Revealing the Hidden Impacts: Insights into Biological Aging and Long-Term Effects in Pauci- and Asymptomatic COVID-19 Healthcare Workers
Abstract
:1. Introduction
2. Results
2.1. Post-COVID Syndrome (PCS) and Symptom Prevalence
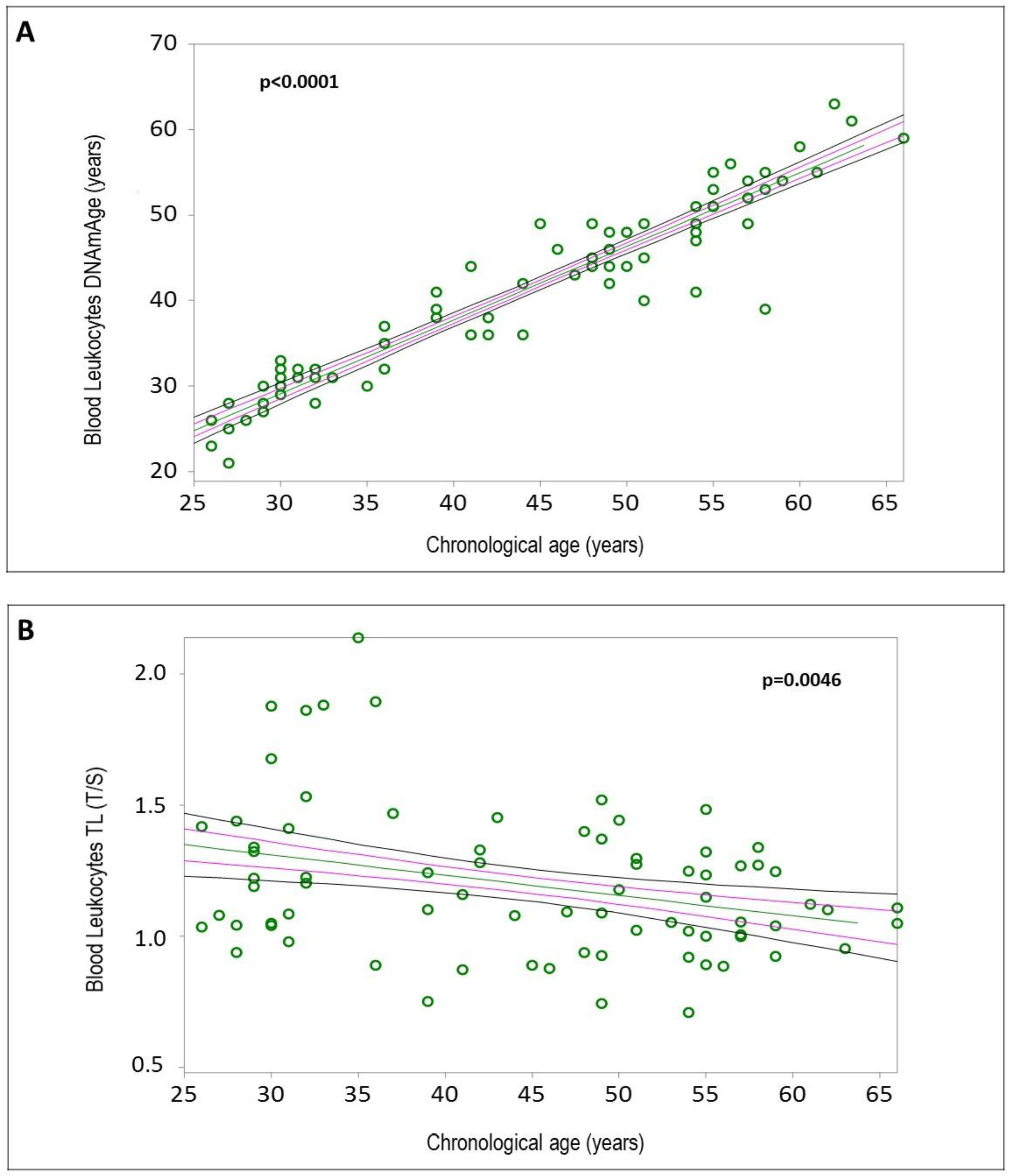
2.2. Blood Leukocyte Biological Age
2.3. Determinants of Blood Leukocyte DNAmAge and TL
2.4. Biological Age of Blood Leukocytes, IS Cells, and NCs
2.5. Correlations between Biological Aging Indicators
2.6. Comparison of Biological Aging in HCWs and COPD Patients
3. Discussion
3.1. PCS and Symptom Prevalence
3.2. Determinants of Increased Blood Leukocyte DNAmAge
3.2.1. Sex-Related DNAmAge Differences
3.2.2. Impact of SARS-CoV-2 Infection
3.2.3. Chronic Diseases and DNAmAge
3.2.4. Lung Function and DNAmAge
3.2.5. Lipid Levels and DNAmAge
3.2.6. Blood Glucose and DNAmAge
3.2.7. Work Capacity and DNAmAge
3.2.8. Lymphocyte Counts and DNAmAge
3.2.9. Hemoglobin Levels and DNAmAge
3.2.10. HR, HRV, and DNAmAge
3.3. Determinants of Shorter Blood Leukocyte TL
3.3.1. WAI
3.3.2. LDL Levels and Cardiovascular Disease
3.3.3. Blood Leukocyte TL and Job Position
3.3.4. Lymphocyte Numbers
3.4. Biological Age of the Blood Leukocytes, IS Cells, and NCs Determined by DNAmAge and TL
3.4.1. Tissue-Specific Aging Rates
3.4.2. COVID-19 Impact on DNAmAge and TL
3.4.3. Biological Implications of Telomere Shortening in IS Cells
3.4.4. Epigenetic Aging in IS Cells, NCs, and Implications for Surrogate Tissue Use
3.5. Comparison of Biological Aging (AgeAcc and TL) in HCWs and COPD Patients
3.6. Limitations and Strengths
4. Materials and Methods
4.1. Study Design
4.2. Information Acquired through Questionnaires
4.3. Work Ability Assessment
4.4. Respiratory Function Tests
4.5. Assessment of Autonomic Cardiac Balance and HRV Parameters
4.6. Samples Collection and IS Procedure
4.7. Basic Biochemistry Analyses
4.8. DNA Extraction (from Biological Samples)
4.9. DNAmAge Analysis and AgeAcc Estimation
4.10. TL Analysis
4.11. Statistical Analyses
5. Conclusions
5.1. Persistent Symptoms
5.2. Impact of SARS-CoV-2 Infection Duration on Biological Aging
5.3. Key Determinants of Increased Biological Aging
5.4. Biological Aging in Target Tissues of SARS-CoV-2 Infection
5.5. Comparison with COPD Patients
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgeAcc | Age acceleration |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| COPD | Chronic obstructive pulmonary disease |
| CRP | C-reactive protein |
| CVDs | Cardiovascular diseases |
| DNAmAge | DNA methylation age |
| FEV1 | Forced expiratory volume in the 1st second |
| FVC | Forced vital capacity |
| GGT | Gamma glutamyl transferase |
| HAs | Healthcare assistants |
| HCWs | Healthcare workers |
| HDLs | High-density lipoproteins |
| HF | High frequency |
| HR | Heart rate |
| HRV | Heart rate variability |
| IL-6 | Interleukin 6 |
| IPAQs | International Physical Activity Questionnaires |
| IS | Induced sputum |
| LDLs | Low-density lipoproteins |
| LF | Low frequency |
| LTL | Leukocyte telomere length |
| NCs | Nasal cells |
| nHF | Normalized high frequency |
| nLF | Normalized low frequency |
| NSAID | Non-steroidal anti-inflammatory drug |
| PCS | Post-COVID syndrome |
| RMSSD | Root mean square of successive RR interval differences |
| RV | Residual volume |
| SDNN | Standard deviation of normal-to-normal R-R intervals |
| TL | Telomere length |
| TLC | Total lung capacity |
| VLF | Very low frequency |
| WAI | Work ability index |
References
- COVID-19 Cases|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 17 June 2024).
- Gorna, R.; MacDermott, N.; Rayner, C.; O’Hara, M.; Evans, S.; Agyen, L.; Nutland, W.; Rogers, N.; Hastie, C. Long COVID Guidelines Need to Reflect Lived Experience. Lancet 2021, 397, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef] [PubMed]
- Parotto, M.; Gyöngyösi, M.; Howe, K.; Myatra, S.N.; Ranzani, O.; Shankar-Hari, M.; Herridge, M.S. Post-Acute Sequelae of COVID-19: Understanding and Addressing the Burden of Multisystem Manifestations. Lancet Respir. Med. 2023, 11, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of Post-Acute COVID-19 Syndrome Symptoms at Different Follow-up Periods: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Deng, J.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Long-Term Consequences of Asymptomatic SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1613. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Hummel, T.; Hopkins, C.; Dibattista, M.; Menini, A.; Spinato, G.; Fabbris, C.; Emanuelli, E.; D’Alessandro, A.; Marzolino, R.; et al. High Prevalence of Long-Term Olfactory, Gustatory, and Chemesthesis Dysfunction in Post-COVID-19 Patients: A Matched Case-Control Study with One-Year Follow-up Using a Comprehensive Psychophysical Evaluation. Rhinology 2021, 59, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Guida, F.; Polesel, J.; Marcuzzo, A.V.; Capriotti, V.; D’Alessandro, A.; Zanelli, E.; Marzolino, R.; Lazzarin, C.; Antonucci, P.; et al. Sequelae in Adults at 12 Months after Mild-to-moderate Coronavirus Disease 2019 (COVID-19). Int. Forum Allergy Rhinol. 2021, 11, 1685–1688. [Google Scholar] [CrossRef] [PubMed]
- Andrei Appelt, P.; Taciana Sisconetto, A.; Baldo Sucupira, K.S.M.; Neto, E.d.M.; Chagas, T.d.J.; Bazan, R.; Moura Cabral, A.; Andrade, A.d.O.; de Souza, L.A.P.S.; José Luvizutto, G. Changes in Electrical Brain Activity and Cognitive Functions Following Mild to Moderate COVID-19: A One-Year Prospective Study After Acute Infection. Clin. EEG Neurosci. 2022, 53, 543–557. [Google Scholar] [CrossRef]
- Rank, A.; Tzortzini, A.; Kling, E.; Schmid, C.; Claus, R.; Löll, E.; Burger, R.; Römmele, C.; Dhillon, C.; Müller, K.; et al. One Year after Mild COVID-19: The Majority of Patients Maintain Specific Immunity, But One in Four Still Suffer from Long-Term Symptoms. J. Clin. Med. 2021, 10, 3305. [Google Scholar] [CrossRef]
- Maestre-Muñiz, M.M.; Arias, Á.; Mata-Vázquez, E.; Martín-Toledano, M.; López-Larramona, G.; Ruiz-Chicote, A.M.; Nieto-Sandoval, B.; Lucendo, A.J. Long-Term Outcomes of Patients with Coronavirus Disease 2019 at One Year after Hospital Discharge. J. Clin. Med. 2021, 10, 2945. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.D.M.; Foppiani, A.; Peretti, G.M.; Mangiavini, L.; Battezzati, A.; Bertoli, S.; Martinelli Boneschi, F.; Zuccotti, G.V. Long-Term Coronavirus Disease 2019 Complications in Inpatients and Outpatients: A One-Year Follow-up Cohort Study. Open Forum Infect. Dis. 2021, 8, ofab384. [Google Scholar] [CrossRef]
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year after Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. 2022, 74, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Zhang, L.; Elbéji, A.; Wilmes, P.; Oustric, P.; Staub, T.; Nazarov, P.V.; Ollert, M.; Fagherazzi, G. Long COVID Symptomatology After 12 Months and Its Impact on Quality of Life According to Initial Coronavirus Disease 2019 Disease Severity. Open Forum Infect. Dis. 2022, 9, ofac397. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, W.; Wang, T.; Ran, D.; Davalos, V.; Planas-Serra, L.; Pujol, A.; Esteller, M.; Wang, X.; Yu, H. Accelerated Biological Aging in COVID-19 Patients. Nat. Commun. 2022, 13, 2135. [Google Scholar] [CrossRef] [PubMed]
- Jylhävä, J.; Pedersen, N.L.; Hägg, S. Biological Age Predictors. eBioMedicine 2017, 21, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, S.; Campisi, M.; Tona, F.; Lin, C.D.; Iliceto, S. Exploring Epigenetic Age in Response to Intensive Relaxing Training: A Pilot Study to Slow Down Biological Age. Int. J. Environ. Res. Public Health 2019, 16, 3074. [Google Scholar] [CrossRef] [PubMed]
- Mongelli, A.; Barbi, V.; Gottardi Zamperla, M.; Atlante, S.; Forleo, L.; Nesta, M.; Massetti, M.; Pontecorvi, A.; Nanni, S.; Farsetti, A.; et al. Evidence for Biological Age Acceleration and Telomere Shortening in COVID-19 Survivors. Int. J. Mol. Sci. 2021, 22, 6151. [Google Scholar] [CrossRef] [PubMed]
- Franzen, J.; Nüchtern, S.; Tharmapalan, V.; Vieri, M.; Nikolić, M.; Han, Y.; Balfanz, P.; Marx, N.; Dreher, M.; Brümmendorf, T.H.; et al. Epigenetic Clocks Are Not Accelerated in COVID-19 Patients. Int. J. Mol. Sci. 2021, 22, 9306. [Google Scholar] [CrossRef] [PubMed]
- Campisi, M.; Liviero, F.; Maestrelli, P.; Guarnieri, G.; Pavanello, S. DNA Methylation-Based Age Prediction and Telomere Length Reveal an Accelerated Aging in Induced Sputum Cells Compared to Blood Leukocytes: A Pilot Study in COPD Patients. Front. Med. 2021, 8, 690312. [Google Scholar] [CrossRef]
- COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; National Institute for Health and Care Excellence: Clinical Guidelines; National Institute for Health and Care Excellence (NICE): London, UK, 2020; ISBN 978-1-4731-3943-5.
- Tudorache, E.; Fildan, A.P.; Frandes, M.; Dantes, E.; Tofolean, D.E. Aging and Extrapulmonary Effects of Chronic Obstructive Pulmonary Disease. CIA 2017, 12, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Buonsenso, D.; Carfi, A.; Malorni, W.; Group, T.L.C.K. study Long COVID: An Estrogen-Associated Autoimmune Disease? Cell Death Discov. 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Value Gender and Equity in the Global Health Workforce. Available online: https://www.who.int/activities/value-gender-and-equity-in-the-global-health-workforce (accessed on 19 June 2024).
- Khamsi, R. Rogue Antibodies Could Be Driving Severe COVID-19. Nature 2021, 590, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Ballouz, T.; Menges, D.; Anagnostopoulos, A.; Domenghino, A.; Aschmann, H.E.; Frei, A.; Fehr, J.S.; Puhan, M.A. Recovery and Symptom Trajectories up to Two Years after SARS-CoV-2 Infection: Population Based, Longitudinal Cohort Study. BMJ 2023, 381, e074425. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, C.; Zocchi, C.; Tassetti, L.; Silverii, M.V.; Amato, C.; Livi, L.; Giovannoni, L.; Verrillo, F.; Bartoloni, A.; Marcucci, R.; et al. Factors Associated with Persistence of Symptoms 1 Year after COVID-19: A Longitudinal, Prospective Phone-Based Interview Follow-up Cohort Study. Eur. J. Intern. Med. 2022, 97, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-de-las-Peñas, C.; Notarte, K.I.; Macasaet, R.; Velasco, J.V.; Catahay, J.A.; Ver, A.T.; Chung, W.; Valera-Calero, J.A.; Navarro-Santana, M. Persistence of Post-COVID Symptoms in the General Population Two Years after SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. J. Infect. 2024, 88, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Oblak, L.; van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A Systematic Review of Biological, Social and Environmental Factors Associated with Epigenetic Clock Acceleration. Ageing Res. Rev. 2021, 69, 101348. [Google Scholar] [CrossRef]
- Alberts, S.C.; Archie, E.A.; Gesquiere, L.R.; Altmann, J.; Vaupel, J.W.; Christensen, K. The Male-Female Health-Survival Paradox: A Comparative Perspective on Sex Differences in Aging and Mortality. In Sociality, Hierarchy, Health: Comparative Biodemography: A Collection of Papers; National Academies Press (US): Washington, DC, USA, 2014. [Google Scholar]
- Corley, M.J.; Pang, A.P.S.; Dody, K.; Mudd, P.A.; Patterson, B.K.; Seethamraju, H.; Bram, Y.; Peluso, M.J.; Torres, L.; Iyer, N.S.; et al. Genome-Wide DNA Methylation Profiling of Peripheral Blood Reveals an Epigenetic Signature Associated with Severe COVID-19. J. Leukoc. Biol. 2021, 110, 21–26. [Google Scholar] [CrossRef]
- Schäfer, A.; Baric, R.S. Epigenetic Landscape during Coronavirus Infection. Pathogens 2017, 6, 8. [Google Scholar] [CrossRef]
- Castro de Moura, M.; Davalos, V.; Planas-Serra, L.; Alvarez-Errico, D.; Arribas, C.; Ruiz, M.; Aguilera-Albesa, S.; Troya, J.; Valencia-Ramos, J.; Vélez-Santamaria, V.; et al. Epigenome-Wide Association Study of COVID-19 Severity with Respiratory Failure. eBioMedicine 2021, 66, 103339. [Google Scholar] [CrossRef]
- Menachery, V.D.; Schäfer, A.; Burnum-Johnson, K.E.; Mitchell, H.D.; Eisfeld, A.J.; Walters, K.B.; Nicora, C.D.; Purvine, S.O.; Casey, C.P.; Monroe, M.E.; et al. MERS-CoV and H5N1 Influenza Virus Antagonize Antigen Presentation by Altering the Epigenetic Landscape. Proc. Natl. Acad. Sci. USA 2018, 115, E1012–E1021. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Cantos, A.; Rodríguez-Centeno, J.; Barruz, P.; Alejos, B.; Saiz-Medrano, G.; Nevado, J.; Martin, A.; Gayá, F.; De Miguel, R.; Bernardino, J.I.; et al. Epigenetic Age Acceleration Changes 2 Years after Antiretroviral Therapy Initiation in Adults with HIV: A Substudy of the NEAT001/ANRS143 Randomised Trial. Lancet HIV 2021, 8, e197–e205. [Google Scholar] [CrossRef]
- Horvath, S.; Levine, A.J. HIV-1 Infection Accelerates Age According to the Epigenetic Clock. J. Infect. Dis. 2015, 212, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Stein, D.J.; Phillips, N.; Heany, S.J.; Kobor, M.S.; Lin, D.T.S.; Myer, L.; Zar, H.J.; Levine, A.J.; Hoare, J. Perinatally Acquired HIV Infection Accelerates Epigenetic Aging in South African Adolescents. AIDS 2018, 32, 1465. [Google Scholar] [CrossRef]
- Gale, C.R.; Marioni, R.E.; Harris, S.E.; Starr, J.M.; Deary, I.J. DNA Methylation and the Epigenetic Clock in Relation to Physical Frailty in Older People: The Lothian Birth Cohort 1936. Clin. Epigenet. 2018, 10, 101. [Google Scholar] [CrossRef]
- Dugué, P.-A.; Bassett, J.K.; Joo, J.E.; Jung, C.-H.; Ming Wong, E.; Moreno-Betancur, M.; Schmidt, D.; Makalic, E.; Li, S.; Severi, G.; et al. DNA Methylation-Based Biological Aging and Cancer Risk and Survival: Pooled Analysis of Seven Prospective Studies. Int. J. Cancer 2018, 142, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.D.; Jafari, N.; Hou, L.; Li, Y.; Stewart, J.D.; Zhang, G.; Lamichhane, A.; Manson, J.E.; Baccarelli, A.A.; Whitsel, E.A.; et al. A Longitudinal Study of DNA Methylation as a Potential Mediator of Age-Related Diabetes Risk. GeroScience 2017, 39, 475–489. [Google Scholar] [CrossRef]
- Roetker, N.S.; Pankow, J.S.; Bressler, J.; Morrison, A.C.; Boerwinkle, E. Prospective Study of Epigenetic Age Acceleration and Incidence of Cardiovascular Disease Outcomes in the ARIC Study (Atherosclerosis Risk in Communities). Circ. Genom. Precis. Med. 2018, 11, e001937. [Google Scholar] [CrossRef]
- Horvath, S.; Ritz, B.R. Increased Epigenetic Age and Granulocyte Counts in the Blood of Parkinson’s Disease Patients. Aging 2015, 7, 1130–1142. [Google Scholar] [CrossRef]
- Lowery, E.M.; Brubaker, A.L.; Kuhlmann, E.; Kovacs, E.J. The Aging Lung. CIA 2013, 8, 1489–1496. [Google Scholar] [CrossRef]
- Sharma, G.; Goodwin, J. Effect of Aging on Respiratory System Physiology and Immunology. Clin. Interv. Aging 2006, 1, 253. [Google Scholar] [CrossRef]
- Thomas, E.T.; Guppy, M.; Straus, S.E.; Bell, K.J.L.; Glasziou, P. Rate of Normal Lung Function Decline in Ageing Adults: A Systematic Review of Prospective Cohort Studies. BMJ Open 2019, 9, e028150. [Google Scholar] [CrossRef]
- Ammous, F.; Zhao, W.; Ratliff, S.M.; Mosley, T.H.; Bielak, L.F.; Zhou, X.; Peyser, P.A.; Kardia, S.L.R.; Smith, J.A. Epigenetic Age Acceleration Is Associated with Cardiometabolic Risk Factors and Clinical Cardiovascular Disease Risk Scores in African Americans. Clin. Epigenet. 2021, 13, 55. [Google Scholar] [CrossRef]
- Peters, S.A.E.; Singhateh, Y.; Mackay, D.; Huxley, R.R.; Woodward, M. Total Cholesterol as a Risk Factor for Coronary Heart Disease and Stroke in Women Compared with Men: A Systematic Review and Meta-Analysis. Atherosclerosis 2016, 248, 123–131. [Google Scholar] [CrossRef]
- Nagasawa, S.; Okamura, T.; Iso, H.; Tamakoshi, A.; Yamada, M.; Watanabe, M.; Murakami, Y.; Miura, K.; Ueshima, H. Relation Between Serum Total Cholesterol Level and Cardiovascular Disease Stratified by Sex and Age Group: A Pooled Analysis of 65,594 Individuals From 10 Cohort Studies in Japan. J. Am. Heart Assoc. 2012, 1, e001974. [Google Scholar] [CrossRef]
- The Lipid Research Clinics Coronary Primary Prevention Trial Results: I. Reduction in Incidence of Coronary Heart Disease. JAMA 1984, 251, 351–364. [CrossRef]
- Castelli, W.P.; Garrison, R.J.; Wilson, P.W.F.; Abbott, R.D.; Kalousdian, S.; Kannel, W.B. Incidence of Coronary Heart Disease and Lipoprotein Cholesterol Levels: The Framingham Study. JAMA 1986, 256, 2835–2838. [Google Scholar] [CrossRef]
- Castelli, W.P.; Anderson, K.; Wilson, P.W.F.; Levy, D. Lipids and Risk of Coronary Heart Disease The Framingham Study. Ann. Epidemiol. 1992, 2, 23–28. [Google Scholar] [CrossRef]
- Doucet, J.; Gourdy, P.; Meyer, L.; Benabdelmoumene, N.; Bourdel-Marchasson, I. Management of Glucose-Lowering Therapy in Older Adults with Type 2 Diabetes: Challenges and Opportunities. Clin. Interv. Aging 2023, 18, 1687–1703. [Google Scholar] [CrossRef]
- Alavinia, S.M.; van Duivenbooden, C.; Burdorf, A. Influence of Work-Related Factors and Individual Characteristics on Work Ability among Dutch Construction Workers. Scand. J. Work. Environ. Health 2007, 33, 351–357. [Google Scholar] [CrossRef]
- Ilmarinen, J.E. Aging Workers. Occup. Environ. Med. 2001, 58, 546–552. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, A.K.; Fisher, G.G.; Barnes-Farrell, J.L.; Grosch, J.W. Individual and Work Factors Related to Perceived Work Ability and Labor Force Outcomes. J. Appl. Psychol. 2015, 100, 376–398. [Google Scholar] [CrossRef] [PubMed]
- Alcover, C.-M.; Topa, G. Work Characteristics, Motivational Orientations, Psychological Work Ability and Job Mobility Intentions of Older Workers. PLoS ONE 2018, 13, e0195973. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.; Viotti, S.; Bruno, A.; Converso, D. Teachers’ Work Ability: A Study of Relationships between Collective Efficacy and Self-Efficacy Beliefs. Psychol. Res. Behav. Manag. 2018, 11, 197–206. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Effros, R.B. Immunosenescence: What Does It Mean to Health Outcomes in Older Adults? Curr. Opin. Immunol. 2009, 21, 418–424. [Google Scholar] [CrossRef]
- Zhang, Z.; Reynolds, S.R.; Stolrow, H.G.; Chen, J.-Q.; Christensen, B.C.; Salas, L.A. Deciphering the Role of Immune Cell Composition in Epigenetic Age Acceleration: Insights from Cell-Type Deconvolution Applied to Human Blood Epigenetic Clocks. Aging Cell 2024, 23, e14071. [Google Scholar] [CrossRef] [PubMed]
- Wacka, E.; Nicikowski, J.; Jarmuzek, P.; Zembron-Lacny, A. Anemia and Its Connections to Inflammation in Older Adults: A Review. J. Clin. Med. 2024, 13, 2049. [Google Scholar] [CrossRef] [PubMed]
- Bradycardia: Slow Heart Rate. Available online: https://www.heart.org/en/health-topics/arrhythmia/about-arrhythmia/bradycardia--slow-heart-rate (accessed on 2 July 2024).
- O’Brien, I.A.; O’Hare, P.; Corrall, R.J. Heart Rate Variability in Healthy Subjects: Effect of Age and the Derivation of Normal Ranges for Tests of Autonomic Function. Br. Heart J. 1986, 55, 348–354. [Google Scholar] [CrossRef]
- Fransquet, P.D.; Wrigglesworth, J.; Woods, R.L.; Ernst, M.E.; Ryan, J. The Epigenetic Clock as a Predictor of Disease and Mortality Risk: A Systematic Review and Meta-Analysis. Clin. Epigenet. 2019, 11, 62. [Google Scholar] [CrossRef]
- Liviero, F.; Scapellato, M.L.; Volpin, A.; Battistella, M.; Fabris, L.; Brischigliaro, L.; Folino, F.; Moretto, A.; Mason, P.; Pavanello, S. Long Term Follow-up of Heart Rate Variability in Healthcare Workers with Mild COVID-19. Front. Neurol. 2024, 15, 1403551. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef]
- Tang, B.; Li, X.; Wang, Y.; Sjölander, A.; Johnell, K.; Thambisetty, M.; Ferrucci, L.; Reynolds, C.A.; Finkel, D.; Jylhävä, J.; et al. Longitudinal Associations between Use of Antihypertensive, Antidiabetic, and Lipid-Lowering Medications and Biological Aging. Geroscience 2023, 45, 2065–2078. [Google Scholar] [CrossRef]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 2013, 14, 3156. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.-B.; Gao, Y.; et al. Genome-Wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Müezzinler, A.; Zaineddin, A.K.; Brenner, H. A Systematic Review of Leukocyte Telomere Length and Age in Adults. Ageing Res. Rev. 2013, 12, 509–519. [Google Scholar] [CrossRef]
- Lin, J.; Epel, E. Stress and Telomere Shortening: Insights from Cellular Mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Ornish, D.; Lin, J.; Daubenmier, J.; Weidner, G.; Epel, E.; Kemp, C.; Magbanua, M.J.M.; Marlin, R.; Yglecias, L.; Carroll, P.R.; et al. Increased Telomerase Activity and Comprehensive Lifestyle Changes: A Pilot Study. Lancet Oncol. 2008, 9, 1048–1057. [Google Scholar] [CrossRef]
- Rehkopf, D.H.; Needham, B.L.; Lin, J.; Blackburn, E.H.; Zota, A.R.; Wojcicki, J.M.; Epel, E.S. Leukocyte Telomere Length in Relation to 17 Biomarkers of Cardiovascular Disease Risk: A Cross-Sectional Study of US Adults. PLoS Med. 2016, 13, e1002188. [Google Scholar] [CrossRef]
- Mazidi, M.; Kengne, A.P.; Sahebkar, A.; Banach, M. Telomere Length Is Associated with Cardiometabolic Factors in US Adults. Angiology 2018, 69, 164–169. [Google Scholar] [CrossRef]
- Kim, D.; Li, A.A.; Ahmed, A. Leucocyte Telomere Shortening Is Associated with Nonalcoholic Fatty Liver Disease-Related Advanced Fibrosis. Liver Int. 2018, 38, 1839–1848. [Google Scholar] [CrossRef]
- Révész, D.; Verhoeven, J.E.; Picard, M.; Lin, J.; Sidney, S.; Epel, E.S.; Penninx, B.W.J.H.; Puterman, E. Associations Between Cellular Aging Markers and Metabolic Syndrome: Findings From the CARDIA Study. J. Clin. Endocrinol. Metab. 2018, 103, 148–157. [Google Scholar] [CrossRef]
- Baragetti, A.; Palmen, J.; Garlaschelli, K.; Grigore, L.; Pellegatta, F.; Tragni, E.; Catapano, A.L.; Humphries, S.E.; Norata, G.D.; Talmud, P.J. Telomere Shortening over 6 Years Is Associated with Increased Subclinical Carotid Vascular Damage and Worse Cardiovascular Prognosis in the General Population. J. Intern. Med. 2015, 277, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Karlsson, I.K.; Karlsson, R.; Tillander, A.; Reynolds, C.A.; Pedersen, N.L.; Hägg, S. Exploring the Causal Pathway From Telomere Length to Coronary Heart Disease: A Network Mendelian Randomization Study. Circ. Res. 2017, 121, 214–219. [Google Scholar] [CrossRef]
- Rode, L.; Nordestgaard, B.G.; Bojesen, S.E. Peripheral Blood Leukocyte Telomere Length and Mortality among 64,637 Individuals from the General Population. J. Natl. Cancer Inst. 2015, 107, djv074. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological Stress and Disease. JAMA 2007, 298, 1685–1687. [Google Scholar] [CrossRef]
- Bellon, M.; Nicot, C. Telomere Dynamics in Immune Senescence and Exhaustion Triggered by Chronic Viral Infection. Viruses 2017, 9, 289. [Google Scholar] [CrossRef]
- Chou, J.P.; Effros, R.B. T Cell Replicative Senescence in Human Aging. Curr. Pharm. Des. 2013, 19, 1680–1698. [Google Scholar] [CrossRef]
- Dowd, J.B.; Bosch, J.A.; Steptoe, A.; Jayabalasingham, B.; Lin, J.; Yolken, R.; Aiello, A.E. Persistent Herpesvirus Infections and Telomere Attrition Over 3 Years in the Whitehall II Cohort. J. Infect. Dis. 2017, 216, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Baerlocher, G.M.; Vulto, I.; Poon, S.S.; Lansdorp, P.M. Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes. PLoS Genet. 2012, 8, e1002696. [Google Scholar] [CrossRef] [PubMed]
- Al-Attiyah, R.; Safar, H.A.; Botras, L.; Botras, M.; Al-Kandari, F.; Chehadeh, W.; Mustafa, A.S. Immune Cells Profiles in the Peripheral Blood Of Patients with Moderate To Severe COVID-19 and Healthy Subjects with and without Vaccination with The Pfizer-BioNTech mRNA Vaccine. Front. Immunol. 2022, 13, 851765. [Google Scholar] [CrossRef]
- Berentschot, J.C.; Drexhage, H.A.; Aynekulu Mersha, D.G.; Wijkhuijs, A.J.M.; GeurtsvanKessel, C.H.; Koopmans, M.P.G.; Voermans, J.J.C.; Hendriks, R.W.; Nagtzaam, N.M.A.; de Bie, M.; et al. Immunological Profiling in Long COVID: Overall Low Grade Inflammation and T-Lymphocyte Senescence and Increased Monocyte Activation Correlating with Increasing Fatigue Severity. Front. Immunol. 2023, 14, 1254899. [Google Scholar] [CrossRef]
- Fasching, C.L. Telomere Length Measurement as a Clinical Biomarker of Aging and Disease. Crit. Rev. Clin. Lab. Sci. 2018, 55, 443–465. [Google Scholar] [CrossRef]
- Pavanello, S.; Campisi, M.; Fabozzo, A.; Cibin, G.; Tarzia, V.; Toscano, G.; Gerosa, G. The Biological Age of the Heart Is Consistently Younger than Chronological Age. Sci. Rep. 2020, 10, 10752. [Google Scholar] [CrossRef]
- Pavanello, S.; Campisi, M.; Rigotti, P.; Bello, M.D.; Nuzzolese, E.; Neri, F.; Furian, L. DNA Methylation—And Telomere-Based Biological Age Estimation as Markers of Biological Aging in Donors Kidneys. Front. Med. 2022, 9, 832411. [Google Scholar] [CrossRef] [PubMed]
- Binnie, A.; Walsh, C.J.; Hu, P.; Dwivedi, D.J.; Fox-Robichaud, A.; Liaw, P.C.; Tsang, J.L.Y.; Batt, J.; Carrasqueiro, G.; Gupta, S.; et al. Epigenetic Profiling in Severe Sepsis: A Pilot Study of DNA Methylation Profiles in Critical Illness. Crit. Care Med. 2020, 48, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Güiza, F.; Vanhorebeek, I.; Verstraete, S.; Verlinden, I.; Derese, I.; Ingels, C.; Dulfer, K.; Verbruggen, S.C.; Garcia Guerra, G.; Joosten, K.F.; et al. Effect of Early Parenteral Nutrition during Paediatric Critical Illness on DNA Methylation as a Potential Mediator of Impaired Neurocognitive Development: A Pre-Planned Secondary Analysis of the PEPaNIC International Randomised Controlled Trial. Lancet Respir. Med. 2020, 8, 288–303. [Google Scholar] [CrossRef]
- Balnis, J.; Madrid, A.; Hogan, K.J.; Drake, L.A.; Chieng, H.C.; Tiwari, A.; Vincent, C.E.; Chopra, A.; Vincent, P.A.; Robek, M.D.; et al. Blood DNA Methylation and COVID-19 Outcomes. Clin. Epigenet. 2021, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Morselli, M.; Farrell, C.; Montoya, D.; Gören, T.; Sabırlı, R.; Türkçüer, İ.; Kurt, Ö.; Köseler, A.; Pellegrini, M. DNA Methylation Profiles in Pneumonia Patients Reflect Changes in Cell Types and Pneumonia Severity. Epigenetics 2022, 17, 1646–1660. [Google Scholar] [CrossRef] [PubMed]
- Saiz, M.L.; DeDiego, M.L.; López-García, D.; Corte-Iglesias, V.; Baragaño Raneros, A.; Astola, I.; Asensi, V.; López-Larrea, C.; Suarez-Alvarez, B. Epigenetic Targeting of the ACE2 and NRP1 Viral Receptors Limits SARS-CoV-2 Infectivity. Clin. Epigenet. 2021, 13, 187. [Google Scholar] [CrossRef]
- Wang, Q.; Codd, V.; Raisi-Estabragh, Z.; Musicha, C.; Bountziouka, V.; Kaptoge, S.; Allara, E.; Angelantonio, E.D.; Butterworth, A.S.; Wood, A.M.; et al. Shorter Leukocyte Telomere Length Is Associated with Adverse COVID-19 Outcomes: A Cohort Study in UK Biobank. eBioMedicine 2021, 70, 103485. [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Sanaie, S.; Roudbari, F.; Sabzevari, T.; Sohrabifar, N.; Kazeminasab, S. Understanding the Role of Telomere Attrition and Epigenetic Signatures in COVID-19 Severity. Gene 2022, 811, 146069. [Google Scholar] [CrossRef]
- Aviv, A. Telomeres and COVID-19. FASEB J 2020, 34, 7247–7252. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vazquez, R.; Guío-Carrión, A.; Zapatero-Gaviria, A.; Martínez, P.; Blasco, M.A. Shorter Telomere Lengths in Patients with Severe COVID-19 Disease. Aging 2021, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Froidure, A.; Mahieu, M.; Hoton, D.; Laterre, P.-F.; Yombi, J.C.; Koenig, S.; Ghaye, B.; Defour, J.-P.; Decottignies, A. Short Telomeres Increase the Risk of Severe COVID-19. Aging 2020, 12, 19911–19922. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Sánchez-Vazquez, R.; Saha, A.; Rodriguez-Duque, M.S.; Naranjo-Gonzalo, S.; Osorio-Chavez, J.S.; Villar-Ramos, A.V.; Blasco, M.A. Short Telomeres in Alveolar Type II Cells Associate with Lung Fibrosis in Post COVID-19 Patients with Cancer. Aging 2023, 15, 4625–4641. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Flores-Gonzalez, J.; Buendia-Roldan, I.; Chavez-Galan, L. Telomere Shortening and Its Association with Cell Dysfunction in Lung Diseases. Int. J. Mol. Sci. 2022, 23, 425. [Google Scholar] [CrossRef]
- Bejaoui, Y.; Humaira Amanullah, F.; Saad, M.; Taleb, S.; Bradic, M.; Megarbane, A.; Ait Hssain, A.; Abi Khalil, C.; El Hajj, N. Epigenetic Age Acceleration in Surviving versus Deceased COVID-19 Patients with Acute Respiratory Distress Syndrome Following Hospitalization. Clin. Epigenet. 2023, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, S.; Donkin, I.; Versteyhe, S.; Barrès, R.; Simar, D. The Emerging Role of Epigenetics in Inflammation and Immunometabolism. Trends Endocrinol. Metab. 2016, 27, 782–795. [Google Scholar] [CrossRef]
- Bayarsaihan, D. Epigenetic Mechanisms in Inflammation. J. Dent. Res. 2011, 90, 9–17. [Google Scholar] [CrossRef]
- Ahmad, S.; Manzoor, S.; Siddiqui, S.; Mariappan, N.; Zafar, I.; Ahmad, A.; Ahmad, A. Epigenetic Underpinnings of Inflammation: Connecting the Dots between Pulmonary Diseases, Lung Cancer and COVID-19. Semin. Cancer Biol. 2022, 83, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Stukas, S.; Hoiland, R.L.; Cooper, J.; Thiara, S.; Griesdale, D.E.; Thomas, A.D.; Orde, M.M.; English, J.C.; Chen, L.Y.C.; Foster, D.; et al. The Association of Inflammatory Cytokines in the Pulmonary Pathophysiology of Respiratory Failure in Critically Ill Patients with Coronavirus Disease 2019. Crit. Care Explor. 2020, 2, e0203. [Google Scholar] [CrossRef] [PubMed]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 Entry Factors Are Highly Expressed in Nasal Epithelial Cells Together with Innate Immune Genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Wellford, S.A.; Moseman, E.A. Olfactory Immune Response to SARS-CoV-2. Cell Mol. Immunol. 2024, 21, 134–143. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2021, 27, 582–603. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inflammatory Mechanisms in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Barnes, P.J. Cellular and Molecular Mechanisms of Asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.O.; de Souza Nogueira, J.; do Nascimento, A.P.; Victoni, T.; Bártholo, T.P.; da Costa, C.H.; Costa, A.M.A.; Valença, S.d.S.; Schmidt, M.; Porto, L.C. COPD Patients Exhibit Distinct Gene Expression, Accelerated Cellular Aging, and Bias to M2 Macrophages. Int. J. Mol. Sci. 2023, 24, 9913. [Google Scholar] [CrossRef]
- Kukrety, S.P.; Parekh, J.D.; Bailey, K.L. Chronic Obstructive Pulmonary Disease and the Hallmarks of Aging. Lung India 2018, 35, 321–327. [Google Scholar] [CrossRef]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the Long Term Effects of COVID-19: Summary of NICE, SIGN, and RCGP Rapid Guideline. BMJ 2021, 372, n136. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, S.; Stendardo, M.; Mastrangelo, G.; Bonci, M.; Bottazzi, B.; Campisi, M.; Nardini, M.; Leone, R.; Mantovani, A.; Boschetto, P. Inflammatory Long Pentraxin 3 Is Associated with Leukocyte Telomere Length in Night-Shift Workers. Front. Immunol. 2017, 8, 516. [Google Scholar] [CrossRef]
- Pavanello, S.; Stendardo, M.; Mastrangelo, G.; Casillo, V.; Nardini, M.; Mutti, A.; Campisi, M.; Andreoli, R.; Boschetto, P. Higher Number of Night Shifts Associates with Good Perception of Work Capacity and Optimal Lung Function but Correlates with Increased Oxidative Damage and Telomere Attrition. Biomed. Res. Int. 2019, 2019, 8327629. [Google Scholar] [CrossRef]
- Ilmarinen, J. The Work Ability Index (WAI). Occup. Med. 2007, 57, 160. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of Spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Commission des Communautés Européennes. Tables de Reference Pour Les Examens Spirometriques; Office Des Publications Officielle Des Communautes Europeennes: Luxembourg, 1971. [Google Scholar]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart Rate Variability Analysis Software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Koizumi, K.; Terui, N.; Kollai, M. Effect of Cardiac Vagal and Sympathetic Nerve Activity on Heart Rate in Rhythmic Fluctuations. J. Auton. Nerv. Syst. 1985, 12, 251–259. [Google Scholar] [CrossRef]
- Moak, J.P.; Goldstein, D.S.; Eldadah, B.A.; Saleem, A.; Holmes, C.; Pechnik, S.; Sharabi, Y. Supine Low-Frequency Power of Heart Rate Variability Reflects Baroreflex Function, Not Cardiac Sympathetic Innervation. Heart Rhythm. 2007, 4, 1523–1529. [Google Scholar] [CrossRef]
- Rahman, F.; Pechnik, S.; Gross, D.; Sewell, L.; Goldstein, D.S. Low Frequency Power of Heart Rate Variability Reflects Baroreflex Function, Not Cardiac Sympathetic Innervation. Clin. Auton. Res. 2011, 21, 133–141. [Google Scholar] [CrossRef]
- Campisi, M.; Mastrangelo, G.; Mielżyńska-Švach, D.; Hoxha, M.; Bollati, V.; Baccarelli, A.A.; Carta, A.; Porru, S.; Pavanello, S. The Effect of High Polycyclic Aromatic Hydrocarbon Exposure on Biological Aging Indicators. Environ. Health 2023, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, S.; Campisi, M.; Grassi, A.; Mastrangelo, G.; Durante, E.; Veronesi, A.; Gallucci, M. Longer Leukocytes Telomere Length Predicts a Significant Survival Advantage in the Elderly TRELONG Cohort, with Short Physical Performance Battery Score and Years of Education as Main Determinants for Telomere Elongation. J. Clin. Med. 2021, 10, 3700. [Google Scholar] [CrossRef] [PubMed]





| PCS > 12 Weeks | PCS~1 Year | P_Trend | |
|---|---|---|---|
| HCWs | 0.4605 | 0.3026 | 0.0663 |
| Women | 0.5741 | 0.3333 | 0.0204 |
| Men | 0.1818 | 0.2273 | 0.9999 |
| P_Trend | 0.0043 | 0.5238 |
| Blood Leukocyte DNAmAge (years) | b | r | t Value | p | |
| Age | b1 = 0.74399 | r = 0.911647 | t = 17.884209 | <0.0001 | |
| Sex (male) | b2 = 2.703646 | r = 0.298937 | t = 2.525593 | 0.014 | |
| Chronic diseases (0 = no; 1 = yes) | b3 = 1.860584 | r = 0.266824 | t = 2.23213 | 0.0291 | |
| FEV1 (L) | b4 = −2.219589 | r = −0.382602 | t = −3.338666 | 0.0014 | |
| Lymphocytes (109/L) | b5 = −2.017434 | r = −0.36957 | t = −3.206587 | 0.0021 | |
| Blood Leukocyte TL (T/S) | Age | b1 = −0.011446 | r = −0.363521 | t = −3.146031 | 0.0025 |
| Sex (male) | b2 = 0.004352 | r = 0.005765 | t = 0.046482 | 0.9631 | |
| Chronic diseases (0 = no; 1 = yes) | b3 = 0.131573 | r = 0.218459 | t = 1.804868 | 0.0757 | |
| FEV1 (L) | b4 = −0.023505 | r = −0.05008 | t = −0.404266 | 0.6873 | |
| Lymphocytes (109/L) | b5 = 0.120086 | r = 0.261295 | t = 2.182445 | 0.0327 |
| Blood Leukocyte DNAmAge (years) | b | r | t Value | p | |
| Occupation (0 = HA; 1 = N; 2 = D; 3 = R; 4 = T and A) | b1 = −0.727891 | r = −0.097697 | t = −0.785337 | 0.4352 | |
| Night shift work (0 = no; 1 = yes) | b2 = −5.367146 | r = −0.263551 | t = −2.185686 | 0.0325 | |
| WAI | b3 = −0.617807 | r = −0.382432 | t = −3.311157 | 0.0015 | |
| Blood Leukocyte TL (T/S) | Occupation (0 = HA; 1 = N; 2 = D; 3 = R; 4 = T and A) | b1 = 0.064564 | r = 0.268124 | t = 2.226516 | 0.0295 |
| Night shift work (0 = no; 1 = yes) | b2 = 0.102616 | r = 0.164683 | t = 1.3357 | 0.1864 | |
| WAI | b3 = 0.007125 | r = 0.150818 | t = 1.220504 | 0.2268 |
| Blood Leukocyte DNAmAge (years) | b | r | t Value | p | |
| Hemoglobin (g/dL) | b1 = −0.205578 | r = −0.300885 | t = −2.484288 | 0.0157 | |
| Glycaemia (mg/dL) | b2 = 0.2006 | r = 0.329513 | t = 2.748063 | 0.0078 | |
| Cholesterol (mg/dL) | b3 = 0.006919 | r = 0.022835 | t = 0.179853 | 0.8579 | |
| Triglycerides (mg/dL) | b4 = 0.003211 | r = 0.01292 | t = 0.101744 | 0.9193 | |
| HDL (mg/dL) | b5 = 0.063761 | r = 0.098495 | t = 0.779342 | 0.4387 | |
| LDL (mg/dL) | b6 = 0.172631 | r = 0.388744 | t = 3.322289 | 0.0015 | |
| Creatinine (mg/dL) | b7 = 0.267526 | r = 0.205048 | t = 1.649598 | 0.1041 | |
| Bilirubin (mg/dL) | b8 = 0.022068 | r = 0.028541 | t = 0.224826 | 0.8229 | |
| Blood Leukocyte TL (T/S) | Hemoglobin (g/dL) | b1 = 0.003138 | r = 0.141452 | t = 1.125107 | 0.2649 |
| Glycaemia (mg/dL) | b2 = −0.00175 | r = −0.089968 | t = −0.711294 | 0.4796 | |
| Cholesterol (mg/dL) | b3 = −0.000016 | r = −0.001611 | t = −0.012687 | 0.9899 | |
| Triglycerides (mg/dL) | b4 = 0.001514 | r = 0.177891 | t = 1.42342 | 0.1596 | |
| HDL (mg/dL) | b5 = −0.000176 | r = −0.008083 | t = −0.06365 | 0.9495 | |
| LDL (mg/dL) | b6 = −0.003492 | r = −0.245463 | t = −1.993777 | 0.0506 | |
| Creatinine (mg/dL) | b7 = −0.0017 | r = −0.039478 | t = −0.311094 | 0.7568 | |
| Bilirubin (mg/dL) | b8 = −0.000485 | r = −0.018608 | t = −0.146549 | 0.884 |
| Blood Leukocyte DNAmAge (years) | b | r | t Value | p | |
| SDNN | b1 = −0.311272 | r = −0.186422 | t = −1.564708 | 0.1223 | |
| RMSSD | b2 = 0.14605 | r = 0.114644 | t = 0.95165 | 0.3446 | |
| Mean HR | b3 = −0.403812 | r = −0.356467 | t = −3.14618 | 0.0025 | |
| Drugs affecting HRV (0 = no; 1 = yes) | b4 = 8.905208 | r = 0.297306 | t = 2.567761 | 0.0124 | |
| Blood Leukocyte TL (T/S) | SDNN | b1 = 0.008358 | r = 0.166005 | t = 1.388176 | 0.1696 |
| MSSD | b2 = −0.006522 | r = −0.167858 | t = −1.404117 | 0.1648 | |
| Mean HR | b3 = 0.005025 | r = 0.154982 | t = 1.293644 | 0.2002 | |
| Drugs affecting HRV (0 = no; 1 = yes) | b4 = 0.154968 | r = 0.176234 | t = 1.476368 | 0.1445 |
| Post-COVID-19 Subjects | |||||
|---|---|---|---|---|---|
| N = 17 | Age | Blood AgeAcc (years) | IS AgeAcc (years) | Predicted Blood TL (T/S) | Predicted IS TL (T/S) |
| Mean ± SD | 46.00 ± 12.88 | −2.59 ± 3.47 *§ | 1.12 ± 4.37 § | 1.20 ± 0.06 *§ | 0.93 ± 0.02 § |
| COPD patients | |||||
| N = 7 | Age | Blood AgeAcc (years) | IS cells AgeAcc (years) | Predicted Blood TL (T/S) | Predicted IS TL (T/S) |
| Mean ± SD | 72.43 ± 6.00 | −10.29 ± 3.50 * | −4.29 ± 5.15 | 1.31 ± 0.03 * | 0.97 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campisi, M.; Cannella, L.; Bordin, A.; Moretto, A.; Scapellato, M.L.; Mason, P.; Liviero, F.; Pavanello, S.; on behalf of Occupational Medicine Working Group. Revealing the Hidden Impacts: Insights into Biological Aging and Long-Term Effects in Pauci- and Asymptomatic COVID-19 Healthcare Workers. Int. J. Mol. Sci. 2024, 25, 8056. https://doi.org/10.3390/ijms25158056
Campisi M, Cannella L, Bordin A, Moretto A, Scapellato ML, Mason P, Liviero F, Pavanello S, on behalf of Occupational Medicine Working Group. Revealing the Hidden Impacts: Insights into Biological Aging and Long-Term Effects in Pauci- and Asymptomatic COVID-19 Healthcare Workers. International Journal of Molecular Sciences. 2024; 25(15):8056. https://doi.org/10.3390/ijms25158056
Chicago/Turabian StyleCampisi, Manuela, Luana Cannella, Anna Bordin, Angelo Moretto, Maria Luisa Scapellato, Paola Mason, Filippo Liviero, Sofia Pavanello, and on behalf of Occupational Medicine Working Group. 2024. "Revealing the Hidden Impacts: Insights into Biological Aging and Long-Term Effects in Pauci- and Asymptomatic COVID-19 Healthcare Workers" International Journal of Molecular Sciences 25, no. 15: 8056. https://doi.org/10.3390/ijms25158056
APA StyleCampisi, M., Cannella, L., Bordin, A., Moretto, A., Scapellato, M. L., Mason, P., Liviero, F., Pavanello, S., & on behalf of Occupational Medicine Working Group. (2024). Revealing the Hidden Impacts: Insights into Biological Aging and Long-Term Effects in Pauci- and Asymptomatic COVID-19 Healthcare Workers. International Journal of Molecular Sciences, 25(15), 8056. https://doi.org/10.3390/ijms25158056






