, S.; Okada, K.; Matsuo, O. The Role of the Pericellular Fibrinolytic System in Angiogenesis. Jpn. J. Physiol. 1997, 47, 161–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harley, S.L.; Sturge, J.; Powell, J.T. Regulation by fibrinogen and its products of intercellular adhesion molecule-1 expression in human saphenous vein endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Hermans, P.; Hazelzet, J.A. Plasminogen activator inhibitor type 1 gene polymorphism and sepsis. Clin. Infect. Dis. 2005, 41 (Suppl. 7), S453–S458. [Google Scholar] [CrossRef] [PubMed]
- Van Hinsbergh, V.W.; Collen, A.; Koolwijk, P. Role of fibrin matrix in angiogenesis. Ann. N. Y. Acad. Sci. 2001, 936, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Nehls, V.; Herrmann, R. The configuration of fibrin clots determines capillary morphogenesis and endothelial cell migration. Microvasc. Res. 1996, 51, 347–364. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Degen, J.L.; Ginsburg, D. Reduced thrombin generation increases host susceptibility to group A streptococcal infection. Blood J. Am. Soc. Hematol. 2009, 113, 1358–1364. [Google Scholar] [CrossRef]
- Degen, J.; Bugge, T.; Goguen, J. Fibrin and fibrinolysis in infection and host defense. J. Thromb. Haemost. 2007, 5, 24–31. [Google Scholar] [CrossRef]
- Prasad, J.M.; Gorkun, O.V.; Raghu, H.; Thornton, S.; Mullins, E.S.; Palumbo, J.S.; Ko, Y.-P.; Höök, M.; David, T.; Coughlin, S.R.; et al. Mice expressing a mutant form of fibrinogen that cannot support fibrin formation exhibit compromised antimicrobial host defense. Blood J. Am. Soc. Hematol. 2015, 126, 2047–2058. [Google Scholar] [CrossRef]
- Bayer, A.; Höntsch, G.; Kaschwich, M.; Dell, A.; Siggelkow, M.; Berndt, R.; Rusch, R.; Harder, J.; Gläser, R.; Cremer, J. Vivostat Platelet-Rich Fibrin® for complicated or chronic wounds—A pilot study. Biomedicines 2020, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.B.S.; Lago, C.A.P.; Almeida, R.A.C.; Barbirato, D.D.S.; Vasconcelos, B.C.D.E. Biological and Cellular Properties of Advanced Platelet-Rich Fibrin (A-PRF) Compared to Other Platelet Concentrates: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 25, 482. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Dhadse, P.; Bajaj, P.; Sethiya, K.; Subhadarsanee, C.; Oza, R. Comparative evaluation of platelet rich fibrin matrix (PRFM) membrane and platelet rich fibrin (PRF) membrane using the vestibular incision subperiosteal tunnel access (VISTA) approach technique for the treatment of multiple gingival recession in humans: A double-blind, parallel-group, randomized controlled clinical trial. F1000Research 2023, 12, 872. [Google Scholar]
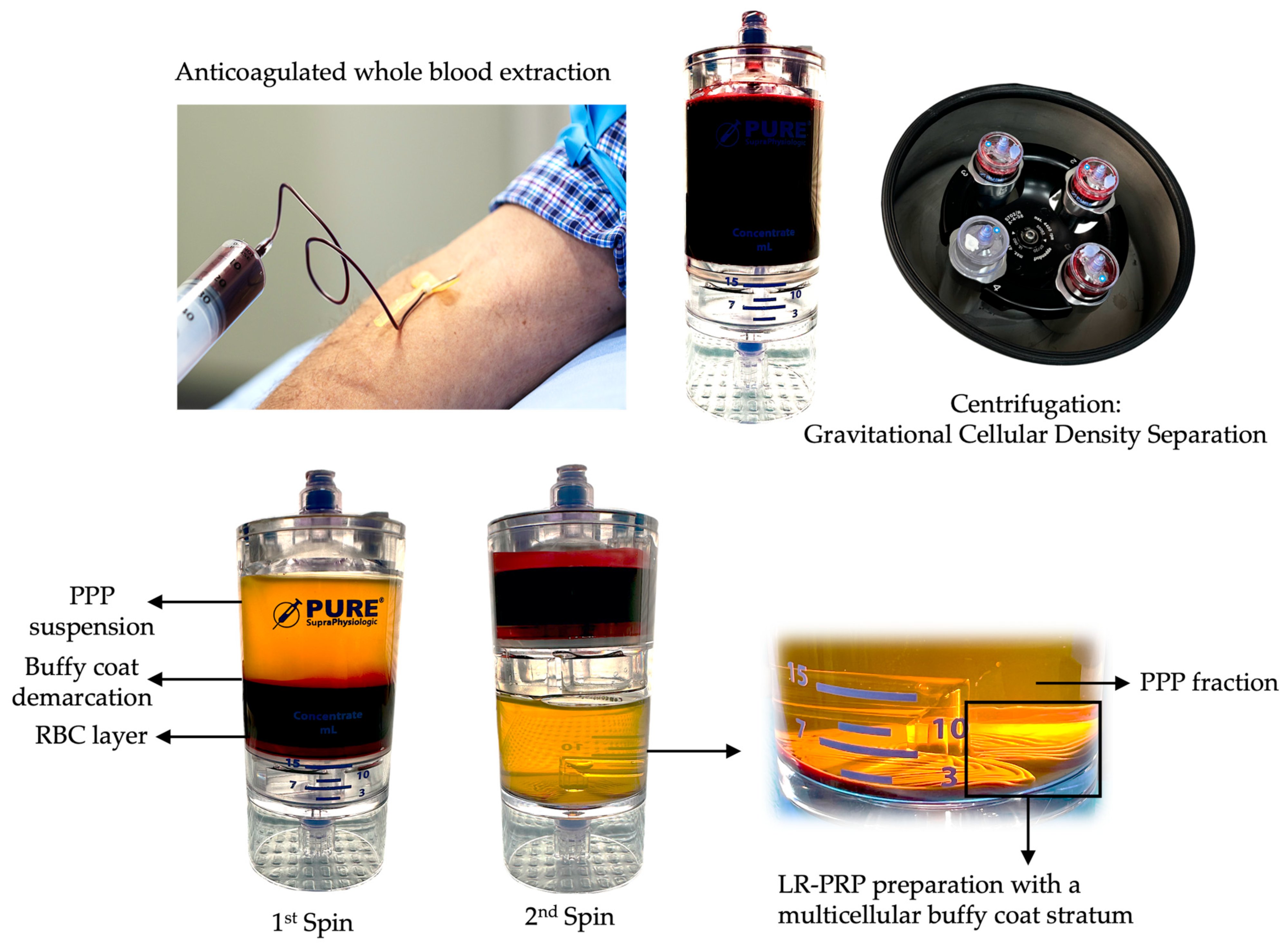
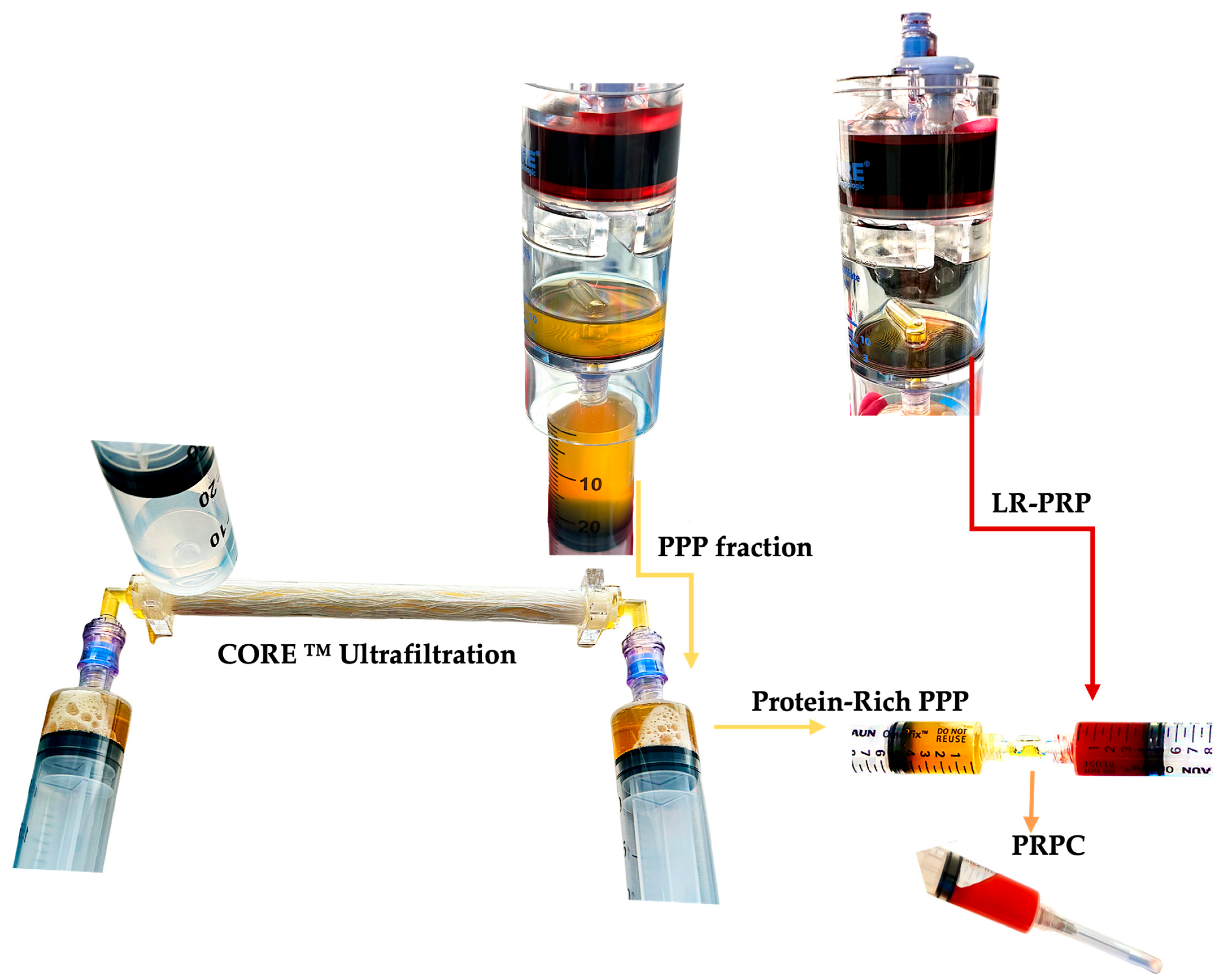


| PR-PRP Component | Structure | Key Content | Main Functions |
|---|---|---|---|
| Platelet | α-granules | Growth Factors: PDGF (AA-BB-AB-CC), VEGF, TGF (α-β), FGF (a-b), EGF, CTGF | Growth factor-based regulation of tissue repair via cell proliferation, differentiation, mitogenesis, chemotaxis, and epithelial repair. |
| Adhesive Proteins: Fibronectin, vitronectin, fibrinogen, vWF, P-selectin, integrins αIIbβ, Phosphatidylserine | Platelet aggregation, platelet–endothelial cell interaction, and thrombus formation. | ||
| Coagulation Factors: Factors IV, XI, XIII, plasminogen, plasmin, antithrombin, tissue factor | Hemostasis and thrombus formation. | ||
| Angiogenic Regulators: IL8, thrombospondin, Angiostatin, PF-4, TIMP-1,4, MMP-1,2,9, Angiopoietin, Endostatin, SDF-1, PMP | Angiogenesis cascades and re-establishing vasculature. | ||
| Cytokines: IL1, IL4, IL6, TFNα, SDF-1 | Chemotaxis, inflammatory response modulation, and antimicrobial activity. | ||
| Chemokines: RANTES, CXCL4, CXCL7, CCL2, CCL3, CCL5, β-TG | Inflammation, antimicrobial, and bactericidal activity. | ||
| Complement Proteins: C3, C4 | Phagocytosis, chemotaxis, and platelet activation. | ||
| Exosomes: mRNA, miRNA, CXCL4, CXCL7 | Cell adhesion, paracrine communication, regulation of cell fate, and modulation of inflammatory response. | ||
| Dense granules | ADP, ATP, TFNα calcium, serotonin, epinephrine, pyrophosphates | Platelet activation and vasoconstriction. | |
| Lysosomes | Collagenase, elastase, Cathepsin, α-arabinoside, β-galactosidase | Matrix degradation and antimicrobial activity. | |
| Multivescicular Bodies | Exosomes Extracellular vesicles | Cell proliferation, PGF transportation, and platelet–cell communication. | |
| PPP | Plasma Proteins (>300) | Albumen, fibrinogen, Alpha-2-macroglobulin, | Blood clotting, maintain blood pressure, carrier functions, immunity, and pH regulation |
| Coagulation factors | Tissue factor, Factor I, II, IV, V, VII, vWF | Intrinsic and extrinsic coagulation pathways and clot formation | |
| Growth Factors | IGF-1, HGF | Bone growth, glucose transport in fat and muscle, muscle production, mitogenesis, cell growth, and cell proliferation |
| Plasma Protein Chains | Concentration (g/L) | Molecular Weight (kDa) |
|---|---|---|
| Albumin | 40 | 66 |
| IgG γ-chain | 12 | 50 |
| Transferrin | 2.3 | 25 |
| IgA α-chain | 2 | 60 |
| Apolipoprotein A1 | 1.4 | 28 |
| α2-macroglobulin | 1.4 | 190 |
| α-1antitripsin | 1.1 | 52 |
| Fibrinogen α-chain | 0.95 | 95 |
| IgM µ chains | 0.75 | 75 |
| Hemopexin | 0.75 | 60 |
| Apolipoprotein B | 0.72 | 250 |
| α1-acid glycoprotein | 0.61 | 41 |
| Fibrinogen β-chain | 0.56 | 56 |
| Apolipoprotein AII | 0.3 | 110 |
| Fibrinogen γ-chain | 0.5 | 50 |
| Complement C3 β-chain | 0.39 | 75 |
| Antithrombin III | 0.32 | 58 |
| Apolipoprotein AII | 0.3 | 17 |
| Haptoglobin α-chain | 0.29 | 40 |
| Pre-albumin | 0.26 | 16 |
| Ceruloplasmin | 0.21 | 132 |
| Haptoglobin β-chain | 0.14 | 20 |
| Fibronectin | 0.11 | 230 |
| Plasminogen α-chain | 0.099 | 60 |
| Complement C4 α-chain | 0.082 | 98 |
| Complement C4 β-chain | 0.061 | 73 |
| Plasminogen β-chain | 0.041 | 25 |
| Complement C4 γ-chain | 0.028 | 33 |
| Other | 0.038 | N/A |
| Device Category | PRPv mL | PLTc ×103/µL | PLTd ×106 | WBCc ×103/µL | MONc ×103/µL | NEUc ×103/µL | RBCc ×109/µL | References |
|---|---|---|---|---|---|---|---|---|
| P-PRP | 4.8 | 170 | 887 | 0.3 | 0 | 0 | 0 | [197,198,199] |
| PRF | 5 | 205 | 1.025 | 0.1 | 0 | 0 | 0 | [195,200,201] |
| LP-PRP | 4.6 | 1280 | 5.686 | 11.5 | 3.1 | 0.8 | 0.2 | [111,202,203] |
| LR-PRP | 6.3 | 1603 | 9.212 | 24.7 | 4.7 | 5.4 | 1.6 | [202,204,205] |
| Matrix Formulation | PLTc ×106/µL | PLTs Available in Matrix, ×109 | MONc ×103/µL | NEUc ×103/µL | RBCc ×109/µL |
|---|---|---|---|---|---|
| PRF | 0.275 | 1.375 | 0 | 0 | 0 |
| LP-PR-PRP | 2.3 | 6.9 | 4.5 | 6.1 | 0.3 |
| LR-PR-PRP | 3.9 | 11.8 | 8.2 | 13.5 | 2.4 |
| Treatment Modality and Biocomponent | Biological Function of Matrix | Therapeutic Goals |
|---|---|---|
| Sealant/Glue Fibrin(ogen) | Post-surgical hemostasis control Oozing tissue sealing Controllable biodegradation | Avoid seromatous wound leakage Avoid blood loss Decrease surgical adhesions Periodontal membrane |
| + | ||
| Regenerative Glue Fibrin(ogen) and PRP | ECM cellular support Anti-inflammatory Active platelet-mediated angiogenesis | Support surgical tissue healing Prevent infection Chronic wound and bone healing Scar reduction |
| Fibrin Matrix Fibrin(ogen) | Temporary matrix for invading platelets and leukocytes EC migration Cell adhesion Promote angiogenesis process Collagen production Growth factor carrier Promote immunomodulation Fibrinolytic sustained release | Tissue repair Tissue regeneration Tissue engineering heart valves and patches Hydrogel transport Intra-osseous infiltration Nerve injuries Myogenesis |
| + | ||
| PR-PRP matrix Fibrin(ogen) and PRP | Osteoblast stimulation Temporary matrix with embedded concentrated platelets and leukocytes Platelet growth factor reservoir Plasma growth factor reservoir Tissue ingrowth stimulation Instigator immunomodulation Cell proliferation–differentiation Organizer angiogenesis process MSC and HF stem cell paracrine effects Antimicrobial pathways Nociception A2M enzymatic processes | Bone defect augmentation Epithelialization chronic wounds Tissue infection management Tissue repair, including nerves Tissue regeneration Skin grafting Wound healing Partial-Full thickness tendon/ligament biofiller Immunomodulation Osteoarthritis Joint protease inhibitor Joint cushioning Hair growth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Everts, P.A.; Lana, J.F.; Alexander, R.W.; Dallo, I.; Kon, E.; Ambach, M.A.; van Zundert, A.; Podesta, L. Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing. Int. J. Mol. Sci. 2024, 25, 7914. https://doi.org/10.3390/ijms25147914
Everts PA, Lana JF, Alexander RW, Dallo I, Kon E, Ambach MA, van Zundert A, Podesta L. Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing. International Journal of Molecular Sciences. 2024; 25(14):7914. https://doi.org/10.3390/ijms25147914
Chicago/Turabian StyleEverts, Peter A., José Fábio Lana, Robert W. Alexander, Ignacio Dallo, Elizaveta Kon, Mary A. Ambach, André van Zundert, and Luga Podesta. 2024. "Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing" International Journal of Molecular Sciences 25, no. 14: 7914. https://doi.org/10.3390/ijms25147914
APA StyleEverts, P. A., Lana, J. F., Alexander, R. W., Dallo, I., Kon, E., Ambach, M. A., van Zundert, A., & Podesta, L. (2024). Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing. International Journal of Molecular Sciences, 25(14), 7914. https://doi.org/10.3390/ijms25147914








