Plastic Fly: What Drosophila melanogaster Can Tell Us about the Biological Effects and the Carcinogenic Potential of Nanopolystyrene
Abstract
:1. Introduction
2. Results
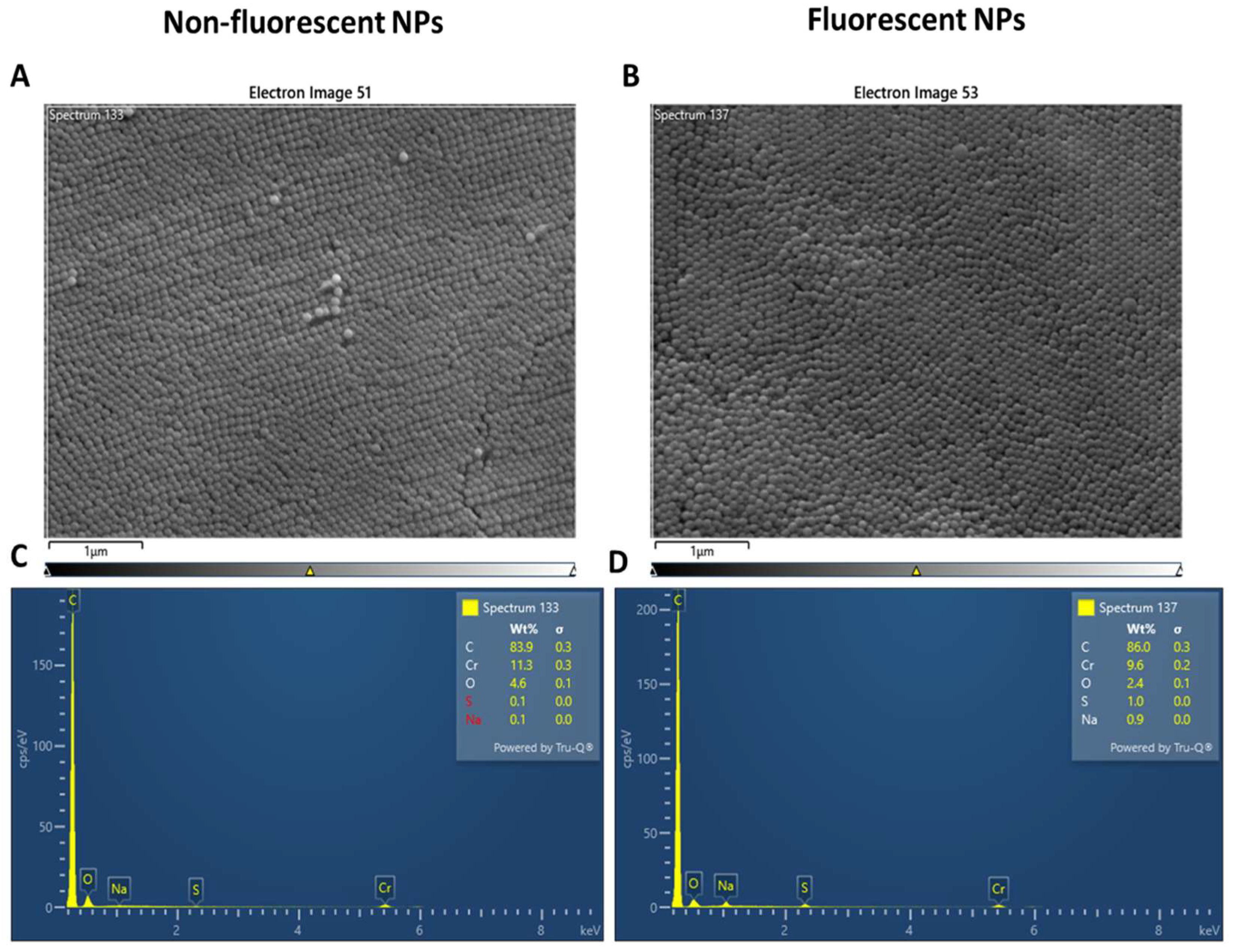
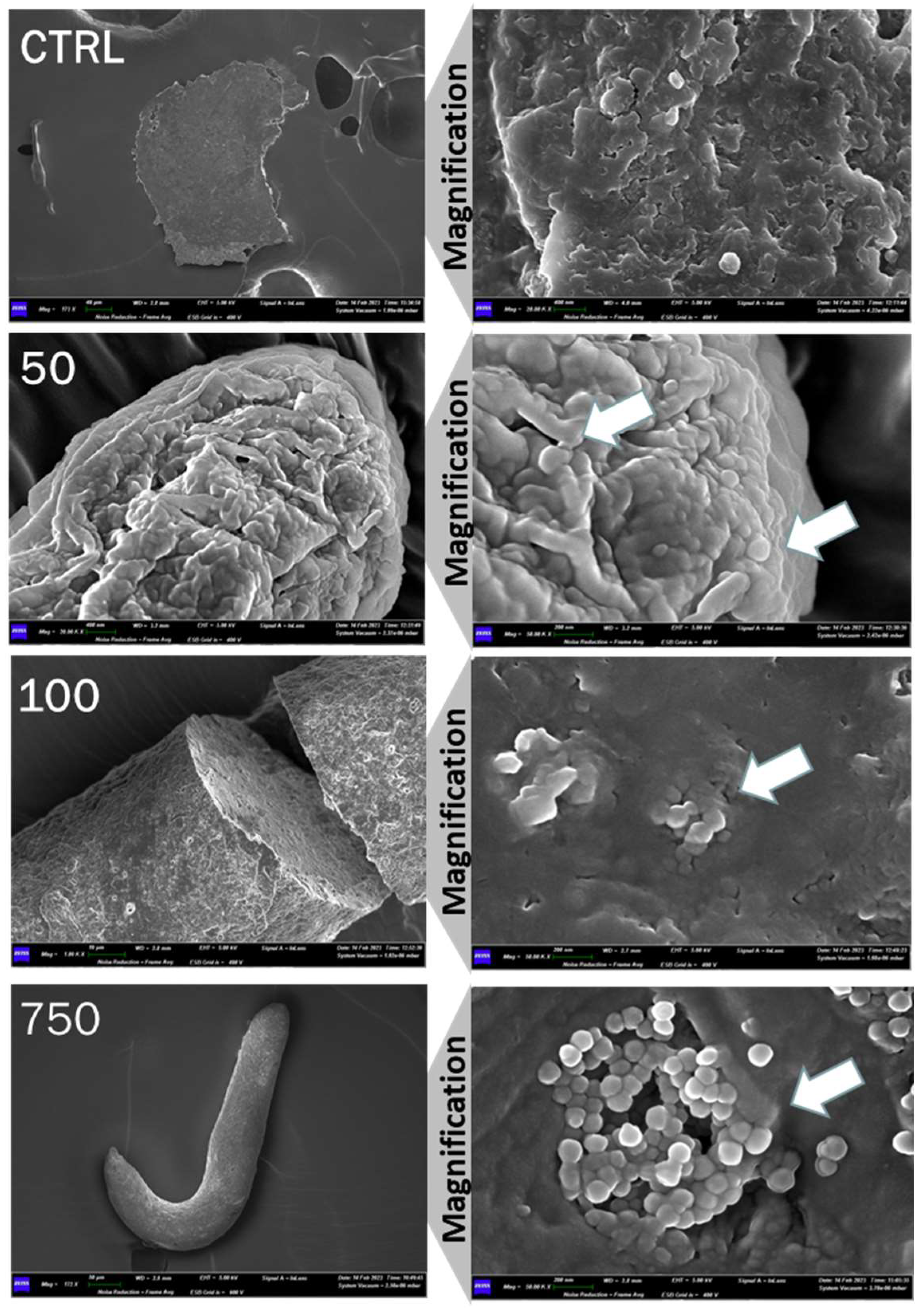
2.1. Analysis of Nanopolystyrene with Scanning Electron Microscopy (SEM)
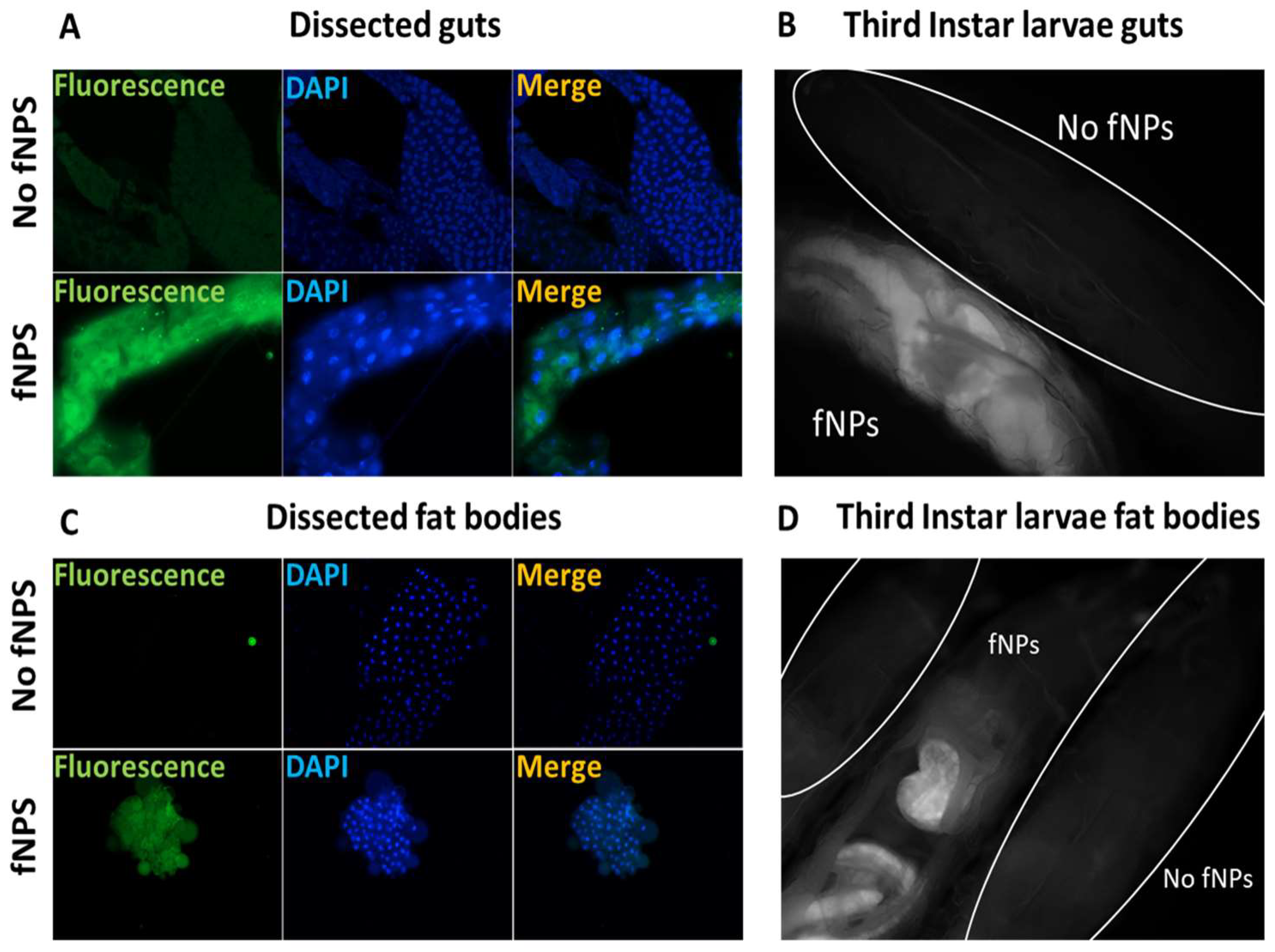
2.2. Biodistribution and Absorption
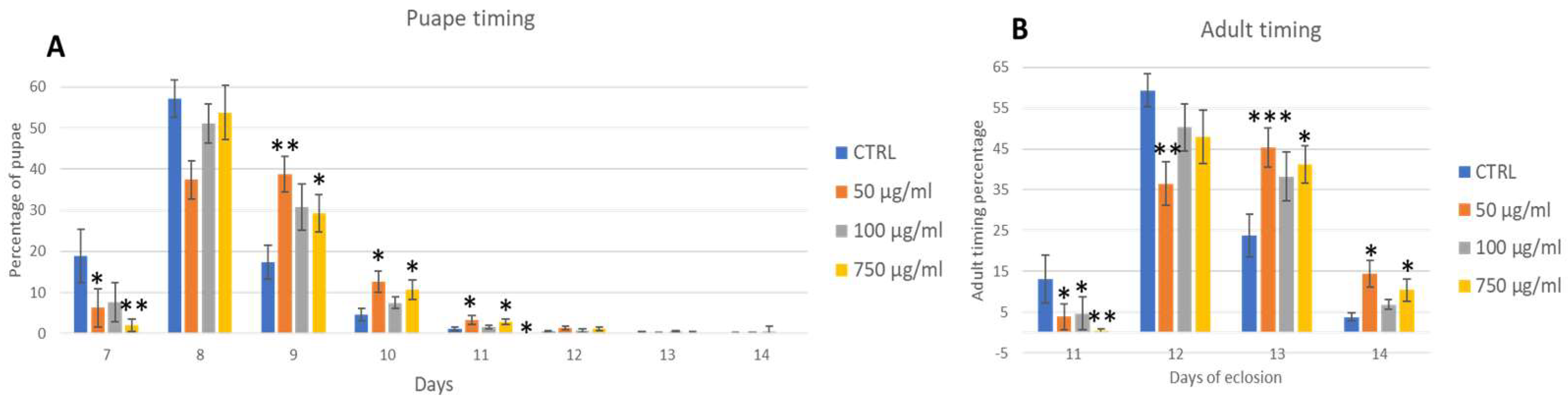
2.3. Development Traits (Life Cycle and Eclosion)
2.4. Weight
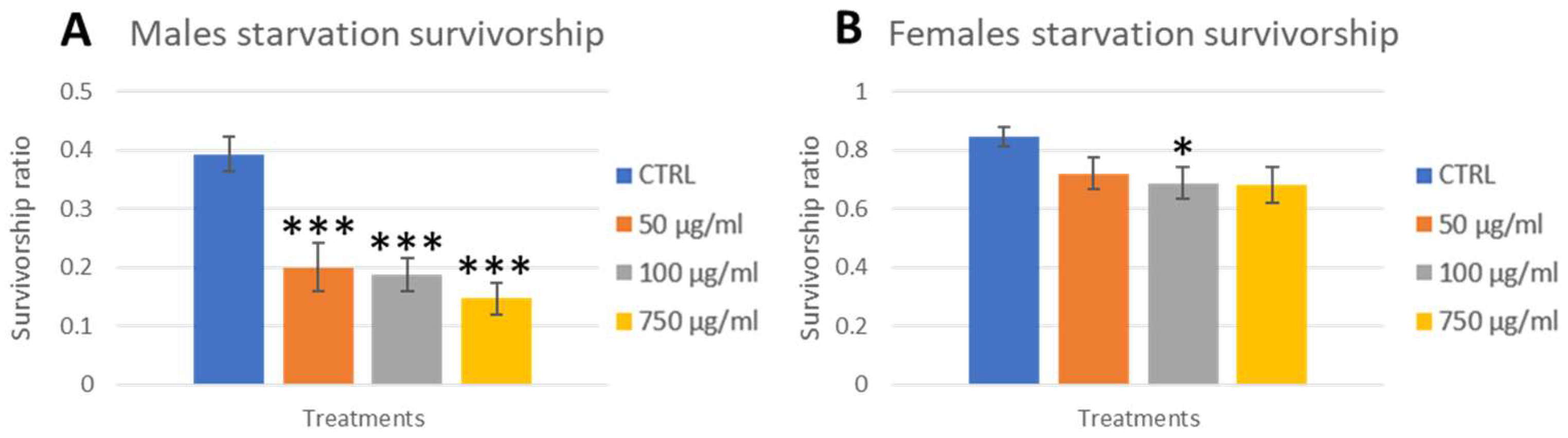
2.5. Stress Response
2.6. Larval Crawling and Wall Body Contraction
2.7. Adults Climbing
2.8. Trypan Blue and Smurf Assay
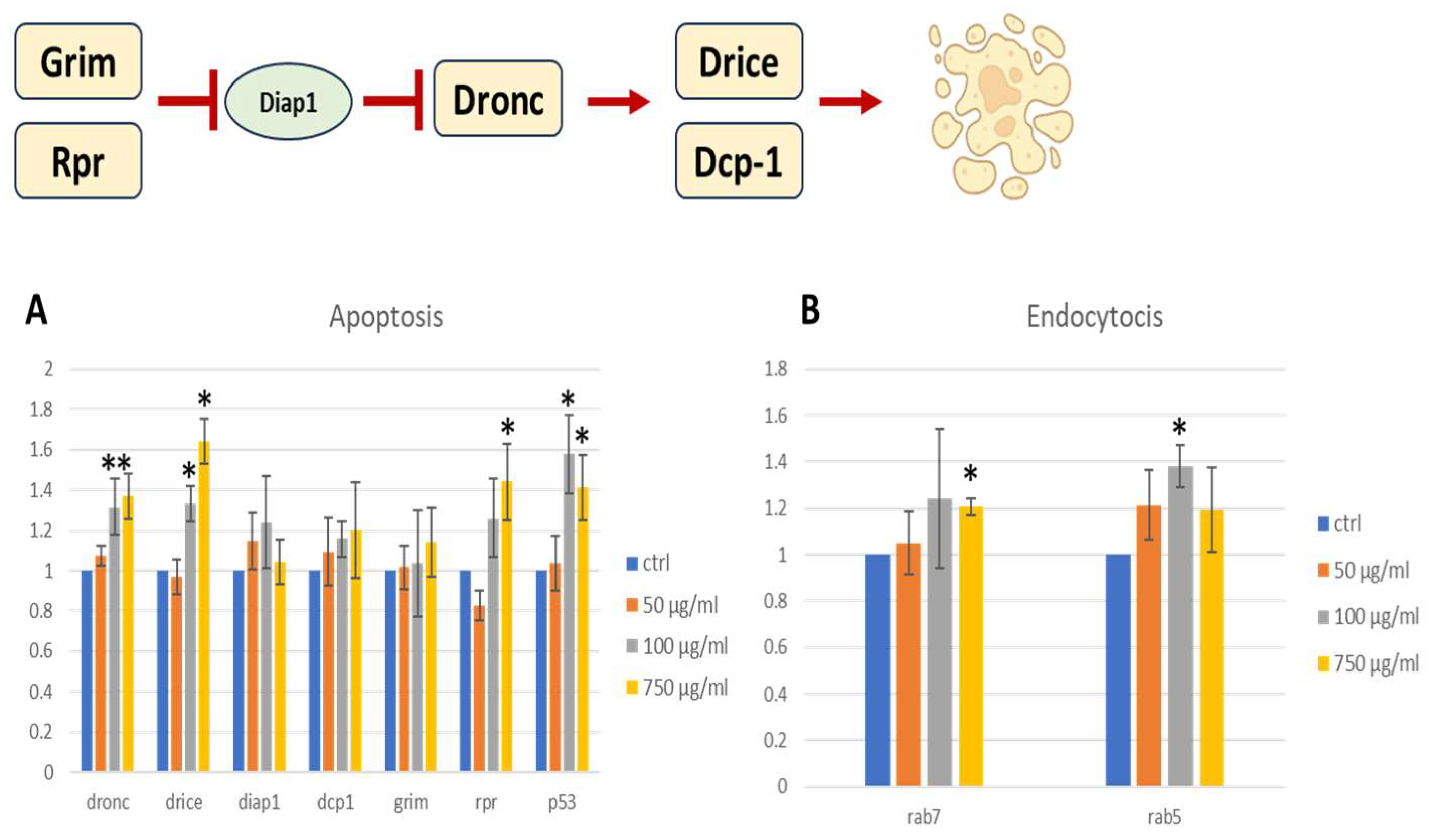
2.9. Pro-Apoptotic Gene Expression
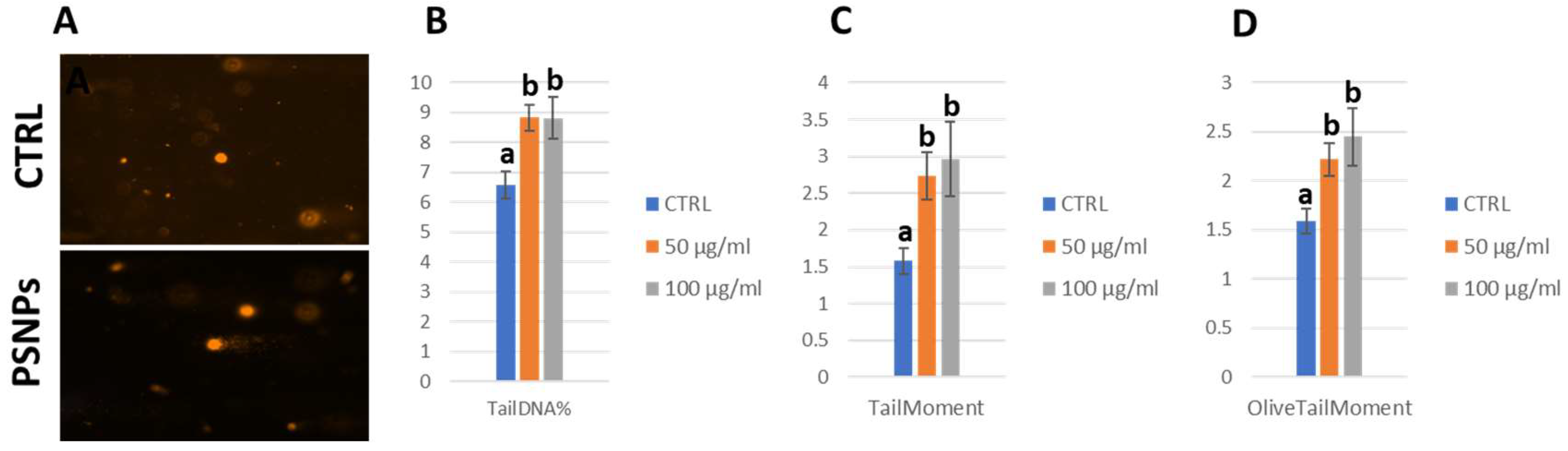
2.10. Comet Assay
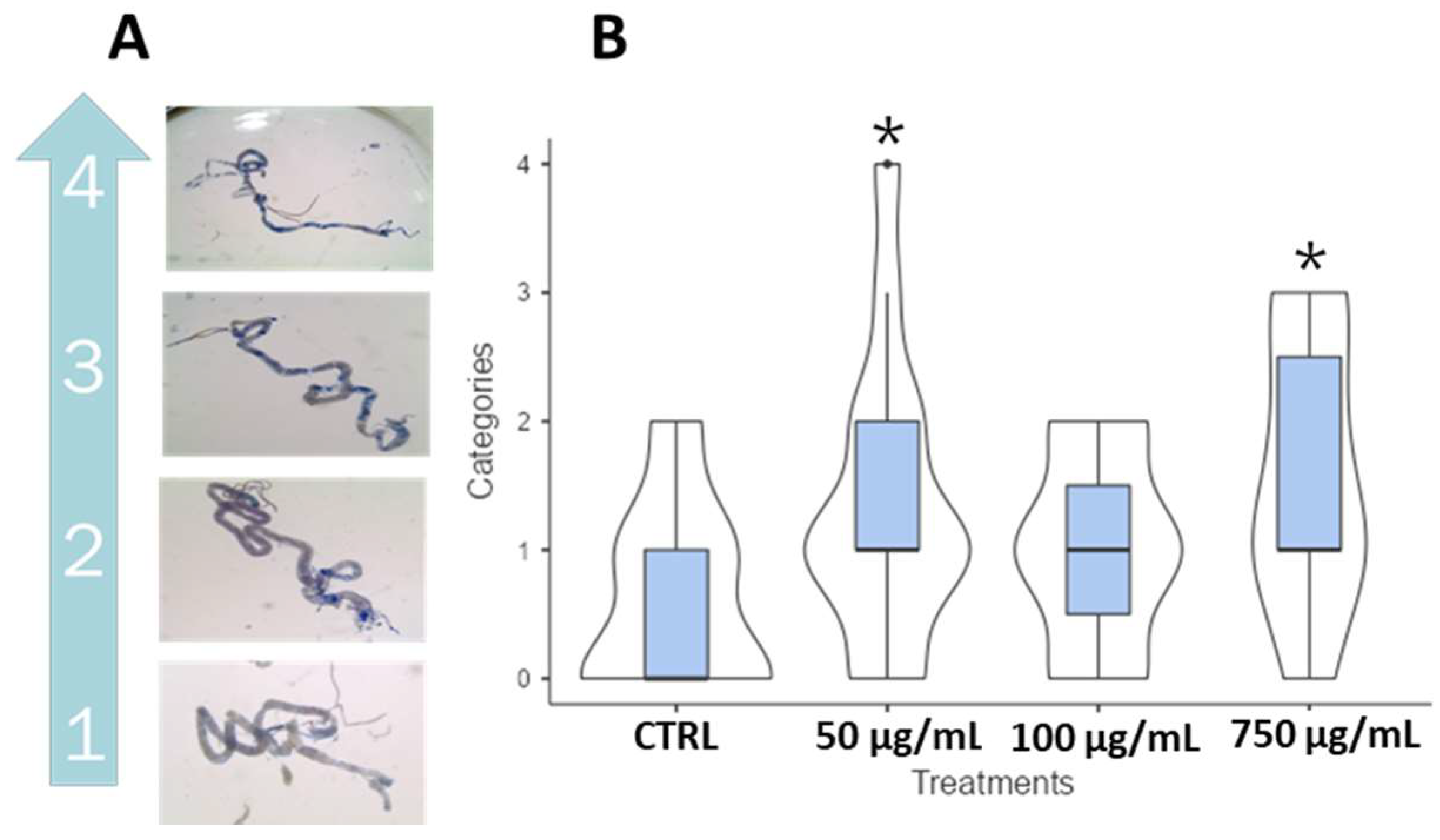
2.11. Carcinogenic Potential
3. Discussion
4. Materials and Methods
4.1. Flies Husbandry and Nanopolystyrene Exposure
4.2. Poyistyrene Nanoparticles
4.3. Scannng Electron Microscopy and EDX Analysis
4.4. Fluorescence Microscopy
4.5. Development Traits and Weight
4.6. Stress Tolerance
4.7. Climbing Assay
4.8. Larval Crawling Assay and Wall Body Contraction
4.9. Smurf Assay (Intestinal Integrity Test)
4.10. Trypan Blue Assay
4.11. Total RNA Extraction and RT-qPCR
4.12. Comet Assay
4.13. Carcinogenic Potential
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostle, C.; Thompson, R.C.; Broughton, D.; Gregory, L.; Wootton, M.; Johns, D.G. The rise in ocean plastics evidenced from a 60-year time series. Nat. Commun. 2019, 10, 1622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Frond, H.; Hampton, L.T.; Kotar, S.; Gesulga, K.; Matuch, C.; Lao, W.; Weisberg, S.B.; Wong, C.S.; Rochman, C.M. Monitoring microplastics in drinking water: An interlaboratory study to inform effective methods for quantifying and characterizing microplastics. Chemosphere 2022, 298, 134282. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Marine pollution. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.R.; Spierling, S.; Endres, H.J.; Barner, L. Plastics and the Environment-Current Status and Challenges in Germany and Australia. Macromol. Rapid Commun. 2020, 41, e2000351. [Google Scholar] [CrossRef] [PubMed]
- Čerkasova, N.; Enders, K.; Lenz, R.; Oberbeckmann, S.; Brandt, J.; Fischer, D.; Fischer, F.; Labrenz, M.; Schernewski, G. A Public Database for Microplastics in the Environment. Microplastics 2023, 2, 132–146. [Google Scholar] [CrossRef]
- Cerasa, M.; Teodori, S.; Pietrelli, L. Searching Nanoplastics: From Sampling to Sample Processing. Polymers 2021, 13, 3658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Plastics—The Facts 2019 An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/wp-content/uploads/2023/10/Plasticsthefastfacts2023-1.pdf (accessed on 14 July 2024).
- Eriksen, M.; Cowger, W.; Erdle, L.M.; Coffin, S.; Villarrubia-Gómez, P.; Moore, C.J.; Carpenter, E.J.; Day, R.H.; Thiel, M.; Wilcox, C. A growing plastic smog, now estimated to be over 170 trillion plastic particles afloat in the world’s oceans-Urgent solutions required. PLoS ONE 2023, 8, e0281596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. Int. 2018, 25, 36046–36063. [Google Scholar] [CrossRef] [PubMed]
- van Wijnen, J.; Ragas, A.M.J.; Kroeze, C. Modelling global river export of microplastics to the marine environment: Sources and future trends. Sci. Total Environ. 2019, 673, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Sun, Y.; Wang, J.; Chen, X.; Zhang, S.; Wu, D. Microplastics in composting of rural domestic waste: Abundance, characteristics, and release from the surface of macroplastics. Environ. Pollut. 2021, 274, 116553. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. Int. 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Liss, P.S. Microplastics: All up in the air? Mar. Pollut. Bull. 2020, 153, 110952. [Google Scholar] [CrossRef] [PubMed]
- Halle, L.L.; Palmqvist, A.; Kampmann, K.; Khan, F.R. Ecotoxicology of micronized tire rubber: Past, present and future considerations. Sci. Total Environ. 2020, 706, 135694. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Kwak, M.; Lee, T.G.; Lee, J.Y. Quantifying the level of nanoparticle uptake in mammalian cells using flow cytometry. Nanoscale 2020, 12, 15743–15751. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailänder, V.; Landfester, K.; et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Budarz, J.F.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, J.; Cheng, J.; Odobel, C.; Pandin, C.; Conan, P.; Pujo-Pay, M.; Barbe, V.; Meistertzheim, A.L.; Ghiglione, J.F. Microbial Ecotoxicology of Marine Plastic Debris: A Review on Colonization and Biodegradation by the “Plastisphere”. Front. Microbiol. 2019, 10, 865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Tender, C.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal Dynamics of Bacterial and Fungal Colonization on Plastic Debris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Kim, S.W.; An, Y.J. Polystyrene nanoplastics inhibit reproduction and induce abnormal embryonic development in the freshwater crustacean Daphnia galeata. Sci. Rep. 2017, 7, 12095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelkhaliq, A.; van der Zande, M.; Punt, A.; Helsdingen, R.; Boeren, S.; Vervoort, J.J.M.; Rietjens, I.M.C.M.; Bouwmeester, H. Impact of nanoparticle surface functionalization on the protein corona and cellular adhesion, uptake and transport. J. Nanobiotechnol. 2018, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.; Tromp, P.; Helsper, J.P.; van der Zande, M.; Rietjens, I.M.; Bouwmeester, H. Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology 2015, 9, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2015, 221, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Wohlleben, W.; Septiadi, D.; Landsiedel, R.; Petri-Fink, A.; Rothen-Rutishauser, B. A novel 3D intestine barrier model to study the immune response upon exposure to microplastics. Arch. Toxicol. 2020, 94, 2463–2479. [Google Scholar] [CrossRef]
- Roursgaard, M.; Hezareh Rothmann, M.; Schulte, J.; Karadimou, I.; Marinelli, E.; Møller, P. Genotoxicity of Particles from Grinded Plastic Items in Caco-2 and HepG2 Cells. Front. Public Health 2022, 10, 906430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vecchiotti, G.; Colafarina, S.; Aloisi, M.; Zarivi, O.; Di Carlo, P.; Poma, A. Genotoxicity and oxidative stress induction by polystyrene nanoparticles in the colorectal cancer cell line HCT116. PLoS ONE 2021, 16, e0255120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bredeck, G.; Halamoda-Kenzaoui, B.; Bogni, A.; Lipsa, D.; Bremer-Hoffmann, S. Tiered testing of micro- and nanoplastics using intestinal in vitro models to support hazard assessments. Environ. Int. 2022, 158, 106921. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cheng, Y.; Chen, Z.; Liu, T.; Yin, L.; Pu, Y.; Liang, G. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol. Environ. Saf. 2021, 226, 112837. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Micro- and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halimu, G.; Zhang, Q.; Liu, L.; Zhang, Z.; Wang, X.; Gu, W.; Zhang, B.; Dai, Y.; Zhang, H.; Zhang, C.; et al. Toxic effects of nanoplastics with different sizes and surface charges on epithelial-to-mesenchymal transition in A549 cells and the potential toxicological mechanism. J. Hazard. Mater. 2022, 430, 128485. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Vecchiotti, G.; Colafarina, S.; Zarivi, O.; Aloisi, M.; Arrizza, L.; Chichiriccò, G.; Di Carlo, P. In Vitro Genotoxicity of Polystyrene Nanoparticles on the Human Fibroblast Hs27 Cell Line. Nanomaterials 2019, 9, 1299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Y.; Shrestha, N.; Préat, V.; Beloqui, A. An overview of in vitro, ex vivo and in vivo models for studying the transport of drugs across intestinal barriers. Adv. Drug Deliv. Rev. 2021, 175, 113795. [Google Scholar] [CrossRef] [PubMed]
- Kelpsiene, E.; Torstensson, O.; Ekvall, M.T.; Hansson, L.A.; Cedervall, T. Long-term exposure to nanoplastics reduces life-time in Daphnia magna. Sci. Rep. 2020, 10, 5979. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ekvall, M.T.; Gimskog, I.; Hua, J.; Kelpsiene, E.; Lundqvist, M.; Cedervall, T. Size fractionation of high-density polyethylene breakdown nanoplastics reveals different toxic response in Daphnia magna. Sci. Rep. 2022, 12, 3109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heinlaan, M.; Viljalo, K.; Richter, J.; Ingwersen, A.; Vija, H.; Mitrano, D.M. Multi-generation exposure to polystyrene nanoplastics showed no major adverse effects in Daphnia magna. Environ. Pollut. 2023, 323, 121213. [Google Scholar] [CrossRef] [PubMed]
- Alaraby, M.; Villacorta, A.; Abass, D.; Hernández, A.; Marcos, R. The hazardous impact of true-to-life PET nanoplastics in Drosophila. Sci. Total Environ. 2023, 863, 160954. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhong, L.; Xu, Y.; Jin, Z.; Pan, Z.; Shen, J. Polypropylene microplastics affect the physiology in Drosophila model. Bull. Entomol. Res. 2023, 113, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liang, B.; Zhang, D.; Li, Y.; Tang, H.; Zhong, L.; Xu, Y. Effects of PET microplastics on the physiology of Drosophila. Chemosphere 2021, 283, 131289, Erratum in Chemosphere 2022, 303, 134678. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- De Marco, G.; Oliveri Conti, G.; Giannetto, A.; Cappello, T.; Galati, M.; Iaria, C.; Pulvirenti, E.; Capparucci, F.; Angela Mauceri, A.; Ferrante, M.; et al. Embryotoxicity of polystyrene microplastics in zebrafish Danio rerio. Environ. Res. 2022, 208, 112552. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 2019, 255 Pt 1, 113122. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poma, A.M.G.; Morciano, P.; Aloisi, M. Beyond genetics: Can micro and nanoplastics induce epigenetic and gene expression modifications? Front. Epigenet. Epigenom. 2023, 1, 1241583. [Google Scholar] [CrossRef]
- Cedervall, T.; Hansson, L.A.; Lard, M.; Frohm, B.; Linse, S. Food chain transport of nanoparticles affects behaviour and fat metabolism in fish. PLoS ONE 2012, 7, e32254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santos, D.; Luzio, A.; Bellas, J.; Monteiro, S.M. Microplastics and copper-induced changes in neurogenesis and DNA methyltransferases in the early life stages of zebrafish. Chem. Biol. Interact. 2022, 363, 110021. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.; Yung, L.Y.; Cai, Y.; Bay, B.H.; Baeg, G.H. Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology 2015, 9, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Yu, L.E.; Ong, C.N.; Bay, B.H.; Baeg, G.H. The use of Drosophila melanogaster as a model organism to study immune-nanotoxicity. Nanotoxicology 2019, 13, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Millet-Boureima, C.; Ennis, C.C.; Jamison, J.; McSweeney, S.; Park, A.; Gamberi, C. Empowering Melatonin Therapeutics with Drosophila Models. Diseases 2021, 9, 67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DeLoriea, J.; Millet-Boureima, C.; Gamberi, C. Protocol to build a drug-testing pipeline using large populations of Drosophila melanogaster. STAR Protoc. 2023, 4, 102747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorski, M.M.; Eeken, J.C.; de Jong, A.W.; Klink, I.; Loos, M.; Romeijn, R.J.; van Veen, B.L.; Mullenders, L.H.; Ferro, W.; Pastink, A. The Drosophila melanogaster DNA Ligase IV gene plays a crucial role in the repair of radiation-induced DNA double-strand breaks and acts synergistically with Rad54. Genetics 2003, 165, 1929–1941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joshi, R.; Banerjee, S.J.; Curtiss, J.; Ashley, A.K. DNA ligase IV mutations confer shorter lifespan and increased sensitivity to nutrient stress in Drosophila melanogaster. J. Appl. Genet. 2022, 63, 141–144. [Google Scholar] [CrossRef]
- Denton, D.; Kumar, S. Studying Apoptosis in Drosophila. Cold Spring Harb. Protoc. 2015, 7, 609–613. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the complexity of p53 in a new era of tumor suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borchers, A.C.; Langemeyer, L.; Ungermann, C. Who’s in control? Principles of Rab GTPase activation in endolysosomal membrane trafficking and beyond. J. Cell Biol. 2021, 220, e202105120. [Google Scholar] [CrossRef]
- Gaivão, I.; Sierra, L.M. Drosophila comet assay: Insights, uses, and future perspectives. Front. Genet. 2014, 5, 304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhat, M.A.; Gedik, K.; Gaga, E.O. (Atmospheric micro (nano) plastics: Future growing concerns for human health. Air Qual. Atmos. Health 2023, 16, 233–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022, 806 Pt 3, 151312. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, F.; Rowenczyk, L.; Minet, A.; Clergeaud, F.; Silvestre, J.; Pinelli, E.; Ferriol, J.; Leflaive, J.; Ten-Hage, L.; Gigault, J.; et al. Ecotoxicity of Heteroaggregates of Polystyrene Nanospheres in Chironomidae and Amphibian. Nanomaterials 2022, 12, 2730. [Google Scholar] [CrossRef] [PubMed]
- Kahane-Rapport, S.R.; Czapanskiy, M.F.; Fahlbusch, J.A.; Friedlaender, A.S.; Calambokidis, J.; Hazen, E.L.; Goldbogen, J.A.; Savoca, M.S. Field measurements reveal exposure risk to microplastic ingestion by filter-feeding megafauna. Nat. Commun. 2022, 13, 6327. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, B.; Yao, Q.; Feng, X.; Shen, T.; Guo, P.; Wang, P.; Bai, Y.; Li, B.; Wang, P.; et al. Toxicological effects of micro/nano-plastics on mouse/rat models: A systematic review and meta-analysis. Front. Public. Health 2023, 11, 1103289. [Google Scholar] [CrossRef]
- Charlton-Howard, H.S.; Bond, A.L.; Rivers-Auty, J.; Lavers, J.L. ‘Plasticosis’: Characterising macro- and microplastic-associated fibrosis in seabird tissues. J. Hazard. Mater. 2023, 450, 131090. [Google Scholar] [CrossRef]
- Kelpsiene, E.; Ekvall, M.T.; Lundqvist, M.; Torstensson, O.; Hua, J.; Cedervall, T. Review of ecotoxicological studies of widely used polystyrene nanoparticles. Environ. Sci. Process. Impacts 2022, 24, 8–16. [Google Scholar] [CrossRef]
- Tu, Q.; Deng, J.; Di, M.; Lin, X.; Chen, Z.; Li, B.; Tian, L.; Zhang, Y. Reproductive toxicity of polystyrene nanoplastics in Drosophila melanogaster under multi-generational exposure. Chemosphere 2023, 330, 138724. [Google Scholar] [CrossRef] [PubMed]
- Kauts, S.; Mishra, Y.; Yousuf, S.; Bhardwaj, R.; Singh, S.K.; Alshabrmi, F.M.; Abdurahman, M.; Vamanu, E.; Singh, M.P. Toxicological Profile of Polyethylene Terephthalate (PET) Microplastic in Ingested Drosophila melanogaster (Oregon R+) and Its Adverse Effect on Behavior and Development. Toxics 2023, 11, 782. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, D.; Liu, X.; Xu, Y.; Tang, H.; Li, Y.; Shen, J. Sex-specific effects of PET-MPs on Drosophila lifespan. Arch. Insect Biochem. Physiol. 2022, 110, e21909. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, É.; Minois, N.; Bullens, P.; Bearet, P. A mild stress due to hypergravity exposure at youngage increases longevity in Drosophila melanogaster males. Biogerontology 2000, 1, 145–155. [Google Scholar] [CrossRef]
- Sørensen, J.; Kristensen, T.N.; Kristensen, K.; Loeschcke, V. Sex specific effects of heat induced hormesis in Hsf-deficient Drosophila melanogaster. Exp. Gerontol. 2007, 42, 1123–1129. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Koshel, N.M.; Litoshenko, A.Y.; Mozzhukhina, T.G.; Voitenko, V.P. Effect of X-irradiation in early ontogenesis on the longevity and amount of S1 nuclease-sensitive DNA sites in adult Drosophila melanogaster. Biogerontology 2003, 4, 9–14. [Google Scholar] [CrossRef]
- Moskalev, A.A.; Plyusnina, E.N.; Shaposhnikov, M.V. Radiation hormesis and radioadaptive response in Drosophila melanogaster flies with different genetic backgrounds: The role of cellular stress-resistance mechanisms. Biogerontology 2011, 12, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Porrazzo, A.; Cipressa, F.; De Gregorio, A.; De Pittà, C.; Sales, G.; Ciapponi, L.; Morciano, P.; Esposito, G.; Tabocchini, M.A.; Cenci, G. Low dose rate γ-irradiation protects fruit fly chromosomes from double strand breaks and telomere fusions by reducing the esi-RNA biogenesis factor Loquacious. Commun. Biol. 2022, 5, 905. [Google Scholar] [CrossRef] [PubMed]
- Guruharsha, K.G.; Rual, J.F.; Zhai, B.; Mintseris, J.; Vaidya, P.; Vaidya, N.; Beekman, C.; Wong, C.; Rhee, D.Y.; Cenaj, O.; et al. A protein complex network of Drosophila melanogaster. Cell 2011, 147, 690–703. [Google Scholar] [CrossRef]
- Steller, H. Regulation of apoptosis in Drosophila. Cell Death Differ. 2008, 15, 1132–1138. [Google Scholar] [CrossRef]
- Polesello, C.; Tapon, N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. CB 2007, 17, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Minakhina, S.; Steward, R. Melanotic mutants in Drosophila: Pathways and phenotypes. Genetics 2006, 174, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Cappucci, U.; Proietti, M.; Casale, A.M.; Schiavo, S.; Chiavarini, S.; Accardo, S.; Manzo, S.; Piacentini, L. Assessing genotoxic effects of plastic leachates in Drosophila melanogaster. Chemosphere 2024, 361, 142440. [Google Scholar] [CrossRef] [PubMed]
- Urbisz, A.Z.; Małota, K.; Chajec, L.; Marta, K.; Sawadro, M.K. Size-dependent and sex-specific negative effects of micro- and nano-sized polystyrene particles in the terrestrial invertebrate model Drosophila melanogaster. Micron 2024, 176, 103560. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Larschan, E.N. Dosage Compensation: How to Be Compensated…Or Not? Curr. Biol. CB 2019, 29, R1229–R1231. [Google Scholar] [CrossRef] [PubMed]
- Catalán, A.; Hutter, S.; Parsch, J. Population and sex differences in Drosophila melanogaster brain gene expression. BMC Genom. 2012, 13, 654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Ye, Y.; Rihan, N.; Jiang, Q.; Liu, X.; Zhao, Y.; Che, X. Polystyrene nanoplastics decrease nutrient accumulation, disturb sex hormones, and inhibit reproductive development in juvenile Macrobrachium nipponense. Sci. Total Environ. 2023, 891, 164481. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xian, H.; Ye, R.; Zhong, Y.; Liang, B.; Huang, Y.; Dai, M.; Guo, J.; Tang, S.; Ren, X.; et al. Gender-specific effects of polystyrene nanoplastic exposure on triclosan-induced reproductive toxicity in zebrafish (Danio rerio). Sci. Total Environ. 2024, 932, 172876. [Google Scholar] [CrossRef] [PubMed]
- Shaposhnikov, M.; Proshkina, E.; Shilova, L.; Zhavoronkov, A.; Moskalev, A. Lifespan and Stress Resistance in Drosophila with Overexpressed DNA Repair Genes. Sci. Rep. 2015, 5, 15299. [Google Scholar] [CrossRef]
- Mituzaite, J.; Petersen, R.; Claridge-Chang, A.; Baines, R.A. Characterization of Seizure Induction Methods in Drosophila. eNeuro 2021, 8, ENEURO.0079-21.2021. [Google Scholar] [CrossRef]
- Iuso, A.; Sibon, O.C.; Gorza, M.; Heim, K.; Organisti, C.; Meitinger, T.; Prokisch, H. Impairment of Drosophila orthologs of the human orphan protein C19orf12 induces bang sensitivity and neurodegeneration. PLoS ONE 2014, 9, e89439. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.D.; Becnel, J.; Pandey, U.B. Methods to assay Drosophila behavior. J. Vis. Exp. JoVE 2012, 61, 3795. [Google Scholar] [CrossRef]
- Rera, M.; Bahadorani, S.; Cho, J.; Koehler, C.L.; Ulgherait, M.; Hur, J.H.; Ansari, W.S.; Lo, T., Jr.; Jones, D.L.; Walker, D.W. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011, 14, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Staats, S.; Lüersen, K.; Wagner, A.E.; Rimbach, G. Drosophila melanogaster as a Versatile Model Organism in Food and Nutrition Research. J. Agric. Food Chem. 2018, 66, 3737–3753. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.R.; Escobar, B.; Vales, G.; Marcos, R. Genotoxic testing of titanium dioxide anatase nanoparticles using the wing-spot test and the comet assay in Drosophila. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 778, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2^DDCT method. Methods 2001, 25, 402–440. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Mini mum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 61122. [Google Scholar] [CrossRef] [PubMed]
- Ponton, F.; Chapuis, M.P.; Pernice, M.; Sword, G.A.; Simpson, S.J. Evaluation of potential reference genes for reverse transcription-qPCR studies of physiological responses in Drosophila melanogaster. J. Insect Physiol. 2011, 57, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Ihry, R.J.; Sapiro, A.L.; Bashirullah, A. Translational control by the DEAD Box RNA helicase belle regulates ecdysone-triggered transcriptional cascades. PLoS Genet. 2012, 8, e1003085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, P.; Yang, X.; Sun, L.; Han, X.; Xu, L.; Gu, W.; Zhang, M. Effects of cadmium on oxidative stress and cell apoptosis in Drosophila melanogaster larvae. Sci. Rep. 2022, 12, 4762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Florentin, A.; Arama, E. Caspase levels and execution efficiencies determine the apoptotic potential of the cell. J. Cell Biol. 2012, 196, 513–527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.N.; Nguyen, N.; Aghazarian, M.; Tan, Y.; Sevrioukov, E.A.; Mabuchi, M.; Tang, W.; Monserrate, J.P.; White, K.; Brachmann, C.B. grim promotes programmed cell death of Drosophila microchaete glial cells. Mech Dev. 2010, 127, 407–417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, Y.; Yamada-Mabuchi, M.; Arya, R.; St Pierre, S.; Tang, W.; Tosa, M.; Brachmann, C.; White, K. Coordinated expression of cell death genes regulates neuroblast apoptosis. Development 2011, 138, 2197–2206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moon, N.S.; Di Stefano, L.; Morris, E.J.; Patel, R.; White, K.; Dyson, N.J. E2F and p53 induce apoptosis independently during Drosophila development but intersect in the context of DNA damage. PLoS Genet. 2008, 4, e1000153. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Møller, P.; Gajski, G.; Vodenková, S.; Abdulwahed, A.; Anderson, D.; Bankoglu, E.E.; Bonassi, S.; Boutet-Robinet, E.; Brunborg, G.; et al. Measuring DNA modifications with the comet assay: A compendium of protocols. Nat. Protoc. 2023, 18, 929–989. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Aloisi, M.; Bonfigli, A.; Colafarina, S.; Zarivi, O.; Aimola, P.; Vecchiotti, G.; Arrizza, L.; Di Cola, A.; Cesare, P. Particle Debris Generated from Passenger Tires Induces Morphological and Gene Expression Alterations in the Macrophages Cell Line RAW 264.7. Nanomaterials 2023, 13, 756. [Google Scholar] [CrossRef]
- Gnocchini, E.; Pilesi, E.; Schiano, L.; Vernì, F. Vitamin B6 Deficiency Promotes Loss of Heterozygosity (LOH) at the Drosophila warts (wts) Locus. Int. J. Mol. Sci. 2022, 23, 6087. [Google Scholar] [CrossRef]



| Animal Models | Particle Type and Size | Outcomes | References |
|---|---|---|---|
| Daphnia magna | Polystyrene, 53 nm to 200 nm | Reduced life-time and survival, decreased size, and decreased lipid content. | [39,40,41] |
| Drosophila melanogaster | Polystyrene and polyethylene terephthalate, 10 nm to 800 μm | Oxidative stress, genotoxicity, locomotor activity, egg deposition. | [42,43,44] |
| Danio rerio | Polystyrene, 70 nm to 10 μm | Oxidative stress, metabolic disorders, intestinal inflammation, development delay, and abnormalities. | [45,46,47] |
| Mus musculus | Polystyrene, 500 nm to 50 μm | Oxidative stress, metabolic disorders, dysbiosis, hepatic accumulation, inflammation in kidney and liver, reduction of intestinal mucosa. | [48,49,50,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloisi, M.; Grifoni, D.; Zarivi, O.; Colafarina, S.; Morciano, P.; Poma, A.M.G. Plastic Fly: What Drosophila melanogaster Can Tell Us about the Biological Effects and the Carcinogenic Potential of Nanopolystyrene. Int. J. Mol. Sci. 2024, 25, 7965. https://doi.org/10.3390/ijms25147965
Aloisi M, Grifoni D, Zarivi O, Colafarina S, Morciano P, Poma AMG. Plastic Fly: What Drosophila melanogaster Can Tell Us about the Biological Effects and the Carcinogenic Potential of Nanopolystyrene. International Journal of Molecular Sciences. 2024; 25(14):7965. https://doi.org/10.3390/ijms25147965
Chicago/Turabian StyleAloisi, Massimo, Daniela Grifoni, Osvaldo Zarivi, Sabrina Colafarina, Patrizia Morciano, and Anna Maria Giuseppina Poma. 2024. "Plastic Fly: What Drosophila melanogaster Can Tell Us about the Biological Effects and the Carcinogenic Potential of Nanopolystyrene" International Journal of Molecular Sciences 25, no. 14: 7965. https://doi.org/10.3390/ijms25147965








