Moderate Aerobic Exercise Induces Homeostatic IgA Generation in Senile Mice
Abstract
1. Introduction
2. Results
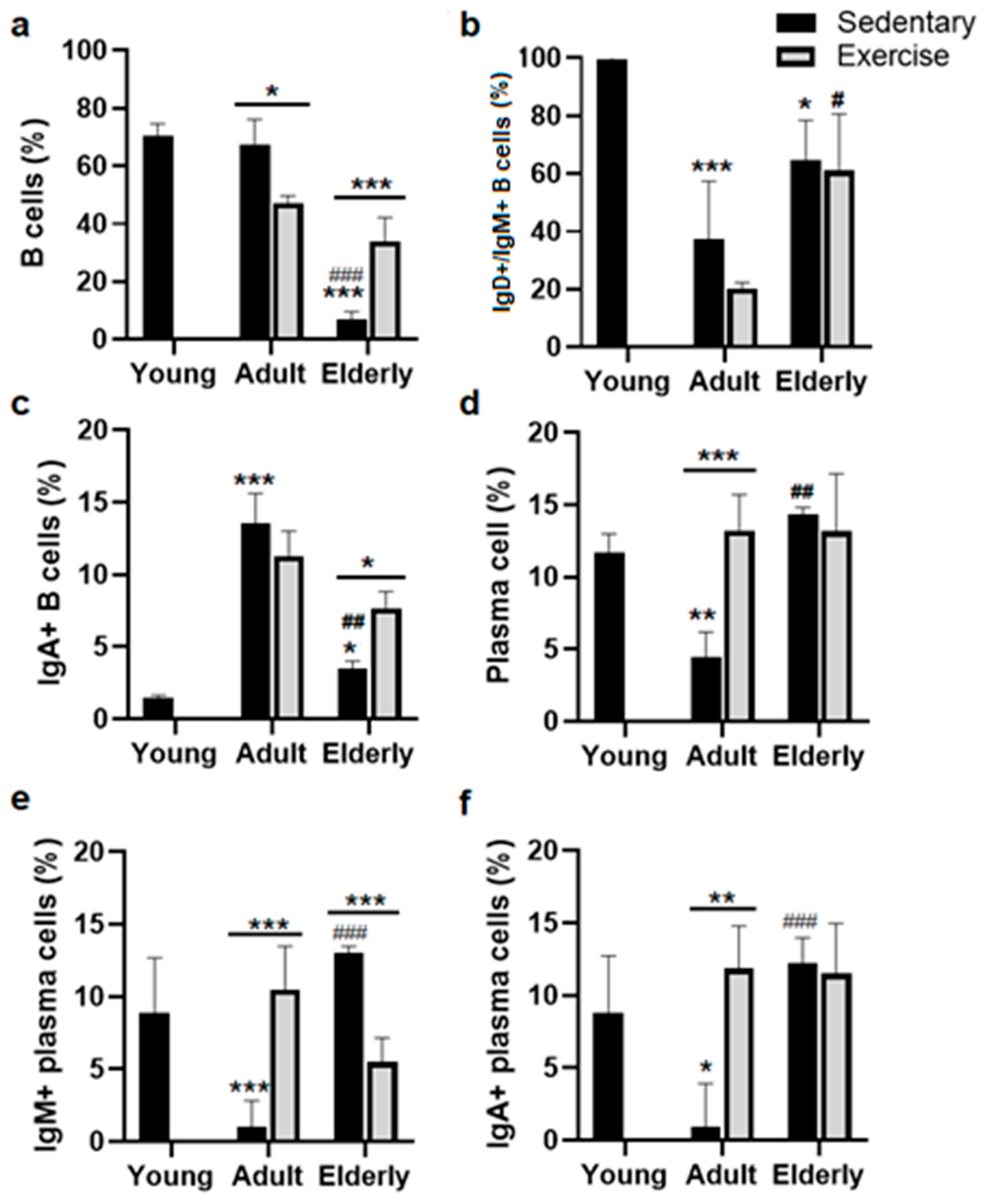
2.1. Exercise Increased Total B Cells in Senile Mice
2.2. Exercise Increased the IgA+ B Cells in Senile Mice
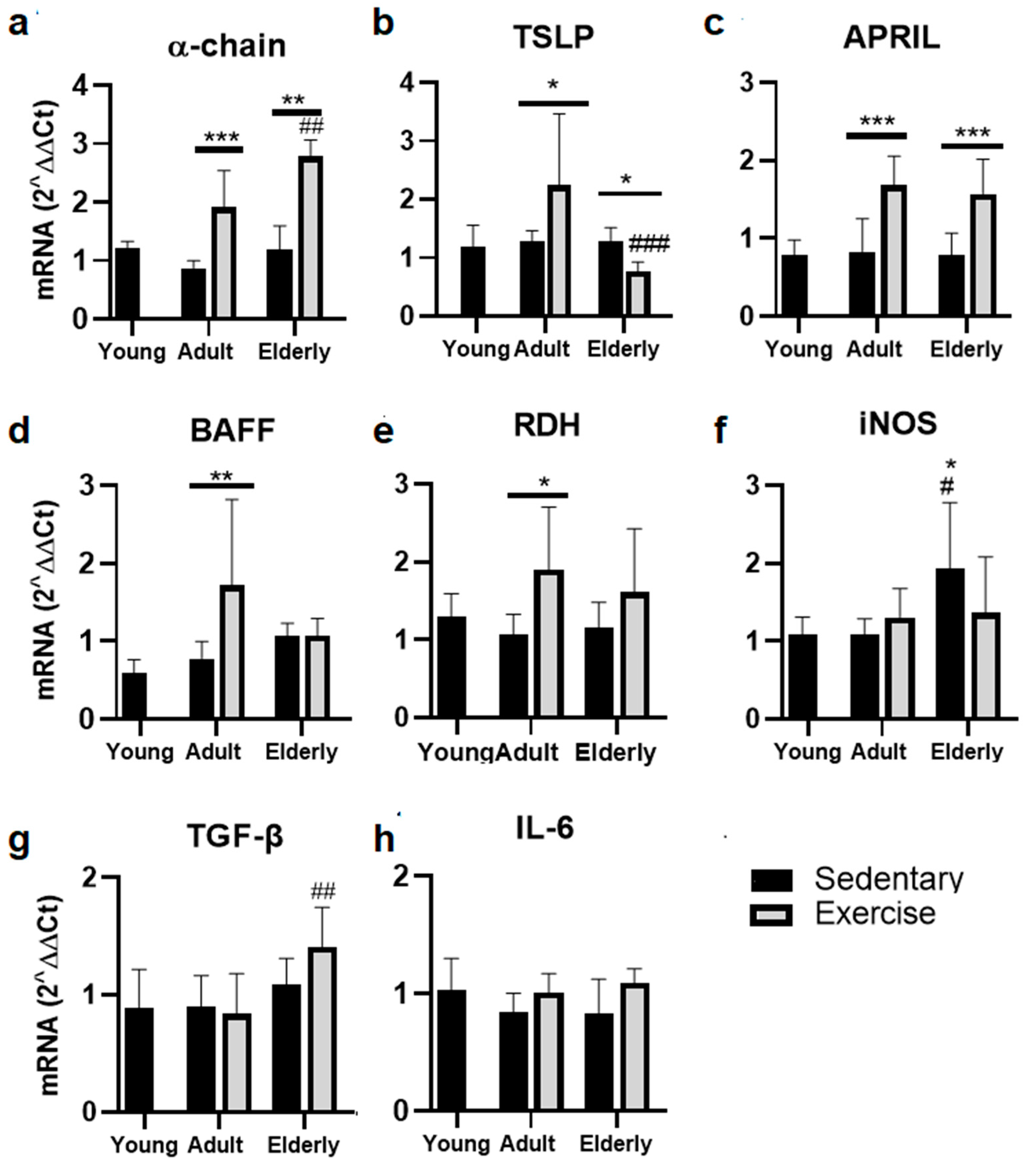
2.3. Exercise Increased the α-Chain and APRIL mRNA in Adult and Senile Mice
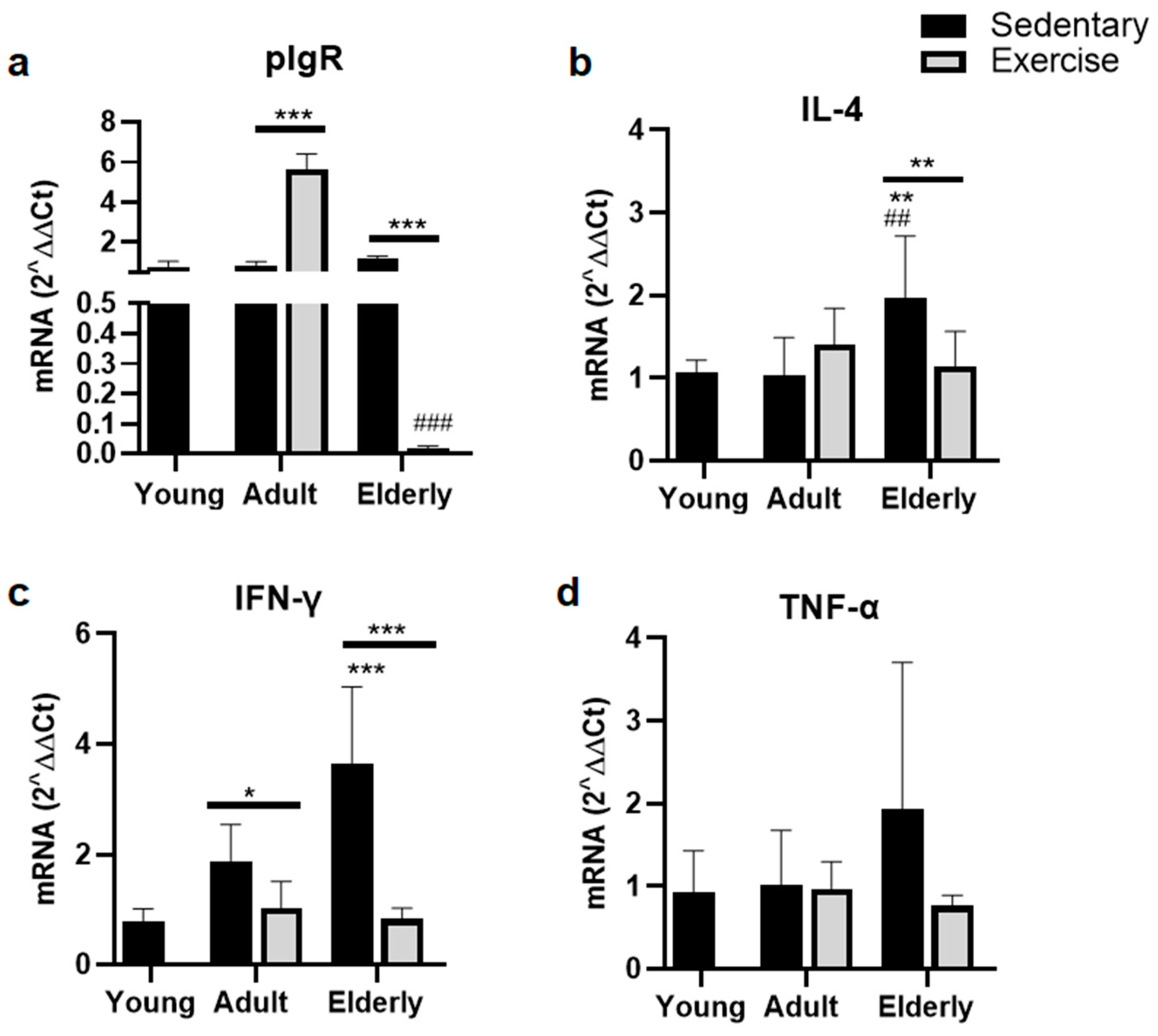
2.4. Factors Related to B Cells Maturation and Differentiation
2.5. Exercise Suppressed Parameters of pIgR for IgA-Transcytosis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Moderate Aerobic Exercise Protocol
4.4. Sampling
4.5. Lymphoid Sub-Populations in Lamina Propria by Cytometry
4.6. Relative Expression of mRNA Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woof, J.M.; Kerr, M.A. The function of immunoglobulin A in immunity. J. Pathol. 2006, 208, 270–282. [Google Scholar] [CrossRef]
- Tezuka, H.; Ohteki, T. Regulation of IgA Production by Intestinal Dendritic Cells and Related Cells. Front. Immunol. 2019, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Bunker, J.J.; Erickson, S.A.; Flynn, T.M.; Henry, C.; Koval, J.C.; Meisel, M.; Jabri, B.; Antonopoulos, D.A.; Wilson, P.C.; Bendelac, A. Natural Polyreactive IgA Antibodies Coat the Intestinal Microbiota. Science 2017, 358, eaan6619. [Google Scholar] [CrossRef] [PubMed]
- León, E.D.; Francino, M.P. Roles of Secretory Immunoglobulin A in Host-Microbiota Interactions in the Gut Ecosystem. Front. Microbiol. 2022, 13, 880484. [Google Scholar] [CrossRef]
- Lindner, C.; Wahl, B.; Föhse, L.; Suerbaum, S.; Macpherson, A.J.; Prinz, I.; Pabst, O. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J. Exp. Med. 2012, 209, 365–377. [Google Scholar] [CrossRef]
- Barone, F.; Patel, P.; Sanderson, J.D.; Spencer, J. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol. 2009, 2, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, S.; Fujimoto, K.; Jang, M.H.; Yang, B.-G.; Jung, Y.-J.; Nishiyama, M.; Sato, S.; Tsujimura, T.; Yamamoto, M.; Yokota, Y.; et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008, 9, 769–776. [Google Scholar] [CrossRef]
- Liu, Y.-J. TSLP in epithelial cell and dendritic cell cross talk. Adv. Immunol. 2009, 101, 1–25. [Google Scholar] [CrossRef]
- Delogu, A.; Schebesta, A.; Sun, Q.; Aschenbrenner, K.; Perlot, T.; Busslinger, M. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity 2006, 24, 269–281. [Google Scholar] [CrossRef]
- Pabst, O.; Slack, E. IgA and the intestinal microbiota: The importance of being specific. Mucosal Immunol. 2020, 13, 12–21. [Google Scholar] [CrossRef]
- Denning, G.M. IL-4 and IFN-gamma synergistically increase total polymeric IgA receptor levels in human intestinal epithelial cells. Role of protein tyrosine kinases. J. Immunol. 1996, 156, 4807–4814. [Google Scholar] [CrossRef] [PubMed]
- Kvale, D.; Brandtzaeg, P.; Løvhaug, D. Up-regulation of the expression of secretory component and HLA molecules in a human colonic cell line by tumour necrosis factor-alpha and gamma interferon. Scand. J. Immunol. 1998, 28, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yang, J.; Jian, Y.; Lei, Y.; Yao, S.; Hu, Z.; Liu, X.; Tang, C.; Liu, W. Treadmill Exercise Modulates Intestinal Microbes and Suppresses LPS Displacement to Alleviate Neuroinflammation in the Brains of APP/PS1 Mice. Nutrients 2022, 14, 4134. [Google Scholar] [CrossRef]
- Sugahara, H.; Okai, S.; Odamaki, T.; Wong, C.B.; Kato, K.; Mitsuyama, E.; Xiao, J.-Z.; Shinkura, R. Decreased Taxon-Specific IgA Response in Relation to the Changes of Gut Microbiota Composition in the Elderly. Front. Microbiol. 2017, 8, 1757. [Google Scholar] [CrossRef]
- van der Lugt, B.; van Beek, A.A.; Aalvink, S.; Meijer, B.; Sovran, B.; Vermeij, W.P.; Brandt, R.M.C.; de Vos, W.M.; Savelkoul, H.F.J.; Steegenga, W.T.; et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1 -/Δ7 mice. Immun. Ageing 2019, 16, 6. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Yang, G.; Meng, B.; Yi, Z.; Yang, G.; Chen, M.; Hou, P.; Wang, H.; Xu, X. Moderate-Intensity Physical Exercise Affects the Exercise Performance and Gut Microbiota of Mice. Front. Cell. Infect. Microbiol. 2021, 11, 712381. [Google Scholar] [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Bragazzi, N.L.; Aboghaba, B.; Elrayess, M.A. The Impact of Acute and Chronic Exercise on Immunoglobulins and Cytokines in Elderly: Insights From a Critical Review of the Literature. Front. Immunol. 2021, 12, 631873. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Sadeghi, M.; Karam, G.A.; Vazirinejad, R. Salivary IgA and IgE levels in healthy subjects: Relation to age and gender. Braz. Oral Res. 2010, 24, 21–27. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Vaerman, J.P.; Soo, C. Influence of severe protein malnutrition on rat lacrimal, salivary and gastrointestinal immune expression during development, adulthood and ageing. Immunology 1993, 78, 308–317. [Google Scholar]
- Senda, S.; Cheng, E.; Kawanishi, H. Aging-associated changes in murine intestinal immunoglobulin A and M secretions. Scand. J. Immunol. 1988, 27, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Schmucker, D.L.; Daniels, C.K.; Wang, R.K.; Smith, K. Mucosal immune response to cholera toxin in ageing rats. I. Anti-body and antibody-containing cell response. Immunology 1988, 64, 691–695. [Google Scholar] [PubMed]
- Thoreux, K.; Owen, R.L.; Schmucker, D.L. Intestinal lymphocyte number, migration and antibody secretion in young and old rats. Immunology 2000, 101, 161–167. [Google Scholar] [CrossRef]
- Yanagihara, T.; Kumagai, Y.; Norose, Y.; Moro, I.; Nanno, M.; Murakami, M.; Takahashi, H. Age-dependent decrease of pol-ymeric Ig receptor expression and IgA elevation in ddY mice: A possible cause of IgA nephropathy. J. Tech. Methods Pathol. 2004, 84, 63–70. [Google Scholar] [CrossRef]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- de Araújo, A.L.; Silva, L.C.R.; Fernandes, J.R.; Benard, G. Preventing or reversing immunosenescence: Can exercise be an immunotherapy? Immunotherapy 2013, 5, 879–893. [Google Scholar] [CrossRef]
- Simpson, R.J.; Lowder, T.W.; Spielmann, G.; Bigley, A.B.; LaVoy, E.C.; Kunz, H. Exercise and the aging immune system. Ageing Res. Rev. 2012, 11, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.-E.; Godínez-Victoria, M.; Lara-Padilla, E.; Resendiz-Albor, A.A.; Reyna-Garfias, H.; Arciniega-Martínez, I.M.; Kormanovski-Kovsova, A.; Campos-Rodriguez, R. Moderate exercise enhances expression of SIgA in mouse ileum. Int. J. Sports Med. 2012, 33, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Viloria, M.; Lara-Padilla, E.; Campos-Rodríguez, R.; Jarillo-Luna, A.; Reyna-Garfias, H.; López-Sánchez, P.; Rivera-Aguilar, V.; Salas-Casas, A.; de la Rosa, F.J.B.; García-Latorre, E. Effect of moderate exercise on IgA levels and lymphocyte count in mouse intestine. Immunol. Investig. 2011, 40, 640–656. [Google Scholar] [CrossRef]
- Godínez-Victoria, M.; Drago-Serrano, M.E.; Reyna-Garfias, H.; Viloria, M.; Lara-Padilla, E.; Resendiz-Albor, A.A.; Sánchez-Torres, L.E.; Cruz-Hernández, T.R.; Campos-Rodriguez, R. Effects on secretory IgA levels in small intestine of mice that underwent moderate exercise training followed by a bout of strenuous swimming exercise. Brain Behav. Immun. 2012, 26, 1300–1309. [Google Scholar] [CrossRef]
- Sierra-Ramírez, J.A.; Saucedo-Bueno, L.; García-Hernández, A.L.; Martínez-Dávalos, A.; Rodríguez-López, C.; Drago-Serrano, M.E.; Godínez-Victoria, M. Moderate aerobic exercise on bone quality changes associated with aging and oxidative stress in BALB/c mice. J. Biomech. 2022, 135, 111035. [Google Scholar] [CrossRef]
- Cook, M.D.; Allen, J.M.; Pence, B.D.; Wallig, M.A.; Gaskins, H.R.; White, B.A.; Woods, J.A. Exercise and gut immune function: Evidence of alterations in colon immune cell homeostasis and microbiome characteristics with exercise training. Immunol. Cell Biol. 2016, 94, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Divella, R.; Palma, G.D.E.; Tufaro, A.; Pelagio, G.; Gadaleta-Caldarola, G.; Bringiotti, R.; Paradiso, A. Diet Probiotics and Physical Activity: The Right Allies for a Healthy Microbiota. Anticancer. Res. 2021, 41, 2759–2772. [Google Scholar] [CrossRef]
- Hernández-Urbán, A.J.; Drago-Serrano, M.E.; Cruz-Baquero, A.; García-Hernández, A.L.; Arciniega-Martínez, I.M.; Pacheco-Yépez, J.; Guzmán-Mejía, F.; Godínez-Victoria, M. Exercise improves intestinal IgA production by T-dependent cell pathway in adults but not in aged mice. Front. Endocrinol. 2023, 14, 1190547. [Google Scholar] [CrossRef]
- McDonald, K.G.; Leach, M.R.; Huang, C.; Wang, C.; Newberry, R.D. Aging impacts isolated lymphoid follicle development and function. Immun. Ageing 2011, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Victoria, M.; Campos-Rodriguez, R.; Rivera-Aguilar, V.; Lara-Padilla, E.; Pacheco-Yepez, J.; Jarillo-Luna, R.A.; Drago-Serrano, M.E. Intermittent fasting promotes bacterial clearance and intestinal IgA production in Salmonella typhimurium-infected mice. Scand. J. Immunol. 2014, 79, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Blomberg, B.B. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016, 17, 7–19. [Google Scholar] [CrossRef]
- Zhao, B.; Xia, Z.; Yang, B.; Guo, Y.; Zhou, R.; Gu, M.; Liu, M.; Li, Q.; Bai, W.; Huang, J.; et al. USP7 promotes IgA class switching through stabilizing RUNX3 for germline transcription activation. Cell Rep. 2024, 43, 114194. [Google Scholar] [CrossRef]
- Gommerman, J.L.; Rojas, O.L.; Fritz, J.H. Re-thinking the functions of IgA(+) plasma cells. Gut Microbes 2014, 5, 652–662. [Google Scholar] [CrossRef]
- Johansen, F.-E.; Brandtzaeg, P. Transcriptional regulation of the mucosal IgA system. Trends Immunol. 2004, 25, 150–157. [Google Scholar] [CrossRef]
- Daniels, C.K.; Schmucker, D.L.; Bazin, H.; Jones, A.L. Immunoglobulin A receptor of rat small intestinal enterocytes is unaffected by aging. Gastroenterology 1988, 94, 1432–1440. [Google Scholar] [CrossRef]
- Shibuya, T.; Kaburagi, T.; Nagai, R.; Oshiro, S. The effects of moderate exercise on secretory IgA production in mice depends on dietary carbohydrate intake. J. Clin. Biochem. Nutr. 2015, 57, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.E.C.; West, R.B.; Schneeman, T.A.; Bresnick, E.H.; Kaetzel, C.S. Upstream stimulatory factor but not c-Myc enhances transcription of the human polymeric immunoglobulin receptor gene. Mol. Immunol. 2004, 40, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.A.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 1985, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, H.; Kiely, J. Immune-related alterations in aged gut-associated lymphoid tissues in mice. Dig. Dis. Sci. 1989, 34, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Gaffey, A.E.; Bergeman, C.S.; Clark, L.A.; Wirth, M.M. Aging and the HPA axis: Stress and resilience in older adults. Neurosci. Biobehav. Rev. 2016, 68, 928–945. [Google Scholar] [CrossRef] [PubMed]
- Reséndiz-Albor, A.A.; Reina-Garfias, H.; Rojas-Hernández, S.; Jarillo-Luna, A.; Rivera-Aguilar, V.; Miliar-García, A.; Campos-Rodríguez, R. Regionalization of pIgR expression in the mucosa of mouse small intestine. Immunol. Lett. 2010, 128, 59–67. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Gen | Sequence 5′→3′ | ID | |
|---|---|---|---|
| Forward | Reverse | ||
| TSLP | cag ctt gtc tcc tga aaa tcg | aaa tgt ttt gtc ggg gag tg | NM_021367.2 |
| BAFF | atg cgg aag gca gat tga | tgc atc ttt tgc tac cct ga | NM_001347309.1 |
| APRIL | agc tgg gca ctg agc ttt ac | aag ttg gcc tcg aac tca tc | NM_023517.2 |
| iNOS | ctt ttc cta tgg ggc aaa aa | ctg gaa ctc tgg gct gtc a | NM_010927.4 |
| RDH11 | tgt act tgg tca cgc caa aa | ccg gga agc tga aca tta ga | NM_021557.5 |
| TGF-β1 | tgg agc aac atg tgg aac tc | gtc agc agc cgg tta cca | NM_011577.2 |
| IL-6 | gct acc aaa ctg gat ata atc agg a | cca ggt agc tat ggt act cca gaa | NM_031168.2 |
| CBFa3 | gct ctc tca gca cca cga g | tca ggt ctg agg agc ctt g | NM_019732.2 |
| USF-1 | tca aga ggt ggg aaa gga tg | cat tgg gcc ccc ttc tac | NM_001305676.1 |
| Pax5 | cct ggg agt gaa ttt tct gg | tgg gga acc tcc aag aat c | NM_008782.2 |
| GAPDH | aag agg gat gct gcc ctt ac | cca ttt tgt cta cgg gac ga | NM_001289726.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Urbán, A.J.; Drago-Serrano, M.-E.; Reséndiz-Albor, A.A.; Sierra-Ramírez, J.A.; Guzmán-Mejía, F.; Oros-Pantoja, R.; Godínez-Victoria, M. Moderate Aerobic Exercise Induces Homeostatic IgA Generation in Senile Mice. Int. J. Mol. Sci. 2024, 25, 8200. https://doi.org/10.3390/ijms25158200
Hernández-Urbán AJ, Drago-Serrano M-E, Reséndiz-Albor AA, Sierra-Ramírez JA, Guzmán-Mejía F, Oros-Pantoja R, Godínez-Victoria M. Moderate Aerobic Exercise Induces Homeostatic IgA Generation in Senile Mice. International Journal of Molecular Sciences. 2024; 25(15):8200. https://doi.org/10.3390/ijms25158200
Chicago/Turabian StyleHernández-Urbán, Angel J., Maria-Elisa Drago-Serrano, Aldo A. Reséndiz-Albor, José A. Sierra-Ramírez, Fabiola Guzmán-Mejía, Rigoberto Oros-Pantoja, and Marycarmen Godínez-Victoria. 2024. "Moderate Aerobic Exercise Induces Homeostatic IgA Generation in Senile Mice" International Journal of Molecular Sciences 25, no. 15: 8200. https://doi.org/10.3390/ijms25158200
APA StyleHernández-Urbán, A. J., Drago-Serrano, M.-E., Reséndiz-Albor, A. A., Sierra-Ramírez, J. A., Guzmán-Mejía, F., Oros-Pantoja, R., & Godínez-Victoria, M. (2024). Moderate Aerobic Exercise Induces Homeostatic IgA Generation in Senile Mice. International Journal of Molecular Sciences, 25(15), 8200. https://doi.org/10.3390/ijms25158200






