Skin Aging and the Upcoming Role of Ferroptosis in Geroscience
Abstract
:1. Introduction
1.1. Normal Skin Morphology
1.2. Effects of Aging
2. Skin Aging
2.1. Intrinsic Aging
2.2. Extrinsic Aging
3. Features of Aging Cells in the Skin
4. Aging in Cutaneous Compartments and in Skin Cells
4.1. Aging in Epidermis
4.2. Aging in Dermis
4.3. Aging in the Subcutaneous Layer
4.4. Aging in the Immune System Cells
5. Pathomechanisms of Skin Aging
5.1. Protein Metabolism
5.2. Glucose Metabolism
5.3. Lipid Metabolism
5.4. Iron Metabolism
6. Molecular Mechanisms in Skin Aging
6.1. Cell Death for Skin Aging
6.1.1. Ferroptosis as a New Cell Death Discover
6.1.2. Ferroptosis in Skin Aging and Diseases
Ferroptosis and Melanoma
Ferroptosis and Autoimmune Diseases
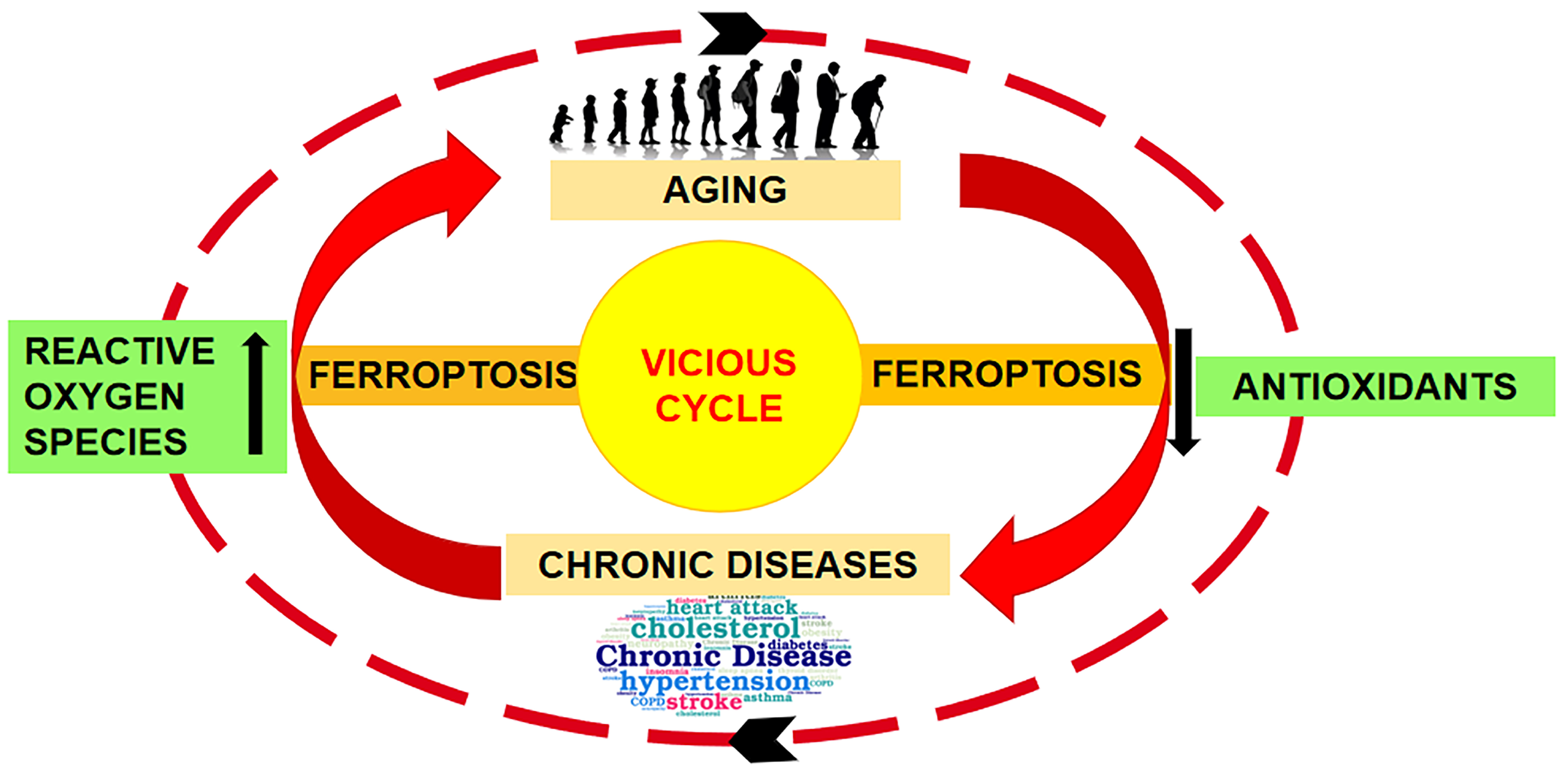
7. Concluding Remarks and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.S.H. Natural Anti-Aging Skincare: Role and Potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.; Mikail, M.A. Anti-Aging Skincare: The Natural and Organic Way. In Anti-Aging Pharmacology; Koltover, V.K.B.T.-A.-A.P., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 269–284. ISBN 978-0-12-823679-6. [Google Scholar]
- Ahmed, I.A.; Mikail, M.A. Diet and Skin Health: The Good and the Bad. Nutrition 2024, 119, 112350. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin Senescence: Mechanisms and Impact on Whole-Body Aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef]
- He, X.; Gao, X.; Xie, W. Research Progress in Skin Aging, Metabolism, and Related Products. Int. J. Mol. Sci. 2023, 24, 15930. [Google Scholar] [CrossRef]
- Ponnappan, S.; Ponnappan, U. Aging and Immune Function: Molecular Mechanisms to Interventions. Antioxid. Redox Signal. 2011, 14, 1551–1585. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, M.; Din, A.U.; Ali, H.; Yang, G.; Ren, W.; Wang, L.; Fan, X.; Yang, S. Implication of Ferroptosis in Aging. Cell Death Discov. 2021, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Wawer, A.A.; Gillings, R.; Jennings, A.; Myint, P.K. Iron Status in the Elderly. Mech. Ageing Dev. 2014, 136–137, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Yanatori, I.; Kong, Y.; Zheng, H.; Motooka, Y.; Jiang, L. Ferroptosis at the Crossroads of Infection, Aging and Cancer. Cancer Sci. 2020, 111, 2665–2671. [Google Scholar] [CrossRef]
- Goyal, M.S.; Vlassenko, A.G.; Blazey, T.M.; Su, Y.; Couture, L.E.; Durbin, T.J.; Bateman, R.J.; Benzinger, T.L.S.; Morris, J.C.; Raichle, M.E. Loss of Brain Aerobic Glycolysis in Normal Human Aging. Cell Metab. 2017, 26, 353–360.e3. [Google Scholar] [CrossRef]
- Kitazoe, Y.; Kishino, H.; Tanisawa, K.; Udaka, K.; Tanaka, M. Renormalized Basal Metabolic Rate Describes the Human Aging Process and Longevity. Aging Cell 2019, 18, e12968. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Conrad, M.; Pratt, D.A. The Chemical Basis of Ferroptosis. Nat. Chem. Biol. 2019, 15, 1137–1147. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Li, T.; Wang, K.; Huang, X.; Shi, J.; Wang, Y. Mechanisms and Inhibitors of Ferroptosis in Psoriasis. Front. Mol. Biosci. 2022, 9, 1019447. [Google Scholar] [CrossRef] [PubMed]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses’ Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Ansari, M.; Darvishi, A. A Review of the Current State of Natural Biomaterials in Wound Healing Applications. Front. Bioeng. Biotechnol. 2024, 12, 1309541. [Google Scholar] [CrossRef]
- Khavkin, J.; Ellis, D.A.F. Aging Skin: Histology, Physiology, and Pathology. Facial Plast. Surg. Clin. North. Am. 2011, 19, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Gianò, M.; Franco, C.; Pinto, D.; van Noorden, C.J.F.; Rinaldi, F.; Rezzani, R. Relation Between Reactive Oxygen Species Production and Transient Receptor Potential Vanilloid1 Expression in Human Skin During Aging. J. Histochem. Cytochem. 2024, 72, 157–171. [Google Scholar] [CrossRef]
- Wu, T.; Xiong, X.; Zhang, W.; Zou, H.; Xie, H.; He, S. Morphogenesis of Rete Ridges in Human Oral Mucosa: A Pioneering Morphological and Immunohistochemical Study. Cells Tissues Organs 2013, 197, 239–248. [Google Scholar] [CrossRef]
- Danielyan, L.; Zellmer, S.; Sickinger, S.; Tolstonog, G.V.; Salvetter, J.; Lourhmati, A.; Reissig, D.D.; Gleiter, C.H.; Gebhardt, R.; Buniatian, G.H. Keratinocytes as Depository of Ammonium-Inducible Glutamine Synthetase: Age- and Anatomy-Dependent Distribution in Human and Rat Skin. PLoS ONE 2009, 4, e4416. [Google Scholar] [CrossRef]
- Brito, S.; Baek, J.M.; Cha, B.; Heo, H.; Lee, S.H.; Lei, L.; Jung, S.Y.; Lee, S.M.; Lee, S.H.; Kwak, B.M.; et al. Nicotinamide Mononucleotide Reduces Melanin Production in Aged Melanocytes by Inhibiting CAMP/Wnt Signaling. J. Dermatol. Sci. 2022, 106, 159–169. [Google Scholar] [CrossRef]
- Losquadro, W.D. Anatomy of the Skin and the Pathogenesis of Nonmelanoma Skin Cancer. Facial Plast. Surg. Clin. N. Am. 2017, 25, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin Barrier Immunity and Ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Wu, C.; He, Y.; Lu, F. Skin-Associated Adipocytes in Skin Barrier Immunity: A Mini-Review. Front. Immunol. 2023, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, Q.; Zhong, Q.; Duan, C.; Krutmann, J.; Wang, J.; Xia, J. Skin Microbiome, Metabolome and Skin Phenome, from the Perspectives of Skin as an Ecosystem. Phenomics 2022, 2, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Hoffmann, A. The Cutaneous Ecosystem: The Roles of the Skin Microbiome in Health and Its Association with Inflammatory Skin Conditions in Humans and Animals. Vet. Dermatol. 2017, 28, 60-e15. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and Maintenance of Skin Barrier Function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Dokoshi, T.; Seidman, J.S.; Cavagnero, K.J.; Li, F.; Liggins, M.C.; Taylor, B.C.; Olvera, J.; Knight, R.; Chang, J.T.; Salzman, N.H.; et al. Skin Inflammation Activates Intestinal Stromal Fibroblasts and Promotes Colitis. J. Clin. Investig. 2021, 131, e147614. [Google Scholar] [CrossRef]
- Oyoshi, M.K.; Elkhal, A.; Scott, J.E.; Wurbel, M.A.; Hornick, J.L.; Campbell, J.J.; Geha, R.S. Epicutaneous Challenge of Orally Immunized Mice Redirects Antigen-Specific Gut-Homing T Cells to the Skin. J. Clin. Investig. 2011, 121, 2210–2220. [Google Scholar] [CrossRef]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The Role of Cellular Senescence in Skin Aging and Age-Related Skin Pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Park, S. Biochemical, Structural and Physical Changes in Aging Human Skin, and Their Relationship. Biogerontology 2022, 23, 275–288. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Quan, T. Molecular Insights of Human Skin Epidermal and Dermal Aging. J. Dermatol. Sci. 2023, 112, 48–53. [Google Scholar] [CrossRef]
- Wang, Z.; Man, M.; Li, T.; Elias, P.M.; Mauro, T.M. Aging-Associated Alterations in Epidermal Function and Their Clinical Significance. Aging 2020, 12, 5551–5565. [Google Scholar] [CrossRef]
- Gunin, A.G.; Kornilova, N.K.; Vasilieva, O.V.; Petrov, V.V. Age-Related Changes in Proliferation, the Numbers of Mast Cells, Eosinophils, and Cd45-Positive Cells in Human Dermis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66 A, 385–392. [Google Scholar] [CrossRef]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From Discoveries in Ageing Research to Therapeutics for Healthy Ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking Aging to Chronic Disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Fabbri, E.; Zoli, M.; Gonzalez-Freire, M.; Salive, M.E.; Studenski, S.A.; Ferrucci, L. Aging and Multimorbidity: New Tasks, Priorities, and Frontiers for Integrated Gerontological and Clinical Research. J. Am. Med. Dir. Assoc. 2015, 16, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef] [PubMed]
- St Sauver, J.L.; Boyd, C.M.; Grossardt, B.R.; Bobo, W.V.; Rutten, L.J.F.; Roger, V.L.; Ebbert, J.O.; Therneau, T.M.; Yawn, B.P.; Rocca, W.A. Risk of Developing Multimorbidity across All Ages in an Historical Cohort Study: Differences by Sex and Ethnicity. BMJ Open 2015, 5, e006413. [Google Scholar] [CrossRef] [PubMed]
- Berneburg, M.; Plettenberg, H.; Krutmann, J. Photoaging of Human Skin. Photodermatol. Photoimmunol. Photomed. 2000, 16, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Dewberry, C.; Norman, R.A. Skin Cancer in Elderly Patients. Dermatol. Clin. 2004, 22, 93–96. [Google Scholar] [CrossRef]
- Puizina-Ivić, N. Skin Aging. Acta Dermatovenerol. Alp. Pannonica Adriat. 2008, 17, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Perricone, N. The Wrinkle Cure; Warner Books: New York, NY, USA, 2001. [Google Scholar]
- Mora Huertas, A.C.; Schmelzer, C.E.H.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-Level Insights into Aging Processes of Skin Elastin. Biochimie 2016, 128–129, 163–173. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Lena, A.M.; Saintigny, G.; Mahé, C.; Di Daniele, N.; Melino, G.; Candi, E. MicroRNAs in Human Skin Ageing. Ageing Res. Rev. 2014, 17, 9–15. [Google Scholar] [CrossRef]
- Montagna, W.; Carlisle, K. Structural Changes in Aging Human Skin. J. Investig. Dermatol. 1979, 73, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Bekou, V.; Zouboulis, C.C. Genetics and Skin Aging. Dermato-Endocrinology 2012, 4, 280–284. [Google Scholar] [CrossRef]
- Cheng, W.; Yan-hua, R.; Fang-gang, N.; Guo-an, Z. The Content and Ratio of Type I and III Collagen in Skin Differ with Age and Injury. Afr. J. Biotechnol. 2011, 10, 2524–2529. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin: Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Gao, J.; Guo, Z.; Zhang, Y.; Liu, Y.; Xing, F.; Wang, J.; Luo, X.; Kong, Y.; Zhang, G. Age-Related Changes in the Ratio of Type I/III Collagen and Fibril Diameter in Mouse Skin. Regen. Biomater. 2023, 10, rbac110. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Natsuga, K.; Nishie, W.; Kobayashi, Y.; Donati, G.; Suzuki, S.; Fujimura, Y.; Tsukiyama, T.; Ujiie, H.; Shinkuma, S.; et al. Type XVII Collagen Coordinates Proliferation in the Interfollicular Epidermis. Elife 2017, 6, e26635. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Mohri, Y.; Thanh Binh, N.; Morinaga, H.; Fukuda, M.; Ito, M.; Kurata, S.; Hoeijmakers, J.; Nishimura, E.K. Stem Cells: Hair Follicle Aging Is Driven by Transepidermal Elimination of Stem Cells via COL17A1 Proteolysis. Science 2016, 351, aad4395. [Google Scholar] [CrossRef] [PubMed]
- Nishie, W.; Sawamura, D.; Goto, M.; Ito, K.; Shibaki, A.; McMillan, J.R.; Sakai, K.; Nakamura, H.; Olasz, E.; Yancey, K.B.; et al. Humanization of Autoantigen. Nat. Med. 2007, 13, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, S.; Tadokoro, Y.; Inomata, K.; Binh, N.T.; Nishie, W.; Yamazaki, S.; Nakauchi, H.; Tanaka, Y.; McMillan, J.R.; Sawamura, D.; et al. Hair Follicle Stem Cells Provide a Functional Niche for Melanocyte Stem Cells. Cell Stem Cell 2011, 8, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kitahata, H.; Kosumi, H.; Watanabe, M.; Fujimura, Y.; Takashima, S.; Osada, S.I.; Hirose, T.; Nishie, W.; Nagayama, M.; et al. Collagen XVII Deficiency Alters Epidermal Patterning. Lab. Investig. 2022, 102, 581–588. [Google Scholar] [CrossRef]
- Tobin, D.J.; Paus, R. Graying: Gerontobiology of the Hair Follicle Pigmentary Unit. Exp. Gerontol. 2001, 36, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Erusalimsky, J.D. Oxidative Stress, Telomeres and Cellular Senescence: What Non-Drug Interventions Might Break the Link? Free Radic. Biol. Med. 2020, 150, 87–95. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Kohl, E.; Torezan, L.; Landthaler, M.; Szeimies, R.M. Aesthetic Effects of Topical Photodynamic Therapy. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1261–1269. [Google Scholar] [CrossRef]
- Ferguson, J.; Dover, J. Photodermatology, 1st ed.; Manson Publishing: London, UK, 2006; ISBN 9781840765168. [Google Scholar]
- Klotz, L.O.; Holbrook, N.J.; Sies, H. UVA and Singlet Oxygen as Inducers of Cutaneous Signaling Events. Curr. Probl. Dermatol. 2001, 29, 95–113. [Google Scholar] [CrossRef]
- Scioli, M.G.; Bielli, A.; Arcuri, G.; Ferlosio, A.; Orlandi, A. Ageing and Microvasculature. Vasc. Cell 2014, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F. Effects of Central Arterial Aging on the Structure and Function of the Peripheral Vasculature: Implications for End-Organ Damage. J. Appl. Physiol. 2008, 105, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; Ritschka, B.; Keyes, W.M. Cellular Senescence in Development, Regeneration and Disease. Development 2019, 146, dev151837. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Laberge, R.M.; Demaria, M.; Campisi, J. Lamin B1 Loss Is a Senescence-Associated Biomarker. Mol. Biol. Cell 2012, 23, 2066–2075. [Google Scholar] [CrossRef]
- Wang, A.S.; Nakamizo, S.; Ishida, Y.; Klassen, G.; Chong, P.; Wada, A.; Lim, J.S.Y.; Wright, G.D.; Kabashima, K.; Dreesen, O. Identification and Quantification of Senescent Cell Types by Lamin B1 and HMGB1 in Actinic Keratosis Lesions. J. Dermatol. Sci. 2022, 105, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, E.W.; Croteau, D.L.; Bohr, V.A. Heterochromatin: An Epigenetic Point of View in Aging. Exp. Mol. Med. 2020, 52, 1466–1474. [Google Scholar] [CrossRef]
- Abercrombie, M.; Dunn, G.A. Adhesions of Fibroblasts to Substratum during Contact Inhibition Observed by Interference Reflection Microscopy. Exp. Cell Res. 1975, 92, 57–62. [Google Scholar] [CrossRef]
- Basisty, N.; Kale, A.; Jeon, O.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Ferrucci, L.; Campisi, J.; et al. A Proteomic Atlas of Senescence-Associated Secretomes for Aging Biomarker Development. SSRN Electron. J. 2020, 18, e3000599. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The Senescence-Associated Secretory Phenotype and Its Physiological and Pathological Implications. Nat. Rev. Mol. Cell Biol. 2024; 1–21, Online ahead of print. [Google Scholar] [CrossRef]
- Jin, P.; Duan, X.; Li, L.; Zhou, P.; Zou, C.G.; Xie, K. Cellular Senescence in Cancer: Molecular Mechanisms and Therapeutic Targets. MedComm 2024, 5, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Lagnado, A.; Halim, J.; Moore, W.; Talbot, D.; Barrett, K.; Chapman, J.; Birch, J.; Ogrodnik, M.; Meves, A.; et al. Senescent Human Melanocytes Drive Skin Ageing via Paracrine Telomere Dysfunction. EMBO J. 2019, 38, e101982. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, T.J.; Kweon, Y.Y.; Baek, D.J.; Lee, J.W.; Kang, H.Y. Age-Dependent Sequential Increase of Senescent Cells in the Skin. J. Invest. Dermatol. 2022, 142, 2521–2523.e1. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Qin, Y.; Huo, F.; Jian, Z.; Li, X.; Geng, J.; Li, Y.; Wu, J. NMN Recruits GSH to Enhance GPX4-Mediated Ferroptosis Defense in UV Irradiation Induced Skin Injury. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166287. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.R.; Tan, C.L.; Chin, T.; Morenc, M.; Ho, C.Y.; Rovito, H.A.; Quek, L.S.; Soon, A.L.; Lim, J.S.Y.; Dreesen, O.; et al. Nicotinamide Prevents UVB- and Oxidative Stress–Induced Photoaging in Human Primary Keratinocytes. J. Investig. Dermatol. 2022, 142, 1670–1681.e12. [Google Scholar] [CrossRef]
- Verdier-Sévrain, S.; Bonté, F. Skin Hydration: A Review on Its Molecular Mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Bae, I.H.; Lee, E.S.; Kim, H.J.; Lee, J.; Lee, C.S. Glucose Exerts an Anti-melanogenic Effect by Indirect Inactivation of Tyrosinase in Melanocytes and a Human Skin Equivalent. Int. J. Mol. Sci. 2020, 21, 1736. [Google Scholar] [CrossRef]
- Yoon, J.E.; Kim, Y.; Kwon, S.; Kim, M.; Kim, Y.H.; Kim, J.H.; Park, T.J.; Kang, H.Y. Senescent Fibroblasts Drive Ageing Pigmentation: A Potential Therapeutic Target for Senile Lentigo. Theranostics 2018, 8, 4620–4632. [Google Scholar] [CrossRef]
- Park, J.H.; Yoon, J.E.; Kim, Y.H.; Kim, Y.; Park, T.J.; Kang, H.Y. The Potential Skin-Lightening Candidate, Senolytic Drug ABT263, for Photoageing Pigmentation. Br. J. Dermatol. 2022, 186, 740–742. [Google Scholar] [CrossRef]
- Shin, J.; Park, J.Y.; Kim, S.J.; Kang, H.Y. Characteristics of Keratinocytes in Facial Solar Lentigo with Flattened Rete Ridges: Comparison with Melasma. Clin. Exp. Dermatol. 2015, 40, 489–494. [Google Scholar] [CrossRef]
- Pereira, B.I.; Devine, O.P.; Vukmanovic-Stejic, M.; Chambers, E.S.; Subramanian, P.; Patel, N.; Virasami, A.; Sebire, N.J.; Kinsler, V.; Valdovinos, A.; et al. Senescent Cells Evade Immune Clearance via HLA-E-Mediated NK and CD8+ T Cell Inhibition. Nat. Commun. 2019, 10, 2387. [Google Scholar] [CrossRef]
- Lago, J.C.; Puzzi, M.B. The Effect of Aging in Primary Human Dermal Fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Hammerberg, C.; Li, Y.; He, T.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Expression of Catalytically Active Matrix Metalloproteinase-1 in Dermal Fibroblasts Induces Collagen Fragmentation and Functional Alterations That Resemble Aged Human Skin. Aging Cell 2013, 12, 661–671. [Google Scholar] [CrossRef]
- Soydas, T.; Sayitoglu, M.; Sarac, E.Y.; Cınar, S.; Solakoglu, S.; Tiryaki, T.; Sultuybek, G.K. Metformin Reverses the Effects of High Glucose on Human Dermal Fibroblasts of Aged Skin via Downregulating RELA/P65 Expression. J. Physiol. Biochem. 2021, 77, 443–450. [Google Scholar] [CrossRef]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced Glycation End Products (AGEs) May Be a Striking Link between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef]
- Fenske, N.A.; Lober, C.W. Structural and Functional Changes of Normal Aging Skin. J. Am. Acad. Dermatol. 1986, 15, 571–585. [Google Scholar] [CrossRef]
- Wollina, U.; Wetzker, R.; Abdel-Naser, M.B.; Kruglikov, I.L. Role of Adipose Tissue in Facial Aging. Clin. Interv. Aging 2017, 12, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.L.; Scherer, P.E. General Theory of Skin Reinforcement. PLoS ONE 2017, 12, e0182865. [Google Scholar] [CrossRef]
- Wernstedt Asterholm, I.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte Inflammation Is Essential for Healthy Adipose Tissue Expansion and Remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Harkins, J.M.; Moustaid-Moussa, N.; Chung, Y.J.; Penner, K.M.; Pestka, J.J.; North, C.M.; Claycombe, K.J. Expression of Interleukin-6 Is Greater in Preadipocytes than in Adipocytes of 3T3-L1 Cells and C57BL/6J and Ob/Ob Mice. J. Nutr. 2004, 134, 2673–2677. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Stout, M.B.; Giorgadze, N.; Wang, L.; Li, P.W.; Heppelmann, C.J.; Bouloumié, A.; Jensen, M.D.; Robert Bergen, H.; et al. Inflammation and the Depot-Specific Secretome of Human Preadipocytes. Obesity 2015, 23, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Kim, J.A.; Lee, J.Y. Mechanisms for the Activation of Toll-like Receptor 2/4 by Saturated Fatty Acids and Inhibition by Docosahexaenoic Acid. Eur. J. Pharmacol. 2016, 785, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Graja, A.; Schulz, T.J. Mechanisms of Aging-Related Impairment of Brown Adipocyte Development and Function. Gerontology 2015, 61, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I. General Theory of Body Contouring: 2. Modulation of Mechanical Properties of Subcutaneous Fat Tissue. J. Cosmet. Dermatol. Sci. Appl. 2014, 4, 117–127. [Google Scholar] [CrossRef]
- Lassen, P.B.; Charlotte, F.; Liu, Y.; Bedossa, P.; Le Naour, G.; Tordjman, J.; Poitou, C.; Bouillot, J.L.; Genser, L.; Zucker, J.D.; et al. The Fat Score, a Fibrosis Score of Adipose Tissue: Predicting Weight-Loss Outcome after Gastric Bypass. J. Clin. Endocrinol. Metab. 2017, 102, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Gao, X.; Xie, W. Research Progress in Skin Aging and Immunity. Int. J. Mol. Sci. 2024, 25, 4101. [Google Scholar] [CrossRef]
- Clark, R.A.; Kupper, T.S. The Vast Majority of CLA+ T Cells Are Resident in Normal Skin. J. Immunol. 2006, 176, 4431–4439. [Google Scholar] [CrossRef]
- Rapozo Guimarães, G.; Almeida, P.P.; Santos, L.D.O.; Rodrigues, L.P.; de Carvalho, J.L.; Boroni, M. Hallmarks of Aging in Macrophages: Consequences to Skin Inflammaging. Cells 2021, 10, 1323. [Google Scholar] [CrossRef]
- Zhang, C.; Merana, G.R.; Harris-Tryon, T.; Scharschmidt, T.C. Skin Immunity: Dissecting the Complex Biology of Our Body’s Outer Barrier. Mucosal Immunol. 2022, 15, 551–561. [Google Scholar] [CrossRef]
- Agrawal, R.; Hu, A.; Bollag, W.B. The Skin and Inflamm-Aging. Biology 2023, 12, 1396. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Oka, T.; Son, H.G.; Oliver-García, V.S.; Azin, M.; Eisenhaure, T.M.; Lieb, D.J.; Hacohen, N.; Demehri, S. Cytotoxic CD4+ T Cells Eliminate Senescent Cells by Targeting Cytomegalovirus Antigen. Cell 2023, 186, 1417–1431.e20. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and Aging: Signaling Pathways and Intervention Therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Pająk, J.; Nowicka, D.; Szepietowski, J.C. Inflammaging and Immunosenescence as Part of Skin Aging—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 7784. [Google Scholar] [CrossRef]
- Chen, B.; Yang, J.; Song, Y.; Zhang, D.; Hao, F. Skin Immunosenescence and Type 2 Inflammation: A Mini-Review With an Inflammaging Perspective. Front. Cell Dev. Biol. 2022, 10, 835675. [Google Scholar] [CrossRef]
- Fekete, M.; Major, D.; Feher, A.; Fazekas-Pongor, V.; Lehoczki, A. Geroscience and Pathology: A New Frontier in Understanding Age-Related Diseases. Pathol. Oncol. Res. 2024, 30, 1611623. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV Radiation-Induced Inflammation and Immunosuppression Accelerate the Aging Process in the Skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Umbayev, B.; Askarova, S.; Almabayeva, A.; Saliev, T.; Masoud, A.R.; Bulanin, D. Galactose-Induced Skin Aging: The Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 23–26. [Google Scholar] [CrossRef]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-Skin Aging Properties of Protocatechuic Acid in Vitro and in Vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.K.; Kim, S.; Kim, J.E.; Tian, Y.D.; Doh, E.J.; Lee, D.H.; Chung, J.H. Adipochemokines Induced by Ultraviolet Irradiation Contribute to Impaired Fat Metabolism in Subcutaneous Fat Cells. Br. J. Dermatol. 2018, 178, 492–501. [Google Scholar] [CrossRef]
- Zoanni, B.; Aiello, G.; Negre-Salvayre, A.; Aldini, G.; Carini, M.; D’Amato, A. Lipidome Investigation of Carnosine Effect on Nude Mice Skin to Prevent UV-A Damage. Int. J. Mol. Sci. 2023, 24, 10009. [Google Scholar] [CrossRef]
- Anisimova, A.S.; Alexandrov, A.I.; Makarova, N.E.; Gladyshev, V.N.; Dmitriev, S.E. Protein Synthesis and Quality Control in Aging. Aging 2018, 10, 4269–4288. [Google Scholar] [CrossRef]
- Schönborn, K.; Willenborg, S.; Schulz, J.N.; Imhof, T.; Eming, S.A.; Quondamatteo, F.; Brinckmann, J.; Niehoff, A.; Paulsson, M.; Koch, M.; et al. Role of Collagen XII in Skin Homeostasis and Repair. Matrix Biol. 2020, 94, 57–76. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Keast, D. Energy Metabolism and The Skin. Int. J. Biochem. 1991, 23, 1175–1183. [Google Scholar] [CrossRef]
- Chen, J.H.; Lin, X.; Bu, C.; Zhang, X. Role of Advanced Glycation End Products in Mobility and Considerations in Possible Dietary and Nutritional Intervention Strategies. Nutr. Metab. 2018, 15, 72. [Google Scholar] [CrossRef]
- Nagarajan, S.R.; Paul-Heng, M.; Krycer, J.R.; Fazakerley, D.J.; Sharland, A.F.; Hoy, A.J. Lipid and Glucose Metabolism in Hepatocyte Cell Lines and Primary Mouse Hepatocytes: A Comprehensive Resource for in Vitro Studies of Hepatic Metabolism. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E578–E589. [Google Scholar] [CrossRef]
- Ji, S.; Xiong, M.; Chen, H.; Liu, Y.; Zhou, L.; Hong, Y.; Wang, M.; Wang, C.; Fu, X.; Sun, X. Cellular Rejuvenation: Molecular Mechanisms and Potential Therapeutic Interventions for Diseases. Signal Transduct. Target. Ther. 2023, 8, 116. [Google Scholar] [CrossRef]
- Fu, H.; Shan, D.; Li, J.; Swallah, M.S.; Yang, X.; Ji, L.; Wang, S.; Gong, H.; Lyu, B.; Yu, H. Potential Functionality of β-Conglycinin with Subunit Deficiencies: Soy Protein May Regulate Glucose and Lipid Metabolism. Food Funct. 2022, 13, 12291–12302. [Google Scholar] [CrossRef]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell Aging and Cellular Senescence in Skin Aging—Recent Advances in Fibroblast and Keratinocyte Biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Zeidan, R.S.; Martenson, M.; Tamargo, J.A.; McLaren, C.; Ezzati, A.; Lin, Y.; Yang, J.J.; Yoon, H.S.; McElroy, T.; Collins, J.F.; et al. Iron Homeostasis in Older Adults: Balancing Nutritional Requirements and Health Risks. J. Nutr. Health Aging 2024, 28, 100212. [Google Scholar] [CrossRef]
- Sukhbaatar, N.; Weichhart, T. Iron Regulation: Macrophages in Control. Pharmaceuticals 2018, 11, 137. [Google Scholar] [CrossRef]
- Pouillot, A.; Polla, A.; Polla, B. Iron and Iron Chelators: A Review on Potential Effects on Skin Aging. Curr. Aging Sci. 2014, 6, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, M.; Land, W.G.; Tonnus, W.; Hugo, C.P.; Linkermann, A. Origin and Consequences of Necroinflammation. Physiol. Rev. 2018, 98, 727–780. [Google Scholar] [CrossRef]
- Poon, I.K.H.; Lucas, C.D.; Rossi, A.G.; Ravichandran, K.S. Apoptotic Cell Clearance: Basic Biology and Therapeutic Potential. Nat. Rev. Immunol. 2014, 14, 166–180. [Google Scholar] [CrossRef]
- Khorsandi, K.; Esfahani, H.S.; Ghamsari, S.K.; Lakhshehei, P. Targeting Ferroptosis in Melanoma: Cancer Therapeutics. Cell Commun. Signal. 2023, 21, 337. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, G.; Zhang, P.; Fan, J. Programmed Cell Death and Its Role in Inflammation. Mil. Med. Res. 2015, 2, 1–12. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, P.; Zhai, B.; Zhang, M.; Xiang, Y.; Fang, J.; Xu, S.; Gao, Y.; Chen, X.; Sui, X.; et al. The Emerging Role of Ferroptosis in Inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef] [PubMed]
- Vats, K.; Kruglov, O.; Mizes, A.; Samovich, S.N.; Amoscato, A.A.; Tyurin, V.A.; Tyurina, Y.Y.; Kagan, V.E.; Bunimovich, Y.L. Keratinocyte Death by Ferroptosis Initiates Skin Inflammation after UVB Exposure. Redox Biol. 2021, 47, 102143. [Google Scholar] [CrossRef]
- Surbek, M.; Sukseree, S.; Eckhart, L. Iron Metabolism of the Skin: Recycling versus Release. Metabolites 2023, 13, 1005. [Google Scholar] [CrossRef] [PubMed]
- Hirschhorn, T.; Stockwell, B.R. The Development of the Concept of Ferroptosis. Free Radic. Biol. Med. 2019, 133, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in Cancer: From Molecular Mechanisms to Therapeutic Strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Yagoda, N.; Von Rechenberg, M.; Zaganjor, E.; Bauer, A.J.; Yang, W.S.; Fridman, D.J.; Wolpaw, A.J.; Smukste, I.; Peltier, J.M.; Boniface, J.J.; et al. RAS-RAF-MEK-Dependent Oxidative Cell Death Involving Voltage-Dependent Anion Channels. Nature 2007, 447, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and Apoptotic Body: Disease Message and Therapeutic Target Potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lian, N.; Shi, L.; Hao, Z.; Chen, K. Ferroptosis: Mechanism and Connections with Cutaneous Diseases. Front. Cell Dev. Biol. 2023, 10, 1079548. [Google Scholar] [CrossRef] [PubMed]
- Riegman, M.; Sagie, L.; Galed, C.; Levin, T.; Steinberg, N.; Dixon, S.J.; Wiesner, U.; Bradbury, M.S.; Niethammer, P.; Zaritsky, A.; et al. Ferroptosis Occurs through an Osmotic Mechanism and Propagates Independently of Cell Rupture. Nat. Cell Biol. 2020, 22, 1042–1048. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, X.; Liu, C.; Ge, W.; Wang, Q.; Hao, X.; Wang, M.; Chen, Y.; Zhang, Q. Identification of a Small Molecule as Inducer of Ferroptosis and Apoptosis through Ubiquitination of GPX4 in Triple Negative Breast Cancer Cells. J. Hematol. Oncol. 2021, 14, 1–21. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The Hallmarks of Ferroptosis. Annu. Rev. Cancer Biol. 2019, 3, 35–54. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Yang, W.S.; Sriramaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. Identification of ACSL4 as a Biomarker and Contributor of Ferroptosis. Biochem. Biophys. Res. Commun. 2016, 478, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Ubellacker, J.M.; Tasdogan, A.; Ramesh, V.; Shen, B.; Mitchell, E.C.; Martin-Sandoval, M.S.; Gu, Z.; McCormick, M.L.; Durham, A.B.; Spitz, D.R.; et al. Lymph Protects Metastasizing Melanoma Cells from Ferroptosis. Nature 2020, 585, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jin, S.; Yang, Y.; Lu, X.; Dai, X.; Xu, Z.; Zhang, C.; Xiang, L.F. Altered Expression of Ferroptosis Markers and Iron Metabolism Reveals a Potential Role of Ferroptosis in Vitiligo. Pigment. Cell Melanoma Res. 2022, 35, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, Y.; Lv, G.; Wang, H. Ferroptosis as a Therapeutic Target for Inflammation-Related Intestinal Diseases. Front. Pharmacol. 2023, 14, 1095366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xiang, Y.; Ma, Q.; Guo, E.; Zeng, X. A Deep Insight into Ferroptosis in Lung Disease: Facts and Perspectives. Front. Oncol. 2024, 14, 1354859. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Gianò, M.; Pinto, D.; Rinaldi, F.; van Noorden, C.J.F.; Favero, G. Hepatic Alterations in a BTBR T + Itpr3tf/J Mouse Model of Autism and Improvement Using Melatonin via Mitigation Oxidative Stress, Inflammation and Ferroptosis. Int. J. Mol. Sci. 2024, 25, 1086. [Google Scholar] [CrossRef]
- Mao, Z.H.; Gao, Z.X.; Pan, S.K.; Liu, D.W.; Liu, Z.S.; Wu, P. Ferroptosis: A Potential Bridge Linking Gut Microbiota and Chronic Kidney Disease. Cell Death Discov. 2024, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Wenzel, J.; Weyd, H. Photosensitivity, Apoptosis, and Cytokines in the Pathogenesis of Lupus Erythematosus: A Critical Review. Clin. Rev. Allergy Immunol. 2014, 47, 148–162. [Google Scholar] [CrossRef]
- Bald, T.; Quast, T.; Landsberg, J.; Rogava, M.; Glodde, N.; Lopez-Ramos, D.; Kohlmeyer, J.; Riesenberg, S.; Van Den Boorn-Konijnenberg, D.; Hömig-Hölzel, C.; et al. Ultraviolet-Radiation-Induced Inflammation Promotes Angiotropism and Metastasis in Melanoma. Nature 2014, 507, 109–113. [Google Scholar] [CrossRef]
- Manzari Tavakoli, G.; Mirzapour, M.H.; Razi, S.; Rezaei, N. Targeting Ferroptosis as a Cell Death Pathway in Melanoma: From Molecular Mechanisms to Skin Cancer Treatment. Int. Immunopharmacol. 2023, 119, 110215. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, X.; Li, H.; Zhang, X.; Liu, X.; Song, Y. Baicalein Inhibits RLS3-Induced Ferroptosis in Melanocytes. Biochem. Biophys. Res. Commun. 2021, 561, 65–72. [Google Scholar] [CrossRef]
- Talty, R.; Bosenberg, M. The Role of Ferroptosis in Melanoma. Pigment. Cell Melanoma Res. 2022, 35, 18–25. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Li, C. Mechanisms of Melanocyte Death in Vitiligo. Med. Res. Rev. 2021, 41, 1138–1166. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Yang, Y.; Xiang, L.; Zhang, C. The Role of Oxidative Stress in the Pathogenesis of Vitiligo: A Culprit for Melanocyte Death. Oxid. Med. Cell. Longev. 2022, 2022, 8498472. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kang, X. xiao ACSL4 Is Overexpressed in Psoriasis and Enhances Inflammatory Responses by Activating Ferroptosis. Biochem. Biophys. Res. Commun. 2022, 623, 1–8. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current State of Melanoma Diagnosis and Treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Stanganelli, I.; De Felici, M.B.; Mandel, V.D.; Caini, S.; Raimondi, S.; Corso, F.; Bellerba, F.; Quaglino, P.; Sanlorenzo, M.; Ribero, S.; et al. The Association between Pesticide Use and Cutaneous Melanoma: A Systematic Review and Meta-Analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 691–708. [Google Scholar] [CrossRef]
- Sawada, Y.; Nakamura, M. Daily Lifestyle and Cutaneous Malignancies. Int. J. Mol. Sci. 2021, 22, 5227. [Google Scholar] [CrossRef]
- Gupta, P.B.; Pastushenko, I.; Skibinski, A.; Blanpain, C.; Kuperwasser, C. Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell 2019, 24, 65–78. [Google Scholar] [CrossRef]
- Tsoi, J.; Robert, L.; Paraiso, K.; Galvan, C.; Sheu, K.M.; Lay, J.; Wong, D.J.L.; Atefi, M.; Shirazi, R.; Wang, X.; et al. Multi-Stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-Dependent Oxidative Stress. Cancer Cell 2018, 33, 890–904.e5. [Google Scholar] [CrossRef]
- Straino, S.; Di Carlo, A.; Mangoni, A.; De Mori, R.; Guerra, L.; Maurelli, R.; Panacchia, L.; Di Giacomo, F.; Palumbo, R.; Di Campli, C.; et al. High-Mobility Group Box 1 Protein in Human and Murine Skin: Involvement in Wound Healing. J. Investig. Dermatol. 2008, 128, 1545–1553. [Google Scholar] [CrossRef]
- Agresti, A.; Bianchi, M.E. HMGB Proteins and Gene Expression. Curr. Opin. Genet. Dev. 2003, 13, 170–178. [Google Scholar] [CrossRef]
- Morgan, A.M.; Lo, J.; Fisher, D.E. How Does Pheomelanin Synthesis Contribute to Melanomagenesis?: Two Distinct Mechanisms Could Explain the Carcinogenicity of Pheomelanin Synthesis. BioEssays 2013, 35, 672–676. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Peng, X.; Fan, Q.; Wei, S.; Yang, S.; Li, X.; Jin, H.; Wu, B.; Huang, M.; et al. Emerging Mechanisms and Applications of Ferroptosis in the Treatment of Resistant Cancers. Biomed. Pharmacother. 2020, 130, 110710. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, C.; Luo, M.; Cen, C.; Qiu, J.; Zhang, S.; Hu, K. Ferroptosis in Cancer Treatment: Another Way to Rome. Front. Oncol. 2020, 10, 571127. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef]
- Sato, M.; Onuma, K.; Domon, M.; Hasegawa, S.; Suzuki, A.; Kusumi, R.; Hino, R.; Kakihara, N.; Kanda, Y.; Osaki, M.; et al. Loss of the Cystine/Glutamate Antiporter in Melanoma Abrogates Tumor Metastasis and Markedly Increases Survival Rates of Mice. Int. J. Cancer 2020, 147, 3224–3235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, L.; Zhang, P.; Luo, M.; Du, J.; Gao, T.; O’Connell, D.; Wang, G.; Wang, H.; Yang, Y. MiR-9 Regulates Ferroptosis by Targeting Glutamic-Oxaloacetic Transaminase GOT1 in Melanoma. Mol. Carcinog. 2018, 57, 1566–1576. [Google Scholar] [CrossRef]
- Jiang, M.; Fang, H.; Shao, S.; Dang, E.; Zhang, J.; Qiao, P.; Yang, A.; Wang, G. Keratinocyte Exosomes Activate Neutrophils and Enhance Skin Inflammation in Psoriasis. FASEB J. 2019, 33, 13241–13253. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Yang, L.; Yang, Y.; Xu, J. Inhibition of Keratinocyte Ferroptosis Suppresses Psoriatic Inflammation. Cell Death Dis. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the Pathogenesis of Psoriasis: From Keratinocyte Perspective. Cell Death Dis. 2022, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Serwin, A.B.; Wasowicz, W.; Gromadzinska, J.; Chodynicka, B. Selenium Status in Psoriasis and Its Relations to the Duration and Severity of the Disease. Nutrition 2003, 19, 301–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Swanda, R.V.; Nie, L.; Liu, X.; Wang, C.; Lee, H.; Lei, G.; Mao, C.; Koppula, P.; Cheng, W.; et al. MTORC1 Couples Cyst(e)Ine Availability with GPX4 Protein Synthesis and Ferroptosis Regulation. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Sengupta, A.; Lichti, U.F.; Carlson, B.A.; Cataisson, C.; Ryscavage, A.O.; Mikulec, C.; Conrad, M.; Fischer, S.M.; Hatfield, D.L.; Yuspa, S.H. Targeted Disruption of Glutathione Peroxidase 4 in Mouse Skin Epithelial Cells Impairs Postnatal Hair Follicle Morphogenesis That Is Partially Rescued through Inhibition of COX-2. J. Investig. Dermatol. 2013, 133, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Li, S.; Luo, X.; Zhang, S.; Su, Y.; Deng, M.; Zhu, Y.; Zhang, P.; Wu, R.; Zhao, M. Ferroptosis Activation Contributes to the Formation of Skin Lesions in Psoriasis Vulgaris. Antioxidants 2023, 12, 310. [Google Scholar] [CrossRef]
- Frisoli, M.L.; Essien, K.; Harris, J.E. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu. Rev. Immunol. 2020, 38, 621–648. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Rashighi, M.; Agarwal, P.; Richmond, J.M.; Harris, T.H.; Dresser, K.; Su, M.W.; Zhou, Y.; Deng, A.; Hunter, C.A.; Luster, A.D.; et al. CXCL10 Is Critical for the Progression and Maintenance of Depigmentation in a Mouse Model of Vitiligo. Sci. Transl. Med. 2014, 6, 223ra23. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T Cells Regulate Tumour Ferroptosis during Cancer Immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, Y.; Xiang, L.; Zhang, C. The Fate of Melanocyte: Mechanisms of Cell Death in Vitiligo. Pigment Cell Melanoma Res. 2021, 34, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, N.; Cao, Y.; Li, M. Differentially Expressed Circular RNAs and Their Therapeutic Mechanism in Non-Segmental Vitiligo Patients Treated with Methylprednisolone. Front. Med. 2022, 9, 839066. [Google Scholar] [CrossRef] [PubMed]
- Man, A.; Correa, J.K.; Ziemek, J.; Simms, R.W.; Felson, D.T.; Lafyatis, R. Development and Validation of a Patient-Reported Outcome Instrument for Skin Involvement in Patients with Systemic Sclerosis. Ann. Rheum. Dis. 2017, 76, 1374–1380. [Google Scholar] [CrossRef]
- Thoreau, B.; Chaigne, B.; Renaud, A.; Mouthon, L. Pathophysiology of Systemic Sclerosis. Press. Medicale 2021, 50, 104087. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D.R.; Werth, V.P.; Pappas-Taffer, L. Systemic Sclerosis: Current Concepts of Skin and Systemic Manifestations. Clin. Dermatol. 2018, 36, 459–474. [Google Scholar] [CrossRef]
- Bukiri, H.; Volkmann, E.R. Current Advances in the Treatment of Systemic Sclerosis. Curr. Opin. Pharmacol. 2022, 64, 102211. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Varga, J. Emerging Targets of Disease-Modifying Therapy for Systemic Sclerosis. Nat. Rev. Rheumatol. 2019, 15, 208–224. [Google Scholar] [CrossRef]
- Zhang, F.; Xiao, Y.; Huang, Z.; Wang, Y.; Wan, W.; Zou, H.; Wang, B.; Qiu, X.; Yang, X. Upregulation of GPX4 Drives Ferroptosis Resistance in Scleroderma Skin Fibroblasts. Free Radic. Biol. Med. 2024, 221, 23–30. [Google Scholar] [CrossRef]
- Alhammadi, N.A.; Alqahtani, H.; Al Hamdan, S.A.; Al Hamdan, J.A.; Hadhir Alalyani, R.T.; Asiri, S.A.A.; Alqahtani, R.S.; Aljari, A.A.M.; Asiri, G.B.M. Dermatological Manifestation of SLE Patients, Living in Aseer. J. Fam. Med. Prim. Care 2024, 13, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Morel, L.; Scindia, Y. Functional Consequence of Iron Dyshomeostasis and Ferroptosis in Systemic Lupus Erythematosus and Lupus Nephritis. Clin. Immunol. 2024, 262, 110181. [Google Scholar] [CrossRef] [PubMed]
- Brunner, H.I.; Gladman, D.D.; Ibañez, D.; Urowitz, M.D.; Silverman, E.D. Difference in Disease Features between Childhood-Onset and Adult-Onset Systemic Lupus Erythematosus. Arthritis Rheum. 2008, 58, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Davidson, A. Taming Lupus-a New Understanding of Pathogenesis Is Leading to Clinical Advances. Nat. Med. 2012, 18, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C. Systemic Lupus Erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Almaani, S.; Meara, A.; Rovin, B.H. Update on Lupus Nephritis for GPs. Clin. J. Am. Soc. Nephrol. 2017, 12, 825–835. [Google Scholar] [CrossRef]
- Davidson, A.; Aranow, C.; Mackay, M. Lupus Nephritis: Challenges and Progress. Curr. Opin. Rheumatol. 2019, 31, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Wlazlo, E.; Mehrad, B.; Morel, L.; Scindia, Y. Iron Metabolism: An Under Investigated Driver of Renal Pathology in Lupus Nephritis. Front. Med. 2021, 8, 643686. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, J.; Xiang, M.; Wang, Y.; Zhang, Z.; Liang, J.; Xu, J. The Potential Role of Ferroptosis in Systemic Lupus Erythematosus. Front. Immunol. 2022, 13, 855622. [Google Scholar] [CrossRef]
- Kienhöfer, D.; Boeltz, S.; Hoffmann, M.H. Reactive Oxygen Homeostasis—The Balance for Preventing Autoimmunity. Lupus 2016, 25, 943–954. [Google Scholar] [CrossRef]
- Lightfoot, Y.L.; Blanco, L.P.; Kaplan, M.J. Metabolic Abnormalities and Oxidative Stress in Lupus. Curr. Opin. Rheumatol. 2017, 29, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, F.; Guo, Y.; Lu, Y.; Ji, W.; Lin, L.; Chen, W.; Xu, T.; Kong, D.; Shen, Q.; et al. Untargeted Lipidomics Reveals Specific Lipid Abnormalities in Systemic Lupus Erythematosus Metabolomics Research in SLE / Y. Wang et Al. Clin. Exp. Rheumatol. 2022, 40, 1011–1018. [Google Scholar] [PubMed]
- Gao, X.; Song, Y.; Wu, J.; Lu, S.; Min, X.; Liu, L.; Hu, L.; Zheng, M.; Du, P.; Yu, Y.; et al. Iron-Dependent Epigenetic Modulation Promotes Pathogenic T Cell Differentiation in Lupus. J. Clin. Investig. 2022, 132, e152345. [Google Scholar] [CrossRef] [PubMed]
- Scindia, Y.; Mehrad, B.; Morel, L. Labile Iron Accumulation Augments T Follicular Helper Cell Differentiation. J. Clin. Investig. 2022, 132, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Choi, S.C.; Park, Y.P.; Zou, X.; Elshikha, A.S.; Gerriets, V.A.; Rathmell, J.C.; Mohamazadeh, M.; Morel, L. Transcriptional and Metabolic Programs Promote the Expansion of Follicular Helper T Cells in Lupus-Prone Mice. iScience 2023, 26, 106774. [Google Scholar] [CrossRef] [PubMed]
- Zilka, O.; Shah, R.; Li, B.; Friedmann Angeli, J.P.; Griesser, M.; Conrad, M.; Pratt, D.A. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent. Sci. 2017, 3, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.E.; Kendig, C.; Le BS, T.; Brenac, C.; Griffin, M.; Guo, J.; Kameni, L.; Dixon, S.J.; Longaker, M.T.; Wan, D. Ferroptosis Inhibition with Deferoxamine Alleviates Radiation-Induced Fibrosis. Res. Sq. 2024; rs.3.rs-4314380, Preprint. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Wang, Y. Ferrostatin-1 Inhibits Fibroblast Fibrosis in Keloid by Inhibiting Ferroptosis. PeerJ 2024, 12, 1–18. [Google Scholar] [CrossRef]
- Yuan, T.; Meijia, L.; Rong, C.; Jian, Y.; Lijun, H. Identification of Novel Biomarkers of Ferroptosis Involved in Keloid Based on Bioinformatics Analysis. Int. Wound J. 2024, 21, e14606. [Google Scholar] [CrossRef]
- Hegedus, F.; Mathew, L.M.; Schwartz, R.A. Radiation Dermatitis: An Overview. Int. J. Dermatol. 2017, 56, 909–914. [Google Scholar] [CrossRef]
- Hymes, S.R.; Strom, E.A.; Fife, C. Radiation Dermatitis: Clinical Presentation, Pathophysiology, and Treatment 2006. J. Am. Acad. Dermatol. 2006, 54, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Al Zahrani, R.A.; Alotaibi, W.N.; Almanasef, Z.M.; Malawi, I.; Mohammed, L.A.; Algahamdi, R.A.; Almohanna, A.A.; AlKhaytan, A.N.; Albishi, R.J.; Alsofyani, Y.A.; et al. Comprehensive Analysis of Current Treatment Approaches for Keloids in Pediatrics: A Systematic Review. Cureus 2023, 15, e50290. [Google Scholar] [CrossRef] [PubMed]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. Hypertrophic Scars and Keloids: Overview of the Evidence and Practical Guide for Differentiating between These Abnormal Scars. Exp. Dermatol. 2021, 30, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Fitsiou, E.; Pulido, T.; Campisi, J.; Alimirah, F.; Demaria, M. Cellular Senescence and the Senescence-Associated Secretory Phenotype as Drivers of Skin Photoaging. J. Invest. Dermatol. 2021, 141, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, H.; Man, M.Q.; Hu, L. Aging in the Dermis: Fibroblast Senescence and Its Significance. Aging Cell 2024, 23, e14054. [Google Scholar] [CrossRef]

























| Age Group | N° Specimens | Type I Collagen (µg/g) | Type III Collagen (µg/g) | Type I/III |
|---|---|---|---|---|
| Fetus | 10 | 264.71 ± 5.88 a | 278.87 ± 6.18 a | 0.95 ± 0.03 a |
| Adolescent | 10 | 279.12 ± 7.65 b | 123.27 ± 5.30 b | 2.27 ± 0.13 b |
| Adult | 10 | 241.79 ± 8.2 c | 98.41 ± 5.58 c | 2.46 ± 0.15 c |
| Elderly | 10 | 209.50 ± 14.31 d | 71.30 ± 7.41 d | 2.97 ± 0.40 d |
| Source | Compounds |
|---|---|
| Aloe vera | Aloe sterols |
| Anemarrhena asphodeloides Bunge | Crude extracts and pure compounds containing steroidal saponins, flavonoids, phenylpropanoids, alkaloids, steroids, organic acids, anthraquinones and other compounds |
| Arctium lappa | Arctiin |
| Chlamydomonas hedleyi | Mycosporine-like amino acids |
| Glycyrrhiza glabra | 18β-Glycyrrhetinic acid |
| Patchouli | Pogostone from patchouli oil |
| Organ System | Mechanism | Associated Pathologies |
|---|---|---|
| Liver | Iron overload-induced hepatic fibrosis; iron deficiency reduces activity of cytochrome P450 enzymes involved in drug metabolism | Liver cirrhosis, hepatic-hypoxia |
| Kidneys | Excess iron accumulation causes iron-mediated oxidative stress and subsequent renal fibrosis; leading to impaired renal cell fraction | Chronic kidney disease |
| Brain | Excessive iron accumulation has been linked to increased risk of neurodegenerative disorders | Parkinson’s disease, Alzheimer’s disease |
| Heart | Iron is involved in oxygen transport and utilization; iron overload causes oxidative stress, inflammation and fibrosis | Atherosclerosis |
| Pancreas | Iron-induced oxidative stress and advanced glycation end products impair insulin signaling and β cell dysfunction with impaired insulin secretion | Type 2 diabetes mellitus |
| Joints | Iron is a component of several enzymes and proteins involded in cartilage and bone formation, maintenance and repair; iron deficiency induces alterations in cartilage and bone formation | Osteoarthritis, joint pain |
| Endocrine glands | Iron deficiency leads to decreased hormone production and secretion | Hypothyroidism, sexual dysfunction, infertility |
| Proteins and Regulators | Intracellular Functions Involved in Ferroptosis |
|---|---|
| Solute Carrier Family 3 Member 2 (SLC3A2) Solute Carrier Family 7 Member 11 (SLC7A11) | The constituents of an amino acid antiporter (Xc- system) that mediates the exchange across the plasma membrane of extracellular cystine and intracellular glutamate. Cystine is then reduced to cysteine and takes part in the synthesis of GSH, the substrate of GPX4; |
| Glutathione peroxidase 4 (GPX4) | A decrease in its activity causes lipid peroxides to be unable to be metabolized, resulting in lipid peroxides accumulation and inducing ferroptosis; |
| Acyl-CoA synthetase long-chain family member/ lysophosphatidylcholine acyltransferase 3 (ACSL4/LPCAT3) | ACSL4 catalyzes the ligation reaction of CoA with AdA/AA forming COA-AdA/AA. LPCAT3 catalyzes the esterification of COA-AdA/AA with lysophospholipids. Its overexpression triggers oxidative stress inducing ferroptosis; |
| Lipoxygenases (LOXs) | A group of iron-containing enzymes responsible for catalyzing the PUFAs oxidation through stereo-specific peroxidation to produce fatty acid hydroperoxides. Suppression or downregulation of LOXs activity leads to inhibition of ferroptosis in certain cell lines; |
| Transferrin receptor 1 (TRF1) | Mediates TF- Fe3+ transport through the membrane; |
| Ferroportin (FPN) | Iron efflux pump that can release intracellular iron by oxidizing it; |
| Divalent metal transporter 1 (DMT1) | Translocates Fe2+ into a labile iron pool (LIP) within the cytoplasm; |
| Six-transmembrane epithelial antigens of the prostate 3 (STEAP3) | Reduces Fe3+ in Fe2+ in the endosomes; |
| Nuclear receptor coactivator 4 (NCOA4) | Autophagic cargo receptor of ferritin; |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezzani, R.; Favero, G.; Cominelli, G.; Pinto, D.; Rinaldi, F. Skin Aging and the Upcoming Role of Ferroptosis in Geroscience. Int. J. Mol. Sci. 2024, 25, 8238. https://doi.org/10.3390/ijms25158238
Rezzani R, Favero G, Cominelli G, Pinto D, Rinaldi F. Skin Aging and the Upcoming Role of Ferroptosis in Geroscience. International Journal of Molecular Sciences. 2024; 25(15):8238. https://doi.org/10.3390/ijms25158238
Chicago/Turabian StyleRezzani, Rita, Gaia Favero, Giorgia Cominelli, Daniela Pinto, and Fabio Rinaldi. 2024. "Skin Aging and the Upcoming Role of Ferroptosis in Geroscience" International Journal of Molecular Sciences 25, no. 15: 8238. https://doi.org/10.3390/ijms25158238





