Epigenetic Modifications of Hormonal Signaling Pathways in Plant Drought Response and Tolerance for Sustainable Food Security
Abstract
:1. Introduction
2. Epigenetic Memory in Drought-Adapted Plants
2.1. Transgenerational Inheritance of Epigenetic Changes in Response to Drought
2.2. Maintenance and Erasure of Epigenetic Marks in Drought-Primed Plants
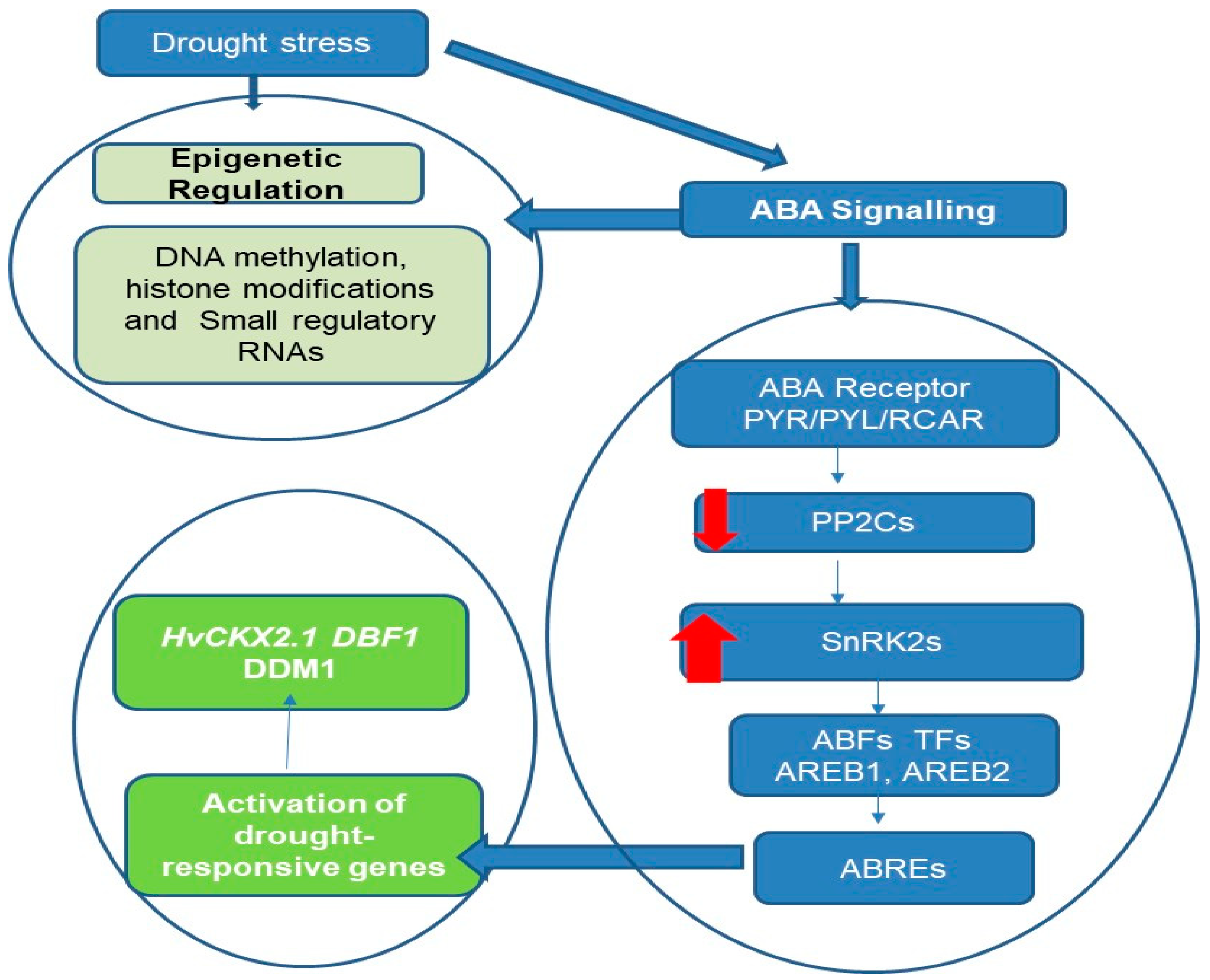
3. Epigenetic Regulation of Hormone Signaling Pathways under Drought Stress
3.1. Influence of DNA Methylation Patterns on Hormonal Signaling Pathways in Plant Adaptation to Drought Stress
| Plants | Findings | Mechanistic Focus | Citation |
|---|---|---|---|
| Tomato plants | Self-grafting induces significant epigenetic changes enhancing drought resilience, observed through modifications in histone and DNA methylation patterns, particularly in drought-responsive genes, followed by broader changes in gene expression and hormone regulation. | Epigenetic alterations during grafting and their impact on drought stress response | [41] |
| Maize | DNA methylation impacts gene regulation and hormone pathways, contributing to plants’ resilience against drought stress. | DNA methylation, hormones | [42] |
| Dendrobium officinale | DNA methylation dynamics, potentially mediated by hormone signaling pathways, contribute to the orchid’s response to drought stress, as evidenced by the differential expression of cytosine-5 DNA methyltransferase (C5-MTase) and DNA demethylase (dMTase) genes. | Role of DNA methylation, including C5-MTase and dMTase genes, in orchids’ adaptation to drought and potential hormone signaling involvement | [44] |
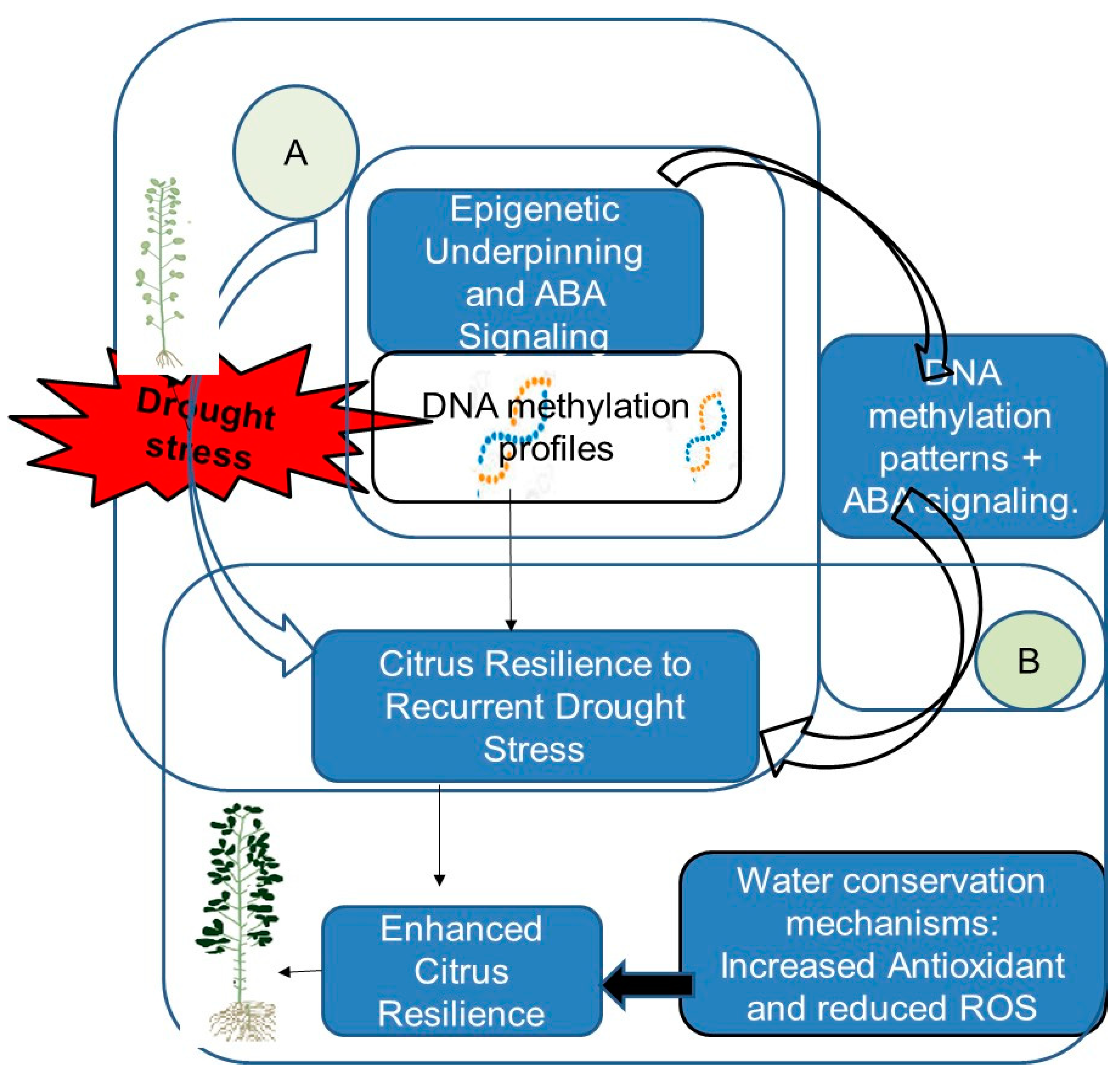
| Citrus | Divergent DNA methylation profiles in scion/rootstock interactions contribute to enhanced citrus resilience to recurring drought stress. | DNA methylation, ABA | [45] |
| Barley | Small RNAs, including 24mer hc-siRNAs, are linked to RNA-directed DNA methylation (RdDM) and gene silencing (TGS) under terminal drought stress during grain filling. | RNA silencing, hormones | [47] |
| Maize | DNA methylation of DBF1 in maize genotypes shows stable upstream methylation under drought conditions, with no specific cytosine methylation patterns in regulatory regions. | DNA methylation, ABA | [48] |
| Mulberry | Altered DNA methylation patterns in mulberry leaves under drought stress impact hormone signaling and biosynthesis pathways. | DNA methylation, hormones | [49] |
| Poplar | Downregulation of chromatin remodeler DDM1 in poplar RNAi lines reveals DNA methylation’s role in phytohormone pathways and stress response regulation during water deficit. | DNA methylation, hormones | [50] |
| Wheat cultivars | Drought-tolerant cultivar “Bolani” exhibits increased abscisic acid (ABA) levels under severe drought conditions, correlating with improved drought tolerance, evidenced by higher radical scavenging activity and maintained relative water content. | Interplay between DNA methylation dynamics, hormonal changes, and drought response | [51] |
| Tea leaves | Dehydration stress induces epigenetic modifications promoting abscisic acid (ABA) biosynthesis, evidenced by increased expression of ABA biosynthesis genes, the accumulation of ABA, and histone acetylation. | Epigenetic regulation of ABA biosynthesis genes during dehydration stress | [52] |
| Rice | Zinc finger proteins (ZFPs) respond to salt and osmotic stresses, with tissue-specific expression patterns under stress conditions, revealing epigenetic regulation of gene expression in response to environmental pressures. | Role of ZFPs in epigenetic regulation of gene expression under salt and osmotic stresses | [53] |
| Cotton plants | Drought stress induces significant DNA methylation changes, including hyper-methylation and asymmetric CHH methylation, potentially regulated by long non-coding RNAs, accompanied by alterations in the methylation of hormone-related genes. | Epigenetic modifications, particularly DNA methylation, and their association with hormone-related genes during drought stress | [54] |
| Rice | Cytochrome P450 (CYP) genes exhibit diverse methylation levels in response to salinity and drought stresses, with implications for gene expression and hormone regulation, suggesting a role in the plant’s stress response mechanisms. | Influence of CYP genes on rice’s stress response, including their epigenetic regulation and interaction with hormone signaling pathways | [55] |
| Populus × euramericana | Phenotypic plasticity in response to soil water availability involves epigenetic mechanisms, with dynamic changes observed in gene expression and DNA methylation, particularly in hormone-related genes, enabling adaptation to water availability. | Coordination between DNA methylation, gene expression, and hormone-related genes in response to drought and rewatering | [56] |
3.2. The Impact of Histone Modifications on Hormonal Signaling Pathways in Plant Adaptation to Drought Stress
3.3. Epi-miRNAs and Their Role in Drought Stress Response in Plants
3.4. Influence of MicroRNA (miRNA) Patterns on Hormonal Signaling Pathways in Plant Adaptation to Drought Stress
4. Technological Advances in Studying Epigenetic Modifications
4.1. High-Throughput Techniques for Profiling Epigenetic Changes
4.2. CRISPR-Cas9 Applications in Engineering Epigenetic Regulation for Drought Tolerance
| Techniques | Plant Species | Key Findings | Citations |
|---|---|---|---|
| ChIP-seq technique | Barley | Identification of drought-induced alterations in histone acetylation and trimethylation patterns, revealing the role of epigenetic modifications in barley’s adaptive response to drought | [57] |
| High-throughput sequencing | Wheat | Identification of drought-responsive miRNAs and targets in drought-tolerant wheat variety XF 20 | [87] |
| High-throughput techniques (HTS) | Rice | Exploration of 5468 drought-responsive genes (DRGs) and their regulatory landscape | [88] |
| High-throughput sequencing | Rice | Establishment of drought-induced epimutation accumulation lines, highlighting the role of epigenetic mechanisms in rice adaptation to drought | [89] |
| High-throughput sequencing | Rice | Investigation of DNA methylation patterns and their correlation with gene expression in three rice cultivars with drought stress tolerance | [90] |
| ChIP-seq analysis | Brachypodium distachyon | Exploration of H3K9ac levels in response to simulated drought stress, revealing a positive correlation with gene expression | [93] |
| ChIP-seq technique | IR64 rice | Investigation of the impact of H3K27 modifications on gene expression during cold stress, highlighting the role of histone modifications in stress-responsive pathways | [94] |
| ChIP-seq technology | Rice | Exploration of the regulatory mechanism of differentially expressed genes (DEGs) under drought stress, uncovering the interplay between histone H3K4me3 modification and the transcription factor OsbZIP23 | [95] |
| ATAC-seq analysis | Haberlea rhodopensis | Investigation of chromatin accessibility dynamics during desiccation stress, providing insights into the epigenetic regulation of desiccation tolerance | [97] |
| ATAC-seq analysis | Rice | Study on OsNMCP1’s role in altering chromatin accessibility in genes associated with drought resistance and root growth | [98] |
| ATAC-seq analysis | Maize | Highlighting the role of DNA methylation in regulating drought tolerance variations in maize roots under water stress | [101] |
| ATAC-seq analysis | Rice | Demonstration of the impact of JMJ710 on the expression of drought-related genes during drought stress, leading to a drought-sensitive phenotype upon overexpression | [102] |
| CRISPR/Cas9 technology | Maize | Investigation of the causal relationship between histone acetylation patterns and drought tolerance by modulating ZmHDT103, revealing enhanced drought tolerance in knockout lines | [110] |
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Kumar Patel, M.; Kumar, N.; Bajpai, A.B.; Siddique, K.H. Metabolomics and molecular approaches reveal drought stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Sati, D.; Pande, V.; Pandey, S.C.; Samant, M. Recent advances in PGPR and molecular mechanisms involved in drought stress resistance. J. Soil Sci. Plant Nut. 2023, 23, 106–124. [Google Scholar] [CrossRef]
- Godwin, J.; Farrona, S. 2020 Plant epigenetic stress memory induced by drought: A physiological and molecular perspective. In Plant Epigenetics and Epigenomics: Methods in Molecular Biology; Spillane, C., McKeown, P., Eds.; Springer: New York, NY, USA, 2020; Volume 2093, pp. 43–259. [Google Scholar] [CrossRef]
- Chung, S.; Kwon, C.; Lee, J.H. Epigenetic control of abiotic stress signaling in plants. Genes Genom. 2021, 44, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Temel, A.; Janack, B.; Humbeck, K. Drought stress-related physiological changes and histone modifications in barley primary leaves at HSP17 gene. Agronomy 2017, 7, 43. [Google Scholar] [CrossRef]
- Czajka, K.; Mehes-Smith, M.; Nkongolo, K. DNA methylation and histone modifications induced by abiotic stressors in plants. Genes Genom. 2021, 44, 279–297. [Google Scholar] [CrossRef]
- Kim, J.M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 2015, 6, 114. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A. The role of abscisic acid in drought stress: How ABA helps plants to cope with drought stress. In Drought Stress Tolerance in Plants, Molecular and Genetic Perspectives; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 2, pp. 123–151. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Kinoshita, T.; Seki, M. Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol. 2014, 55, 1859–1863. [Google Scholar] [CrossRef]
- Sun, C.; Ali, K.; Yan, K.; Fiaz, S.; Dormatey, R.; Bi, Z.; Bai, J. Exploration of epigenetics for improvement of drought and other stress resistance in crops: A review. Plants 2021, 10, 1226. [Google Scholar] [CrossRef]
- Sadhukhan, A.; Prasad, S.S.; Mitra, J.; Siddiqui, N.; Sahoo, L.; Kobayashi, Y.; Koyama, H. How do plants remember drought? Planta 2022, 256, 7. [Google Scholar] [CrossRef]
- Kumar, S.; Seem, K.; Mohapatra, T. Biochemical and Epigenetic Modulations under Drought: Remembering the Stress Tolerance Mechanism in Rice. Life 2023, 13, 1156. [Google Scholar] [CrossRef] [PubMed]
- Yung, W.S.; Huang, C.; Li, M.W.; Lam, H.M. Changes in epigenetic features in legumes under abiotic stresses. Plant Genome 2023, 16, e20237. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Jain, D.; Pandey, J.; Yadav, M.; Bansal, K.C.; Singh, I.K. Deciphering the role of miRNA in reprogramming plant responses to drought stress. Crit. Rev. Biotechnol. 2023, 43, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.T.; Aufsatz, W.; Jonak, C.; Luschnig, C. Transgenerational epigenetic inheritance in plants. BBA-Gene Regul. Mech. 2011, 1809, 459–468. [Google Scholar] [CrossRef]
- Fitz-James, M.H.; Cavalli, G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar] [CrossRef]
- Van Dooren, T.J.; Silveira, A.B.; Gilbault, E.; Jiménez-Gómez, J.M.; Martin, A.; Bach, L.; Colot, V. Mild drought in the vegetative stage induces phenotypic, gene expression, and DNA methylation plasticity in Arabidopsis but no transgenerational effects. J. Exp. Bot. 2020, 71, 3588–3602. [Google Scholar] [CrossRef]
- Cheuquemán, C.; Maldonado, R. Non-coding RNAs and chromatin: Key epigenetic factors from spermatogenesis to transgenerational inheritance. Biol. Res. 2021, 54, 41. [Google Scholar] [CrossRef]
- Larriba, E.; Del Mazo, J. Role of non-coding RNAs in the transgenerational epigenetic transmission of the effects of reprotoxicants. Int. J. Mol. Sci. 2016, 17, 452. [Google Scholar] [CrossRef]
- Moelling, K. Epigenetics and transgenerational inheritance. J. Physiol. 2024, 602, 2537–2545. [Google Scholar] [CrossRef]
- Liu, N.; Fromm, M.; Avramova, Z. H3K27me3 and H3K4me3 chromatin environment at super-induced dehydration stress memory genes of Arabidopsis thaliana. Mol. Plant 2014, 7, 502–513. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Aleksandrushkina, N.I.; Vanyushin, B.F. Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. Int. J. Mol. Sci. 2020, 21, 7457. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.Z.; Liu, J.; Wang, Y.Y.; Deng, X. DNA methylation-mediated modulation of rapid desiccation tolerance acquisition and dehydration stress memory in the resurrection plant Boea hygrometrica. PLoS Genet. 2021, 17, e1009549. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Aufsatz, W.; Zilberman, D.; Mette, M.F.; Huang, M.S.; Matzke, M.; Jacobsen, S.E. Role of the DRM and CMT3 methyltransferases in RNA-directed DNA methylation. Curr. Biol. 2003, 13, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.S.; Karagiannis, J.; Tian, L. HD2A and HD2C co-regulate drought stress response by modulating stomatal closure and root growth in Arabidopsis. Front. Plant Sci. 2022, 13, 1062722. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Hung, F.Y.; Yang, S.; Liu, X.; Wu, K. Histone lysine demethylases and their functions in plants. Plant Mol. Biol. Rep. 2014, 32, 558–565. [Google Scholar] [CrossRef]
- Singh, A.; Pandey, G.K. Protein phosphatases: A genomic outlook to understand their function in plants. J. Plant Biochem. Biot. 2012, 21, 100–107. [Google Scholar] [CrossRef]
- Luo, R.; Yang, K.; Xiao, W. Plant deubiquitinases: From structure and activity to biological functions. Plant Cell Rep. 2023, 42, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lv, S.; Zhang, C.; Yang, C. Histone deacetylases and their functions in plants. Plant Cell Rep. 2013, 32, 465–478. [Google Scholar] [CrossRef]
- Li, S.; He, X.; Gao, Y.; Zhou, C.; Chiang, V.L.; Li, W. Histone acetylation changes in plant response to drought stress. Genes 2021, 12, 1409. [Google Scholar] [CrossRef]
- Choi, K.; Park, C.; Lee, J.; Oh, M.; Noh, B.; Lee, I. Arabidopsis homologs of components of the SWR1 complex regulate flowering and plant development. 2007. [CrossRef]
- Aslam, M.; Fakher, B.; Jakada, B.H.; Cao, S.; Qin, Y. SWR1 chromatin remodeling complex: A key transcriptional regulator in plants. Cells 2019, 8, 1621. [Google Scholar] [CrossRef] [PubMed]
- Morillo-Huesca, M.; Clemente-Ruiz, M.; Andújar, E.; Prado, F. The SWR1 histone replacement complex causes genetic instability and genome-wide transcription misregulation in the absence of H2A. Z. PLoS ONE 2010, 5, e12143. [Google Scholar] [CrossRef] [PubMed]
- Forestan, C.; Farinati, S.; Zambelli, F.; Pavesi, G.; Rossi, V.; Varotto, S. Epigenetic signatures of stress adaptation and flowering regulation in response to extended drought and recovery in Zea mays. Plant Cell Environ. 2020, 43, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Dar, F.A.; Mushtaq, N.U.; Saleem, S.; Rehman, R.U.; Dar, T.U.H.; Hakeem, K.R. Role of epigenetics in modulating phenotypic plasticity against abiotic stresses in plants. Int. J. Genom. 2022, 1092894. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.; Tomovičová, L.; Panzarová, K.; Fajkus, J.; Hejátko, J.; Skalák, J. Epigenetics and plant hormone dynamics-a functional and methodological perspective. J. Exp. Bot. 2024, erae054. [Google Scholar] [CrossRef]
- Zhu, Y. The epigenetic involvement in plant hormone signaling. Chin. Sci. Bull. 2010, 55, 2198–2203. [Google Scholar] [CrossRef]
- Yamamuro, C.; Zhu, J.K.; Yang, Z. Epigenetic modifications and plant hormone action. Mol. Plant 2016, 9, 57–70. [Google Scholar] [CrossRef]
- Fuentes-Merlos, M.I.; Bamba, M.; Sato, S.; Higashitani, A. Self-grafting-induced epigenetic changes leading to drought stress tolerance in tomato plants. DNA Res. 2023, 30, dsad016. [Google Scholar] [CrossRef]
- Sallam, N.; Moussa, M. DNA methylation changes stimulated by drought stress in ABA-deficient maize mutant vp10. Plant Physiol. Biochem. 2021, 160, 218–224. [Google Scholar] [CrossRef]
- Tricker, P.J.; Rodríguez López, C.M.; Hadley, P.; Wagstaff, C.; Wilkinson, M.J. Pre-conditioning the epigenetic response to high vapor pressure deficit increases the drought tolerance of Arabidopsis thaliana. Plant Signal. Behav. 2013, 8, e25974. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, G.; Teixeira da Silva, J.A.; Li, M.; Zhao, C.; He, C.; Duan, J. Genome-wide identification and analysis of DNA methyltransferase and demethylase gene families in Dendrobium officinale reveal their potential functions in polysaccharide accumulation. BMC Plant Biol. 2021, 21, 21. [Google Scholar] [CrossRef]
- Santos, A.S.; Neves, D.M.; Santana-Vieira, D.D.S.; Almeida, L.A.H.; Costa, M.G.C.; Soares Filho, W.S.; Gesteira, A.S. Citrus scion and rootstock combinations show changes in DNA methylation profiles and ABA insensitivity under recurrent drought conditions. Sci. Hortic. 2020, 267, 109313. [Google Scholar] [CrossRef]
- Bhogale, S.; Mahajan, A.S.; Natarajan, B.; Rajabhoj, M.; Thulasiram, H.V.; Banerjee, A.K. MicroRNA156: A potential graft-transmissible microRNA that modulates plant architecture and tuberization in Solanum tuberosum ssp. andigena. Plant Physiol. 2014, 164, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Surdonja, K.; Eggert, K.; Hajirezaei, M.R.; Harshavardhan, V.T.; Seiler, C.; Von Wirén, N.; Kuhlmann, M. Increase of DNA methylation at the HvCKX2. 1 promoter by terminal drought stress in barley. Epigenomes 2017, 1, 9. [Google Scholar] [CrossRef]
- Sallam, N.; Moussa, M.; Yacout, M.; Shakam, H.M. Detection of DNA methylation in DBF1 gene of maize inbred W64A and mutant vp 14 exposed to drought stress. Cereal Res. Commun. 2022, 50, 19–24. [Google Scholar] [CrossRef]
- Ackah, M.; Guo, L.; Li, S.; Jin, X.; Asakiya, C.; Aboagye, E.T.; Zhao, W. DNA methylation changes and its associated genes in mulberry (Morus alba L.) Yu-711 response to drought stress using MethylRAD sequencing. Plants 2022, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Sow, M.D.; Le Gac, A.L.; Fichot, R.; Lanciano, S.; Delaunay, A.; Le Jan, I.; Maury, S. RNAi suppression of DNA methylation affects the drought stress response and genome integrity in transgenic poplar. New Phytol. 2021, 232, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S.; Maali-Amiri, R.; Sadeghi, L.; Hamidi, A. Physio-biochemical and DNA methylation analysis of the defense response network of wheat to drought stress. Plant Physiol. Biochem. 2024, 209, 108516. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Yang, J.; Wu, S.; Liao, Y.; Zeng, L.; Yang, Z. Epigenetic regulation of the phytohormone abscisic acid accumulation under dehydration stress during postharvest processing of tea (Camellia sinensis). J. Agric. Food Chem. 2021, 69, 1039–1048. [Google Scholar] [CrossRef]
- Ahmad, F.; Farman, K.; Waseem, M.; Rana, R.M.; Nawaz, M.A.; Rehman, H.M.; Zhang, H. Genome-wide identification, classification, expression profiling and DNA methylation (5mC) analysis of stress-responsive ZFP transcription factors in rice (Oryza sativa L.). Gene 2019, 718, 144018. [Google Scholar] [CrossRef]
- Lu, X.; Wang, X.; Chen, X.; Shu, N.; Wang, J.; Wang, D.; Wang, S.; Fan, W.; Guo, L.; Guo, X.; et al. Single-base resolution methylomes of upland cotton (Gossypium hirsutum L.) reveal epigenome modifications in response to drought stress. BMC Genom. 2017, 18, 297. [Google Scholar] [CrossRef]
- Waseem, M.; Huang, F.; Wang, Q.; Aslam, M.M.; Abbas, F.; Ahmad, F.; Ke, Y. Identification, methylation profiling, and expression analysis of stress-responsive cytochrome P450 genes in rice under abiotic and phytohormones stresses. GM Crops Food 2021, 12, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Lafon-Placette, C.; Le Gac, A.L.; Chauveau, D.; Segura, V.; Delaunay, A.; Lesage-Descauses, M.C.; Hummel, I.; Cohen, D.; Jesson, B.; Thiec, D.L.; et al. Changes in the epigenome and transcriptome of the poplar shoot apical meristem in response to water availability affect preferentially hormone pathways. J. Exp. Bot. 2018, 69, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Ost, C.; Cao, H.X.; Nguyen, T.L.; Himmelbach, A.; Mascher, M.; Stein, N.; Humbeck, K. Drought-Stress-Related Reprogramming of Gene Expression in Barley Involves Differential Histone Modifications at ABA-Related Genes. Int. J. Mol. Sci. 2023, 24, 12065. [Google Scholar] [CrossRef] [PubMed]
- Manh, M.B.; Ost, C.; Peiter, E.; Hause, B.; Krupinska, K.; Humbeck, K. WHIRLY1 Acts Upstream of ABA-Related Reprogramming of Drought-Induced Gene Expression in Barley and Affects Stress-Related Histone Modifications. Int. J. Mol. Sci. 2023, 24, 6326. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, C.; Long, J.; Bai, S.; Yao, M.; Chen, J.; Li, Q. Genome-wide identification of the jumonji C domain-containing histone demethylase gene family in wheat and their expression analysis under drought stress. Front. Plant Sci. 2022, 13, 987257. [Google Scholar] [CrossRef]
- Yu, X.; Gao, Q.; Chen, G.; Guo, J.E.; Guo, X.; Tang, B.; Hu, Z. SlHDA5, a tomato histone deacetylase gene, is involved in responding to salt, drought, and ABA. Plant Mol. Biol. Rep. 2018, 36, 36–44. [Google Scholar] [CrossRef]
- Xin, X.; Su, T.; Li, P.; Wang, W.; Zhao, X.; Yu, Y.; Zhang, F. A histone H4 gene prevents drought-induced bolting in Chinese cabbage by attenuating the expression of flowering genes. J. Exp. Bot. 2021, 72, 623–635. [Google Scholar] [CrossRef]
- Guo, J.E.; Wang, H.; Yang, Y.; Li, J.; Zhu, Z. Histone deacetylase gene SlHDA3 is involved in drought and salt response in tomato. J. Plant Growth Regul. 2023, 99, 359–372. [Google Scholar] [CrossRef]
- Li, J.; Wei, J.; Song, Y.; Chen, N.; Ni, B.; Zhang, J.; He, C. Histone H3K9 acetylation modulates gene expression of key enzymes in the flavonoid and abscisic acid pathways and enhances drought resistance of sea buckthorn. Physiol. Plant. 2023, 175, e13936. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, B.; Liu, C.; Tong, B.; Guan, T.; Xia, D. Expression of a populus histone deacetylase gene 84KHDA903 in tobacco enhances drought tolerance. Plant Sci. 2017, 265, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Haouel, A.; Georgii, E.; Priego-Cubero, S.; Wurm, C.J.; Hemmler, D.; Lindermayr, C. Histone Deacetylases HD2A and HD2B undergo feedback regulation by ABA and modulate drought tolerance via mediating ABA-induced transcriptional repression. Genes 2023, 14, 1199. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Viswanath, A.; Malipatil, R.; Rathore, A.; Thirunavukkarasu, N. Structural and functional characteristics of miRNAs in five strategic millet species and their utility in drought tolerance. Front. Genet. 2020, 11, 608421. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, M.; Zhang, H.; Duan, L.; Sun, X.; Jiang, Q.; Hu, Z. Continuous salt stress-induced long non-coding RNAs and DNA methylation patterns in soybean roots. BMC Genom. 2019, 20, 730. [Google Scholar] [CrossRef]
- Candar-Cakir, B.; Arican, E.; Zhang, B. Small RNA and degradome deep sequencing reveals drought-and tissue-specific micrornas and their important roles in drought-sensitive and drought-tolerant tomato genotypes. Plant Biotechnol. J. 2016, 14, 1727–1746. [Google Scholar] [CrossRef]
- Tombuloglu, G. Drought-responsive miRNAs in plants: A review. Int. J. Innov. Eng. Appl. 2022, 6, 150–157. [Google Scholar] [CrossRef]
- Gao, W.; Li, M.; Yang, S.; Gao, C.; Su, Y.; Zeng, X.; Xia, K. miR2105 and the kinase OsSAPK10 co-regulate OsbZIP86 to mediate drought-induced ABA biosynthesis in rice. Plant Physiol. 2022, 189, 889–905. [Google Scholar] [CrossRef]
- Kaur, S.; Seem, K.; Kumar, D.; Kumar, S.; Kaundal, R.; Mohapatra, T. Biogenesis to functional significance of microRNAs under drought stress in rice: Recent advances and future perspectives. Plant Stress 2024, 12, 100447. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Wang, X.; Ijaz, M.; Oranab, S.; Ali, M.A.; Fiaz, S. Molecular aspects of microRNAs and phytohormonal signaling in response to drought stress: A review. Curr. Issues Mol. Biol. 2022, 44, 3695–3710. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, J.; Zhang, N.; Wu, J.; Si, H. Roles of microRNAs in abiotic stress response and characteristics regulation of plant. Front. Plant Sci. 2022, 13, 919243. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, J.; Chen, R.; Song, C.; Wei, P.; Cai, Y.; Wang, Y.; Han, B. NA-Based Drought Regulation in the Important Medicinal Plant Dendrobium huoshanense. J. Plant Growth Regul. 2022, 41, 1099–1108. [Google Scholar] [CrossRef]
- Jiao, P.; Ma, R.; Wang, C.; Chen, N.; Liu, S.; Qu, J.; Ma, Y. Integration of mRNA and microRNA analysis reveals the molecular mechanisms underlying drought stress tolerance in maize (Zea mays L.). Front. Plant Sci. 2022, 13, 932667. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L. Multiple regression analysis reveals MicroRNA regulatory networks in Oryza sativa under drought stress. Int. J. Genom. 2018, 2018, 9395261. [Google Scholar] [CrossRef] [PubMed]
- Saminathan, T.; Alvarado, A.; Lopez, C.; Shinde, S.; Gajanayake, B.; Abburi, V.L.; Reddy, U.K. Elevated carbon dioxide and drought modulate physiology and storage-root development in sweet potato by regulating microRNAs. Funct. Integr. Genom. 2019, 19, 171–190. [Google Scholar] [CrossRef]
- He, Z.; Liu, C.; Zhang, Z.; Wang, R.; Chen, Y. Integration of mRNA and miRNA analysis reveals the differentially regulatory network in two different Camellia oleifera cultivars under drought stress. Front. Plant Sci. 2022, 13, 1001357. [Google Scholar] [CrossRef]
- Fard, E.M.; Bakhshi, B.; Farsi, M.; Kakhki, A.M.; Nikpay, N.; Ebrahimi, M.A.; Salekdeh, G.H. MicroRNAs regulate the main events in rice drought stress response by manipulating the water supply to shoots. Mol. Biosyst. 2017, 13, 2289–2302. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, F.; Shiran, B.; Fallahi, H.; Mirakhorli, N.; Budak, H.; Martínez-Gómez, P. In silico search and biological validation of microRNAs related to drought response in peach and almond. Funct. Integr. Genom. 2017, 17, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Cao, Y.; Zhao, Z.; Yang, L.; Zhang, Y.; Dong, Y.; Fan, G. Discovery of microRNAs and their target genes related to drought in Paulownia “Yuza 1” by high-throughput sequencing. Int. J. Genom. 2017, 2017, 3674682. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, C.; Niu, X.; Wang, L.; Li, L.; Yuan, Q.; Pei, X. Research on lncRNA related to drought resistance of Shanlan upland rice. BMC Genom. 2022, 23, 336. [Google Scholar] [CrossRef]
- Das, P.; Adhikari, D. Role of epigenetics and the high-throughput sensing techniques to detect stress adaptation mechanisms in crop plants: A mini review. Innov. Agric. 2023, 6, e32855. [Google Scholar] [CrossRef]
- Minnoye, L.; Marinov, G.K.; Krausgruber, T.; Pan, L.; Marand, A.P.; Secchia, S.; Aerts, S. Chromatin accessibility profiling methods. Nat. Rev. Methods Primers 2021, 1, 10. [Google Scholar] [CrossRef]
- Chenarani, N.; Emamjomeh, A.; Allahverdi, A.; Mirmostafa, S.; Afsharinia, M.H.; Zahiri, J. Bioinformatic tools for DNA methylation and histone modification: A survey. Genomics 2021, 113, 1098–1113. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tollefsbol, T.O. DNA methylation methods: Global DNA methylation and methylomic analyses. Methods 2021, 187, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zhang, C.; Shi, W.; Chen, H. High-throughput sequencing reveals microRNAs and their targets in response to drought stress in wheat (Triticum aestivum L.). Biotechnol. Biotechnol. Equip. 2019, 33, 465–471. [Google Scholar] [CrossRef]
- Shaik, R.; Ramakrishna, W. Bioinformatic analysis of epigenetic and microRNA mediated regulation of drought responsive genes in rice. PLoS ONE 2012, 7, e49331. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, L.; Xia, H.; Wei, H.; Lou, Q.; Li, M.; Luo, L. Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci. Rep. 2017, 7, 39843. [Google Scholar] [CrossRef]
- Garg, R.; Narayana Chevala, V.V.S.; Shankar, R.; Jain, M. Divergent DNA methylation patterns associated with gene expression in rice cultivars with contrasting drought and salinity stress response. Sci. Rep. 2015, 5, 14922. [Google Scholar] [CrossRef] [PubMed]
- Mundade, R.; Ozer, H.G.; Wei, H.; Prabhu, L.; Lu, T. Role of ChIP-seq in the discovery of transcription factor binding sites, differential gene regulation mechanism, epigenetic marks and beyond. Cell Cycle 2014, 13, 2847–2852. [Google Scholar] [CrossRef] [PubMed]
- Fosslie, M.; Manaf, A.; Lerdrup, M.; Hansen, K.; Gilfillan, G.D.; Dahl, J.A. Going low to reach high: Small-scale ChIP-seq maps new terrain. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1465. [Google Scholar] [CrossRef]
- Song, J.; Henry, H.; Tian, L. Drought-inducible changes in the histone modification H3K9ac are associated with drought-responsive gene expression in Brachypodium distachyon. Plant Biol. 2020, 22, 433–440. [Google Scholar] [CrossRef]
- Dasgupta, P.; Prasad, P.; Bag, S.K.; Chaudhuri, S. Dynamicity of histone H3K27ac and H3K27me3 modifications regulate the cold-responsive gene expression in Oryza sativa L. ssp. indica. Genomics 2022, 114, 110433. [Google Scholar] [CrossRef]
- Zong, W.; Yang, J.; Fu, J.; Xiong, L. Synergistic regulation of drought-responsive genes by transcription factor OsbZIP23 and histone modification in rice. J. Integr. Plant Biol. 2020, 62, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, J.; Deng, M.; Wang, C.; Wang, R.; Yan, J.; Xu, J. Integrating ATAC-seq and RNA-seq reveals the dynamics of chromatin accessibility and gene expression in apple response to drought. Int. J. Mol. Sci. 2022, 23, 11191. [Google Scholar] [CrossRef]
- Mladenov, P.; Wang, X.; Yang, Z.; Djilianov, D.; Deng, X. Dynamics of chromatin accessibility and genome wide control of desiccation tolerance in the resurrection plant Haberlea rhodopensis. BMC Plant Biol. 2023, 23, 654. [Google Scholar] [CrossRef]
- Yang, J.; Chang, Y.; Qin, Y.; Chen, D.; Zhu, T.; Peng, K.; Xiong, L. A lamin-like protein OsNMCP1 regulates drought resistance and root growth through chromatin accessibility modulation by interacting with a chromatin remodeller OsSWI3C in rice. New Phytol. 2020, 227, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Arora, I.; Tollefsbol, T.O. Computational methods and next-generation sequencing approaches to analyze epigenetics data: Profiling of methods and applications. Methods 2021, 187, 92–103. [Google Scholar] [CrossRef]
- Sheoran, S.; Kaur, Y.; Kumar, S.; Shukla, S.; Rakshit, S.; Kumar, R. Recent advances for drought stress tolerance in maize (Zea mays L.): Present status and future prospects. Front. Plant Sci. 2022, 13, 872566. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, J.; Pu, X.; Lv, H.; Liu, Y.; Ma, H.; Wu, F.; Wang, Q.; Feng, X.; Liu, T.; et al. Maize DNA methylation in response to drought stress is involved in target gene expression and alternative splicing. Int. J. Mol. Sci. 2021, 22, 8285. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, X.; Zhang, Q.; Zheng, Q.; Yao, H.; Gu, X.; Zhu, Z. H3K36 demethylase JMJ710 negatively regulates drought tolerance by suppressing MYB48-1 expression in rice. Plant Physiol. 2022, 189, 1050–1064. [Google Scholar] [CrossRef]
- Mushtaq, M.; Ahmad Dar, A.; Skalicky, M.; Tyagi, A.; Bhagat, N.; Basu, U.; EL Sabagh, A. CRISPR-based genome editing tools: Insights into technological breakthroughs and future challenges. Genes 2021, 12, 797. [Google Scholar] [CrossRef]
- Thakore, P.I.; D’ippolito, A.M.; Song, L.; Safi, A.; Shivakumar, N.K.; Kabadi, A.M.; Gersbach, C.A. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat. Methods 2015, 12, 1143–1149. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, J.S.; Ko, J.H.; Kim, Y.S. Regulation of gene expression by altered promoter methylation using a CRISPR/Cas9-mediated epigenetic editing system. Sci. Rep. 2019, 9, 11960. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Mazarei, M.; Pfotenhauer, A.C.; Dorrough, A.B.; Poindexter, M.R.; Hewezi, T.; Stewart, C.N., Jr. Epigenetic footprints of CRISPR/Cas9-mediated genome editing in plants. Front. Plant Sci. 2020, 10, 1720. [Google Scholar] [CrossRef]
- Devesa-Guerra, I.; Morales-Ruiz, T.; Pérez-Roldán, J.; Parrilla-Doblas, J.T.; Dorado-León, M.; García-Ortiz, M.V.; Roldán-Arjona, T. DNA methylation editing by CRISPR-guided excision of 5-methylcytosine. J. Mol. Biol. 2020, 432, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Corem, S.; Doron-Faigenboim, A.; Jouffroy, O.; Maumus, F.; Arazi, T.; Bouché, N. Redistribution of CHH methylation and small interfering RNAs across the genome of tomato ddm1 mutants. Plant Cell 2018, 30, 1628–1644. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, J.J.; Wedel, C.; Cosentino, R.O.; Siegel, T.N. Exploiting CRISPR–Cas9 technology to investigate individual histone modifications. Nucleic Acids Res. 2018, 46, e106. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Wang, Y.; Peng, Y.; Zhang, H.; Zheng, J. ZmHDT103 negatively regulates drought stress tolerance in maize seedlings. Agronomy 2024, 14, 134. [Google Scholar] [CrossRef]
| Plants | Genes | Findings | Mechanistic Focus | Citation |
|---|---|---|---|---|
| Tomato | Various genes and gene regions | Epigenomic modifications via self-grafting influence drought response and hormone regulation. | Histone modification, hormones | [41] |
| Barley | Various genes and gene regions | Epigenetic and gene expression changes underlie drought response with ABA involvement. | Histone modification, ABA | [57] |
| Barley | WHIRLY1 | Histone modifications affect ABA-related gene expression and drought response. | Histone modification, hormones | [58] |
| Wheat | JmjC genes | JmjC proteins in wheat contribute to drought response and hormone-related pathways. | Histone modification, hormones | [59] |
| Tomato | SlHDA5 | Histone deacetylation impacts drought response through hormone signaling. | Histone modification, hormones | [60] |
| Chinese Cabbage | BrHIS4.A04 | Histone modification links to hormone signaling in drought-induced flowering adaptation. | Histone modification, hormones | [61] |
| Tomato | SlHDA3 | Histone deacetylation affects salt and drought tolerance via hormone responses. | Histone modification, hormones | [62] |
| Sea Buckthorn Seedlings | Various genes related to ABA signaling | H3K9 acetylation positively regulates drought-related genes and hormone signaling. | Histone modification, hormones | [63] |
| Poplar | 84KHDA903 | Histone modification aids drought stress adaptation via positive regulation. | Histone modification, drought | [64] |
| Arabidopsis | HD2A, HD2B | Histone deacetylation negatively modulates drought resistance via ABA signaling. | Histone modification, hormones | [65] |
| Plants | Genes | Key Findings | Citation |
|---|---|---|---|
| Tomato (drought-responsive genotypes) | Drought-related miRNAs | Drought-tolerant tomato breeding involves miRNA-mediated hormonal regulation and stress-related genes. | [68] |
| Dendrobium huoshanense | miRNAs | MiRNAs play a crucial role in Dendrobium’s response to drought, linked to hormone signaling. | [74] |
| Maize (M8186 variety) | DEMIRs, DEMRs | MiRNAs regulate maize root response to drought, with miR408a impacting reactive oxygen species. | [75] |
| Rice (Nipponbare cultivar) | miRNAs, target mRNAs | MiRNA-mRNA interactions influence rice’s drought response through hormone signaling and other pathways. | [76] |
| Sweet potato | Known and novel miRNAs | MiRNAs under elevated CO2 and drought conditions target genes related to stress, photosynthesis, etc. | [77] |
| Camellia oleifera Abel. | miRNAs, target genes | Differentially expressed genes related to photosynthesis, hormone signaling, and drought tolerance. | [78] |
| Rice (IR64 cultivar) | miRNAs | miRNAs regulate rice’s adaptation to drought, targeting transcription factors and hormonal regulators. | [79] |
| Peach, almond, peach–almond hybrid | miRNAs | Drought-responsive miRNAs impact hormone signaling in peach and almond, aiding drought stress response. | [80] |
| Paulownia “yuza 1” | miRNAs, target genes | miRNAs and target genes contribute to Paulownia’s drought resistance, impacting hormonal regulation. | [81] |
| Rice | lncRNAs, miRNAs, mRNAs | lncRNAs, miRNAs, and mRNAs play roles in rice’s drought resistance, with a focus on hormone signaling. | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, C.; Uğurlar, F.; Adamakis, I.-D.S. Epigenetic Modifications of Hormonal Signaling Pathways in Plant Drought Response and Tolerance for Sustainable Food Security. Int. J. Mol. Sci. 2024, 25, 8229. https://doi.org/10.3390/ijms25158229
Kaya C, Uğurlar F, Adamakis I-DS. Epigenetic Modifications of Hormonal Signaling Pathways in Plant Drought Response and Tolerance for Sustainable Food Security. International Journal of Molecular Sciences. 2024; 25(15):8229. https://doi.org/10.3390/ijms25158229
Chicago/Turabian StyleKaya, Cengiz, Ferhat Uğurlar, and Ioannis-Dimosthenis S. Adamakis. 2024. "Epigenetic Modifications of Hormonal Signaling Pathways in Plant Drought Response and Tolerance for Sustainable Food Security" International Journal of Molecular Sciences 25, no. 15: 8229. https://doi.org/10.3390/ijms25158229






