Upregulated Nuclear Expression of Soluble Epoxide Hydrolase Predicts Poor Outcome in Breast Cancer Patients: Importance of the Digital Pathology Approach
Abstract
1. Introduction
2. Results
2.1. Patient Cohort and Clinicopathological Characteristics
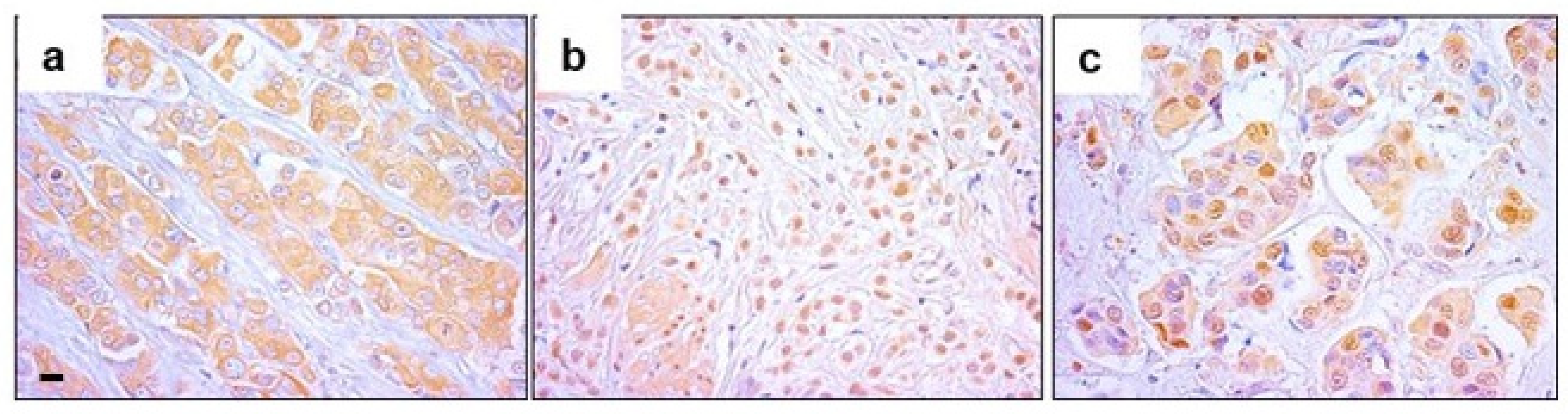
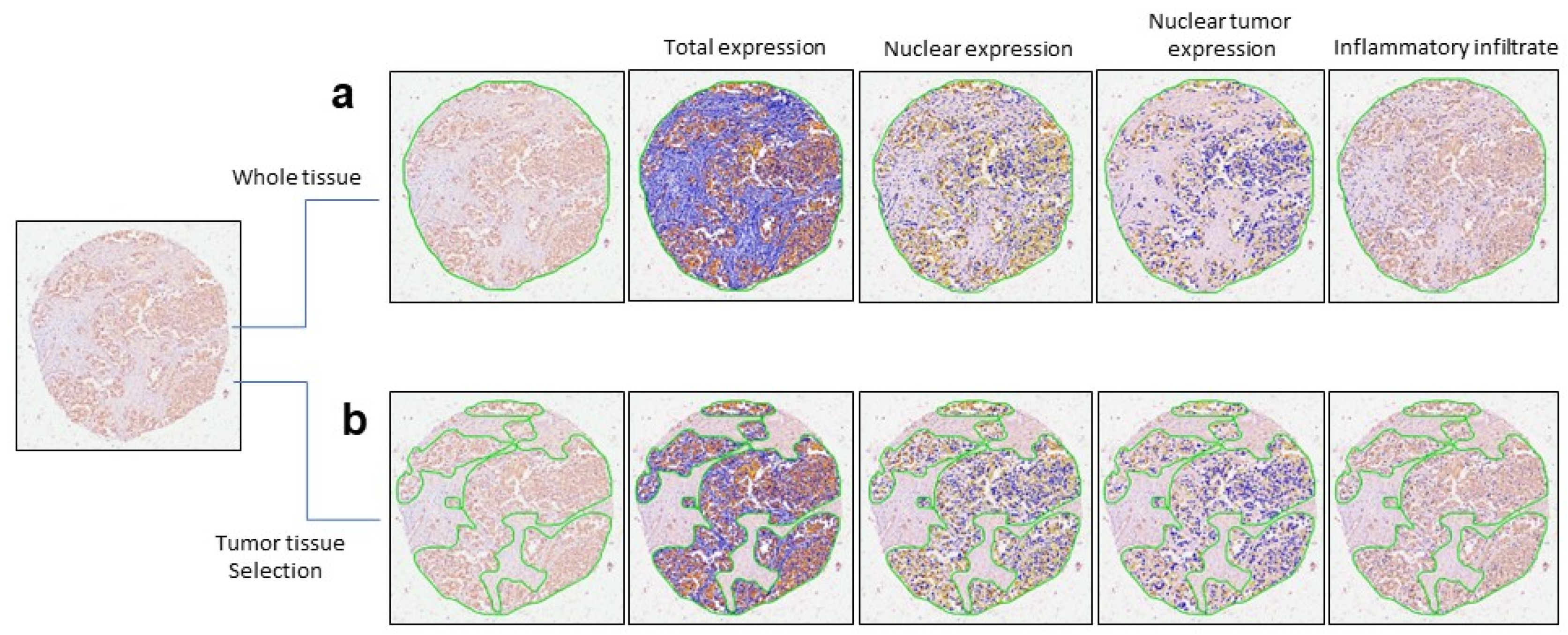
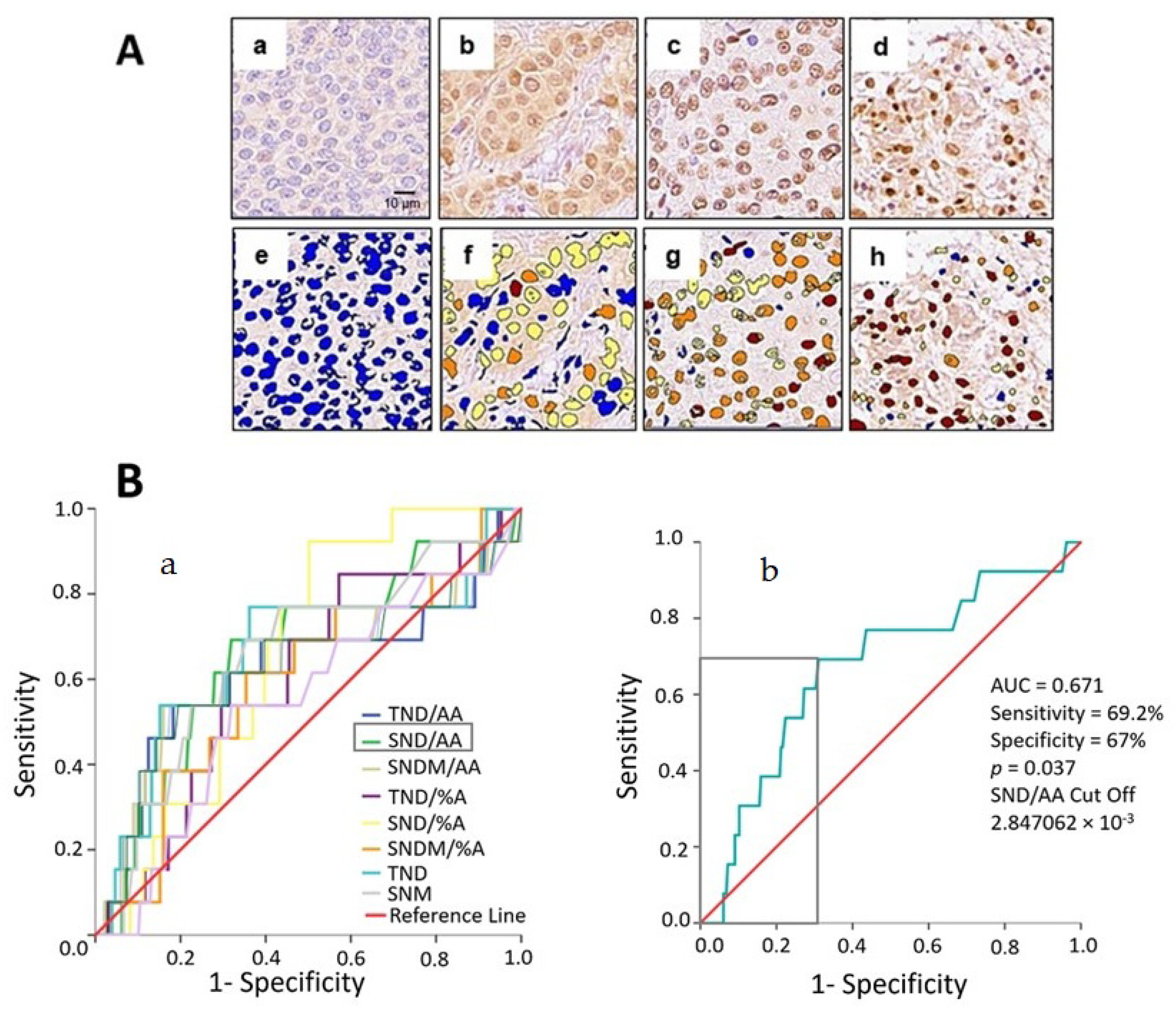
2.2. Nuclear EPHX2 Expression in BC and Digital Pathology Approach
2.3. Color Markup Image of Nuclear Staining Intensity and Biomarker Cutoff
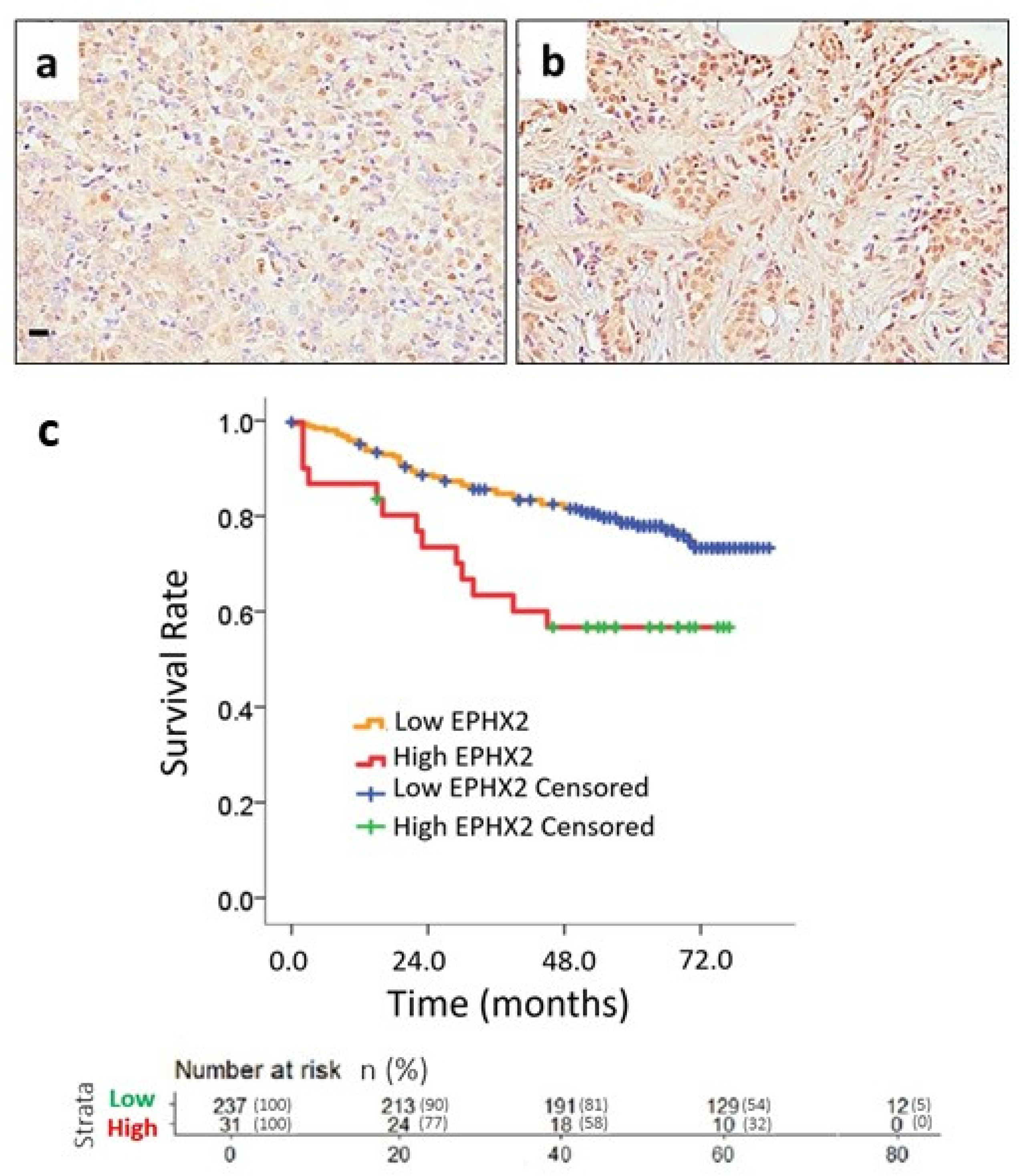
2.4. Uni- and Multivariate Survival Analysis
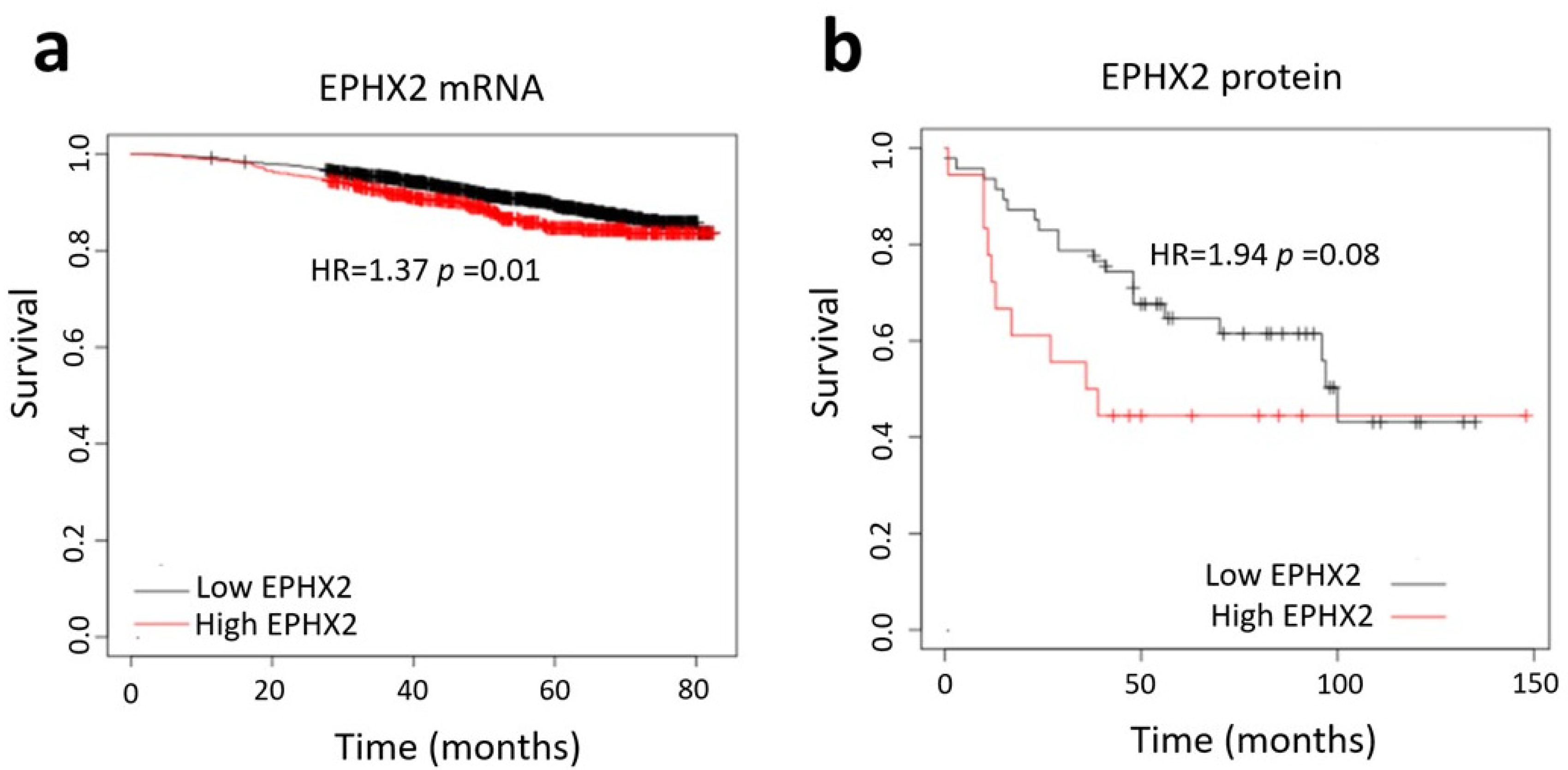
2.5. Database Analysis
3. Discussion
4. Material and Methods
4.1. Patient Selection
4.2. Tissue Microarray
4.3. Immunohistochemical Analysis
4.4. Digital Pathology Analysis and Automated Image Quantification
4.5. Database Search
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Wishart, G.C.; Bajdik, C.D.; Azzato, E.M.; Dicks, E.; Greenberg, D.C.; Rashbass, J.; Caldas, C.; Pharoah, P.D.P. A population-based validation of the prognostic model PREDICT for early breast cancer. Eur. J. Surg. Oncol. 2011, 37, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Monk, J.M.; Robinson, L.E.; Mourtzakis, M. The integrative role of leptin, oestrogen and the insulin family in obesity-associated breast cancer: Potential effects of exercise. Obes. Rev. 2015, 16, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, Y.; Lu, X.; Han, W. Chimeric antigen receptors modified T-cells for cancer therapy. J. Natl. Cancer Inst. 2016, 108, djv439. [Google Scholar] [CrossRef]
- Duan, S.; Buxton, I.L.O. Evolution of Medical Approaches and Prominent Therapies in Breast Cancer. Cancers 2022, 14, 2450. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Yépez, S.; Tirado-Rodriguez, A.B.; Hankinson, O. Role of diets rich in omega-3 and omega-6 in the development of cancer. Bol. Med. Hosp. Infant. Mex. 2016, 73, 446–456. [Google Scholar] [CrossRef]
- Panigrahy, D.; Greene, E.R.; Pozzi, A.; Wang, D.W.; Zeldin, D.C. EET signaling in cancer. Cancer Metastasis Rev. 2011, 30, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Fretland, A.J.; Omiecinski, C.J. Epoxide hydrolases: Biochemistry and molecular biology. Chem. Biol. Interact. 2000, 129, 41–59. [Google Scholar] [CrossRef]
- Newman, J.W.; Morisseau, C.; Harris, T.R.; Hammock, B.D. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc. Natl. Acad. Sci. USA 2003, 100, 1558–1563. [Google Scholar] [CrossRef]
- Gomez, G.A.; Morisseau, C.; Hammock, B.D.; Christianson, D.W. Structure of human epoxide hydrolase reveals mechanistic inferences on bifunctional catalysis in epoxide and phosphate ester hydrolysis. Biochemistry 2004, 43, 4716–4723. [Google Scholar] [CrossRef] [PubMed]
- Enayetallah, A.E.; French, R.A.; Grant, D.F. Distribution of soluble epoxide hydrolase, cytochrome P450 2C8, 2C9 and 2J2 in human malignant neoplasms. J. Mol. Histol. 2006, 37, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, D.; Edin, M.L.; Lee, C.R.; Huang, S.; Bielenberg, D.R.; Butterfield, C.E.; Barnés, C.M.; Mammoto, A.; Mammoto, T.; Luria, A.; et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J. Clin. Investig. 2012, 122, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Li, H.-T.; Yang, Y.; Hussain, S.; Zheng, C.-H.; Xia, J.; Chen, Y. Identification of breast cancer candidate genes using gene co-expression and protein-protein interaction information. Oncotarget 2016, 7, 36092–36100. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, M.; Tan, Q.; Kruse, T.A. Gene expression meta-analysis identifies chromosomal regions and candidate genes involved in breast cancer metastasis. Breast Cancer Res. Treat. 2009, 113, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Su, B.; Yu, P.; He, J.; Meng, L.; Xiao, Q.; Sun, J.; Zhou, K.; Xue, Y.; et al. Transcriptome-wide analysis and modelling of prognostic alternative splicing signatures in invasive breast cancer: A prospective clinical study. Sci. Rep. 2020, 10, 16504. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, D.; Dou, X.; Niu, N.; Huang, W.; Bai, J.; Zhang, G. Elevated 14,15- epoxyeicosatrienoic acid by increasing of cytochrome P450 2C8, 2C9 and 2J2 and decreasing of soluble epoxide hydrolase associated with aggressiveness of human breast cancer. BMC Cancer 2014, 14, 841. [Google Scholar] [CrossRef]
- Tauber, Z.; Koleckova, M.; Cizkova, K. Peroxisome proliferator-activated receptor ɑ (PPARɑ)–cytochrome P450 epoxygenases-soluble epoxide hydrolase axis in ER + PR + HER2− breast cancer. Med. Mol. Morphol. 2020, 53, 141–148. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Breast Cancer: A Molecularly Heterogenous Disease Needing Subtype-Specific Treatments. Med. Sci. 2020, 8, 18. [Google Scholar] [CrossRef]
- Frezza, C. Metabolism and cancer: The future is now. Br. J. Cancer 2020, 122, 133–135. [Google Scholar] [CrossRef]
- Ngoi, N.Y.L.; Eu, J.Q.; Hirpara, J.; Wang, L.; Lim, J.S.J.; Lee, S.-C.; Lim, Y.-C.; Pervaiz, S.; Goh, B.C.; Wong, A.L.A. Targeting Cell Metabolism as Cancer Therapy. Antioxid. Redox Signal. 2020, 32, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Munir, R.; Lisec, J.; Swinnen, J.V.; Zaidi, N. Lipid metabolism in cancer cells under metabolic stress. Br. J. Cancer 2019, 120, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Enayetallah, A.E.; French, R.A.; Barber, M.; Grant, D.F. Cell-specific Subcellular Localization of Soluble Epoxide Hydrolase in Human Tissues. J. Histochem. Cytochem. 2006, 54, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zhan, K.; Bai, Y.; Liao, S.; Chen, H.; Kuang, L.; Luo, Q.; Lv, L.; Qiu, L.; Mei, Z. Identification and validation of EPHX2 as a prognostic biomarker in hepatocellular carcinoma. Mol. Med. Rep. 2021, 24, 650. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-S.; Zhao, H.; Xu, C.-X.; Xie, P.-B.; Wang, W.; Yang, Y.-Y.; Lee, W.-H.; Jin, Y.; Zhou, H.-Q. Clinical significance of EPHX2 deregulation in prostate cancer. Asian J. Androl. 2021, 23, 109. [Google Scholar] [CrossRef] [PubMed]
- Vainio, P.; Gupta, S.; Ketola, K.; Mirtti, T.; Mpindi, J.-P.; Kohonen, P.; Fey, V.; Perälä, M.; Smit, F.; Verhaegh, G.; et al. Arachidonic Acid Pathway Members PLA2G7, HPGD, EPHX2, and CYP4F8 Identified as Putative Novel Therapeutic Targets in Prostate Cancer. Am. J. Pathol. 2011, 178, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Chahed, S.; Tabet, G.; Yang, J.; Morisseau, C.; Griffey, S.; Hammock, B.D.; Haj, F.G. Effects of Soluble Epoxide Hydrolase Deficiency on Acute Pancreatitis in Mice. PLoS ONE 2014, 9, e113019. [Google Scholar] [CrossRef]
- Kesavan, R.; Frömel, T.; Zukunft, S.; Brüne, B.; Weigert, A.; Wittig, I.; Popp, R.; Fleming, I. The Consequences of Soluble Epoxide Hydrolase Deletion on Tumorigenesis and Metastasis in a Mouse Model of Breast Cancer. Int. J. Mol. Sci. 2021, 22, 7120. [Google Scholar] [CrossRef]
- Shi, Z.; He, Z.; Wang, D.W. CYP450 Epoxygenase Metabolites, Epoxyeicosatrienoic Acids, as Novel Anti-Inflammatory Mediators. Molecules 2022, 27, 3873. [Google Scholar] [CrossRef]
- Mullen, R.T.; Trelease, R.N.; Duerk, H.; Arand, M.; Hammock, B.D.; Oesch, F.; Grant, D.F. Differential subcellular localization of endogenous and transfected soluble epoxide hydrolase in mammalian cells: Evidence for isozyme variants. FEBS Lett. 1999, 445, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Enayetallah, A.E.; French, R.A.; Thibodeau, M.S.; Grant, D.F. Distribution of Soluble Epoxide Hydrolase and of Cytochrome P450 2C8, 2C9, and 2J2 in Human Tissues. J. Histochem. Cytochem. 2004, 52, 447–454. [Google Scholar] [CrossRef]
- Pacifici, G.M.; Colizzi, C.; Giuliani, L.; Rane, A. Nuclear Epoxide Hydrolase in the Human Fetal and Adult Liver. Pharmacology 1984, 28, 321–328. [Google Scholar] [CrossRef]
- Pacifici, G.M.; Lindberg, B.; Glaumann, H.; Rane, A. Styrene oxide metabolism in rhesus monkey liver: Enzyme activities in subcellular fractions and in isolated hepatocytes. J. Pharmacol. Exp. Ther. 1983, 226, 869–875. [Google Scholar]
- Brock, T.G.; Maydanski, E.; McNish, R.W.; Peters-Golden, M. Co-localization of Leukotriene A4Hydrolase with 5-Lipoxygenase in Nuclei of Alveolar Macrophages and Rat Basophilic Leukemia Cells but Not Neutrophils. J. Biol. Chem. 2001, 276, 35071–35077. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Hammock, B.D.; Newman, J.W.; Meerarani, P.; Toborek, M.; Hennig, B. Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. J. Am. Coll. Nutr. 2003, 22, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.; Ordóñez-Morán, P.; Allegrucci, C. Challenges for Triple Negative Breast Cancer Treatment: Defeating Heterogeneity and Cancer Stemness. Cancers 2022, 14, 4280. [Google Scholar] [CrossRef]
- Yang, Y.M.; Sun, D.; Kandhi, S.; Froogh, G.; Zhuge, J.; Huang, W.; Hammock, B.D.; Huang, A. Estrogen-dependent epigenetic regulation of soluble epoxide hydrolase via DNA methylation. Proc. Natl. Acad. Sci. USA 2018, 115, 613–618. [Google Scholar] [CrossRef]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial intelligence in digital pathology—New tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef]
- Zhang, G.; Panigrahy, D.; Mahakian, L.M.; Yang, J.; Liu, J.-Y.; Stephen Lee, K.S.; Wettersten, H.I.; Ulu, A.; Hu, X.; Tam, S.; et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 6530–6535. [Google Scholar] [CrossRef]
- Zhang, G.; Panigrahy, D.; Hwang, S.H.; Yang, J.; Mahakian, L.M.; Wettersten, H.I.; Liu, J.-Y.; Wang, Y.; Ingham, E.S.; Tam, S.; et al. Dual inhibition of cyclooxygenase-2 and soluble epoxide hydrolase synergistically suppresses primary tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2014, 111, 11127–11132. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Yepez, S.; Tirado-Rodriguez, A.; Montecillo-Aguado, M.R.; Yang, J.; Hammock, B.D.; Hankinson, O. Aryl Hydrocarbon Receptor-Dependent inductions of omega-3 and omega-6 polyunsaturated fatty acid metabolism act inversely on tumor progression. Sci. Rep. 2020, 10, 7843. [Google Scholar] [CrossRef]
- Kelly, A.G.; Wang, W.; Rothenberger, E.; Yang, J.; Gilligan, M.M.; Kipper, F.C.; Attaya, A.; Gartung, A.; Hwang, S.H.; Gillespie, M.J.; et al. Enhancing cancer immunotherapy via inhibition of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA 2024, 121, e2314085121. [Google Scholar] [CrossRef] [PubMed]
- Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Antonio-Andres, G.; Morales-Martinez, M.; Tong, Z.; Yang, J.; Hammock, B.D.; Hernandez-Pando, R.; Huerta-Yepez, S. Omega-6 Polyunsaturated Fatty Acids Enhance Tumor Aggressiveness in Experimental Lung Cancer Model: Important Role of Oxylipins. Int. J. Mol. Sci. 2022, 23, 6179. [Google Scholar] [CrossRef] [PubMed]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-Tile. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef]
| Variable n (%) | N | EPHX2 Low (n = 234) | EPHX2 High (n = 33) | Total (n = 267) | p-Value |
|---|---|---|---|---|---|
| Age | 267 | 0.710 C | |||
| <52 yr | 111 (47.4%) | 14 (42.4%) | 125 (46.8%) | ||
| ≥52 yr | 123 (52.6%) | 19 (57.6%) | 142 (53.2%) | ||
| Histologic Grade | 265 | 0.751 F | |||
| I | 46 (19.8%) | 8 (24.2%) | 54 (20.4%) | ||
| II | 124 (53.4%) | 18 (54.5%) | 142 (53.6%) | ||
| III | 62 (26.7%) | 7 (21.2%) | 69 (26.0%) | ||
| Nuclear Grade | 265 | 0.505 F | |||
| 1 | 19 (8.2%) | 4 (12.1%) | 23 (8.7%) | ||
| 2 | 145(62.5%) | 17 (51.5%) | 162 (61.1%) | ||
| 3 | 68 (29.3%) | 12 (36.4%) | 80 (30.2%) | ||
| Border Type | 267 | 0.030 F* | |||
| Non- | 172 (73.5%) | 30 (90.9%) | 202 (75.7%) | ||
| Infiltrative | 62 (26.5%) | 3 (9.1%) | 65 (24.3%) | ||
| Tumor Size | 267 | 0.090 C* | |||
| ≤2 cm | 94 (40.2%) | 19 (57.6%) | 113 (42.3%) | ||
| >2 cm | 140 (59.8%) | 14 (42.4%) | 154 (57.7%) | ||
| ALND | 267 | 1.000 C | |||
| No | 84 (35.9%) | 12 (36.4%) | 96 (36.0%) | ||
| Yes | 150 (64.1%) | 21 (63.6%) | 171 (64.0%) | ||
| ER Status | 267 | 0.109 C | |||
| Negative | 70 (29.9%) | 15 (45.5%) | 85 (31.8%) | ||
| Positive | 164 (70.1%) | 18 (54.5%) | 182 (68.2%) | ||
| PR Status | 267 | 1.000 C | |||
| Negative | 98 (41.9%) | 14 (42.4%) | 112 (41.9%) | ||
| Positive | 136 (58.1%) | 19 (57.6%) | 155 (58.1%) | ||
| HER2 Status | 267 | 1.000 C | |||
| Negative | 192 (82.8%) | 27 (81.8%) | 219 (82.6%) | ||
| Positive | 40 (17.2%) | 6 (18.2%) | 46 (17.4%) | ||
| Molecular Subtype | 260 | 0.330 F | |||
| LUM A + B | 163 (71.2%) | 18 (58.1%) | 181 (69.6%) | ||
| HER2 | 23 (10.0%) | 4 (12.9%) | 27 (10.4%) | ||
| TNBC | 43 (18.8%) | 9 (29.0%) | 52 (20.0%) | ||
| AJCC-PS | 264 | 0.323 F | |||
| I | 86 (37.2%) | 12 (36.4%) | 98 (37.1%) | ||
| II | 90 (39.0%) | 10 (30.3%) | 100 (37.9%) | ||
| III | 51 (22.1%) | 9 (27.3%) | 60 (22.7%) | ||
| IV | 4 (1.7%) | 2 (6.1%) | 6 (2.3%) | ||
| Recurrence | 267 | 0.506 C | |||
| No | 183 (78.2%) | 24 (72.7%) | 207 (77.5%) | ||
| Yes | 51 (21.8%) | 9 (27.3%) | 60 (22.5%) |
| Univariate Analysis | ||||
|---|---|---|---|---|
| Variable | Category | HR | 95% CI | p-Value |
| Age (Years) | ≥52 vs. <52 | 1.100 | 0.689–1.755 | 0.690 |
| Histologic Grade | III vs. I + II | 1.262 | 0.750–2.123 | 0.381 |
| Nuclear Grade | 3 vs. 1 + 2 | 1.823 | 1.127–2.948 | 0.014 * |
| Border Type | Infiltrative vs. Non | 1.333 | 0.742–2.393 | 0.336 |
| Tumor Size | >2 cm vs. ≤2 | 1.638 | 1.002–2.680 | 0.049 * |
| ALND | Yes vs. No | 0.481 | 0.302–0.766 | 0.002 * |
| ER Status | + vs. − | 0.558 | 0.348–0.894 | 0.015 * |
| PR Status | + vs. − | 0.561 | 0.352–0.894 | 0.015 * |
| HER2 Status | + vs. − | 0.894 | 0.469–1.701 | 0.732 |
| Molecular Subtype | TNBC vs. LUM A + B | 1.815 | 1.055–3.123 | 0.031 * |
| HER2 vs. LUM A + B | 2.054 | 1.029–4.097 | 0.041 * | |
| AJCC Prognostic Stage | II vs. I | 2.281 | 1.172–4.441 | 0.015 * |
| III vs. I | 5.778 | 2.987–11.180 | <0.001 * | |
| IV vs. I | 5.216 | 1.173–23.189 | 0.030 * | |
| Recurrence | Yes vs. No | 3.939 | 2.465–6.294 | <0.001 * |
| EPHX2 | High vs. Low | 2.316 | 1.261–4.252 | 0.007 * |
| Multivariate Analysis | ||||
| Variable | Category | HR | 95% CI | p-Value |
| Nuclear Grade | 3 vs. 1 + 2 | 1.412 | 0.801–2.487 | 0.232 |
| Tumor Size | >2 cm vs. ≤2 | 1.399 | 0.734–2.665 | 0.308 |
| ALND | Yes vs. No | 0.629 | 0.284–1.392 | 0.253 |
| ALND (Number) | ≥10 vs. <10 | 0.723 | 0.318–1.645 | 0.439 |
| ER Status | + vs. − | 0.041 | 0.005–0.357 | 0.004 * |
| PR Status | + vs. − | 0.772 | 0.328–1.816 | 0.553 |
| Molecular Subtype | TNBC vs. LUM | 0.712 | 0.305–1.661 | 0.432 |
| HER2 vs. LUM | 14.855 | 1.446–152.623 | 0.023 * | |
| AJCC Prognostic Stage | II vs. I | 2.008 | 0.904–4.458 | 0.087 |
| III vs. I | 4.295 | 1.719–10.731 | 0.002 * | |
| IV vs. I | 1.375 | 0.243–7.786 | 0.719 | |
| Recurrence | Yes vs. No | 3.714 | 2.167–6.365 | <0.001 * |
| EPHX2 | High vs. Low | 3.483 | 1.804–6.724 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montecillo-Aguado, M.; Soca-Chafre, G.; Antonio-Andres, G.; Morales-Martinez, M.; Tirado-Rodriguez, B.; Rocha-Lopez, A.G.; Hernandez-Cueto, D.; Sánchez-Ceja, S.G.; Alcala-Mota-Velazco, B.; Gomez-Garcia, A.; et al. Upregulated Nuclear Expression of Soluble Epoxide Hydrolase Predicts Poor Outcome in Breast Cancer Patients: Importance of the Digital Pathology Approach. Int. J. Mol. Sci. 2024, 25, 8024. https://doi.org/10.3390/ijms25158024
Montecillo-Aguado M, Soca-Chafre G, Antonio-Andres G, Morales-Martinez M, Tirado-Rodriguez B, Rocha-Lopez AG, Hernandez-Cueto D, Sánchez-Ceja SG, Alcala-Mota-Velazco B, Gomez-Garcia A, et al. Upregulated Nuclear Expression of Soluble Epoxide Hydrolase Predicts Poor Outcome in Breast Cancer Patients: Importance of the Digital Pathology Approach. International Journal of Molecular Sciences. 2024; 25(15):8024. https://doi.org/10.3390/ijms25158024
Chicago/Turabian StyleMontecillo-Aguado, Mayra, Giovanny Soca-Chafre, Gabriela Antonio-Andres, Mario Morales-Martinez, Belen Tirado-Rodriguez, Angelica G. Rocha-Lopez, Daniel Hernandez-Cueto, Sandra G. Sánchez-Ceja, Berenice Alcala-Mota-Velazco, Anel Gomez-Garcia, and et al. 2024. "Upregulated Nuclear Expression of Soluble Epoxide Hydrolase Predicts Poor Outcome in Breast Cancer Patients: Importance of the Digital Pathology Approach" International Journal of Molecular Sciences 25, no. 15: 8024. https://doi.org/10.3390/ijms25158024
APA StyleMontecillo-Aguado, M., Soca-Chafre, G., Antonio-Andres, G., Morales-Martinez, M., Tirado-Rodriguez, B., Rocha-Lopez, A. G., Hernandez-Cueto, D., Sánchez-Ceja, S. G., Alcala-Mota-Velazco, B., Gomez-Garcia, A., Gutiérrez-Castellanos, S., & Huerta-Yepez, S. (2024). Upregulated Nuclear Expression of Soluble Epoxide Hydrolase Predicts Poor Outcome in Breast Cancer Patients: Importance of the Digital Pathology Approach. International Journal of Molecular Sciences, 25(15), 8024. https://doi.org/10.3390/ijms25158024





