Methylation Modification in Ornamental Plants: Impact on Floral Aroma and Color
Abstract
:1. Introduction
2. Formation Mechanism of Plant Methylation Modification
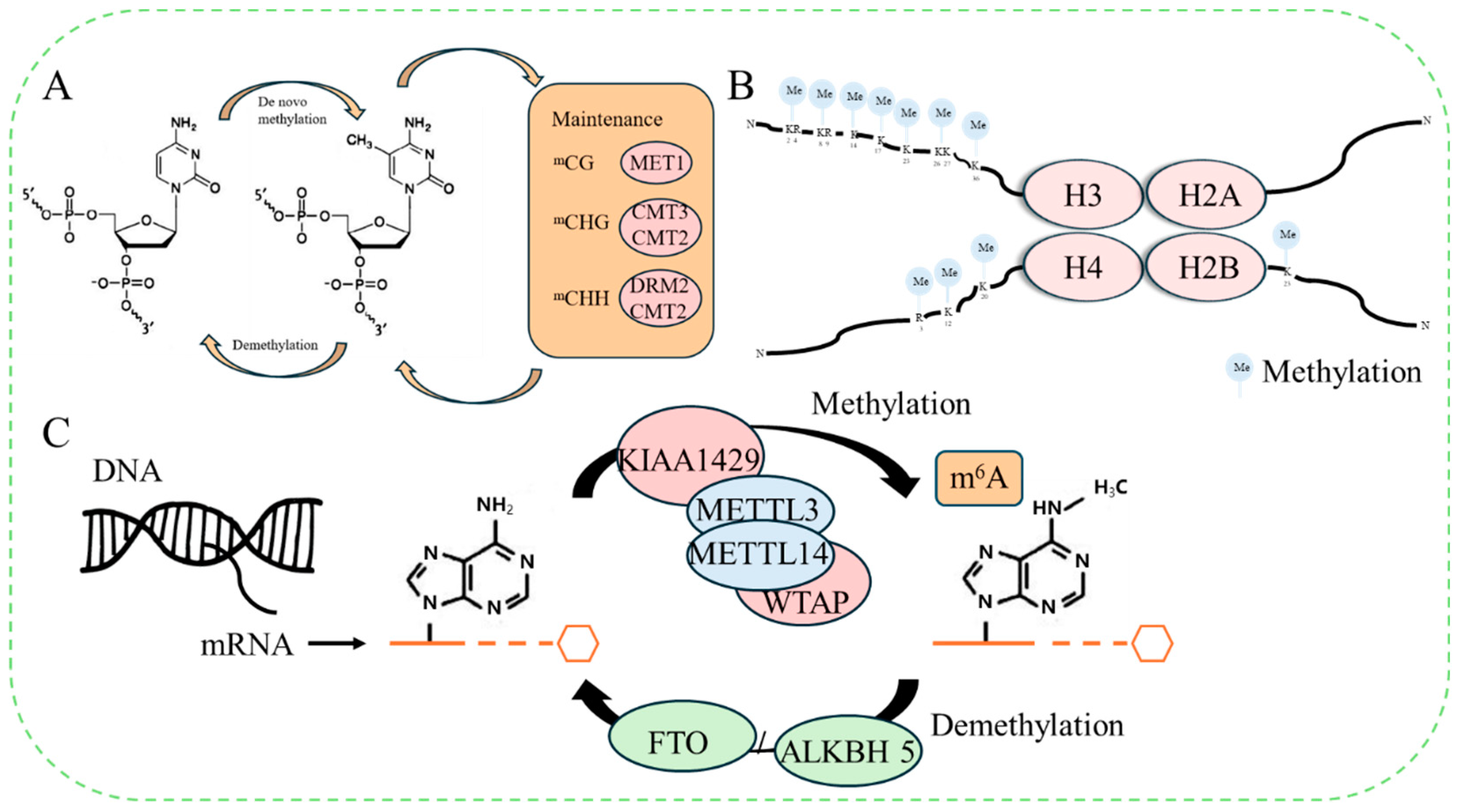
2.1. DNA Methylation
2.2. Histone Methylation
2.3. RNA m6A Methylation
2.4. O-Methylation
3. Impact of Methylation Modification on the Formation of Floral Aroma in Ornamental Plants
3.1. Terpenoids
3.2. Phenylpropanoids/Benzenoids
3.3. Fatty Acid Derivatives
4. Impact of Methylation Modification on the Formation of Floral Color in Ornamental Plants
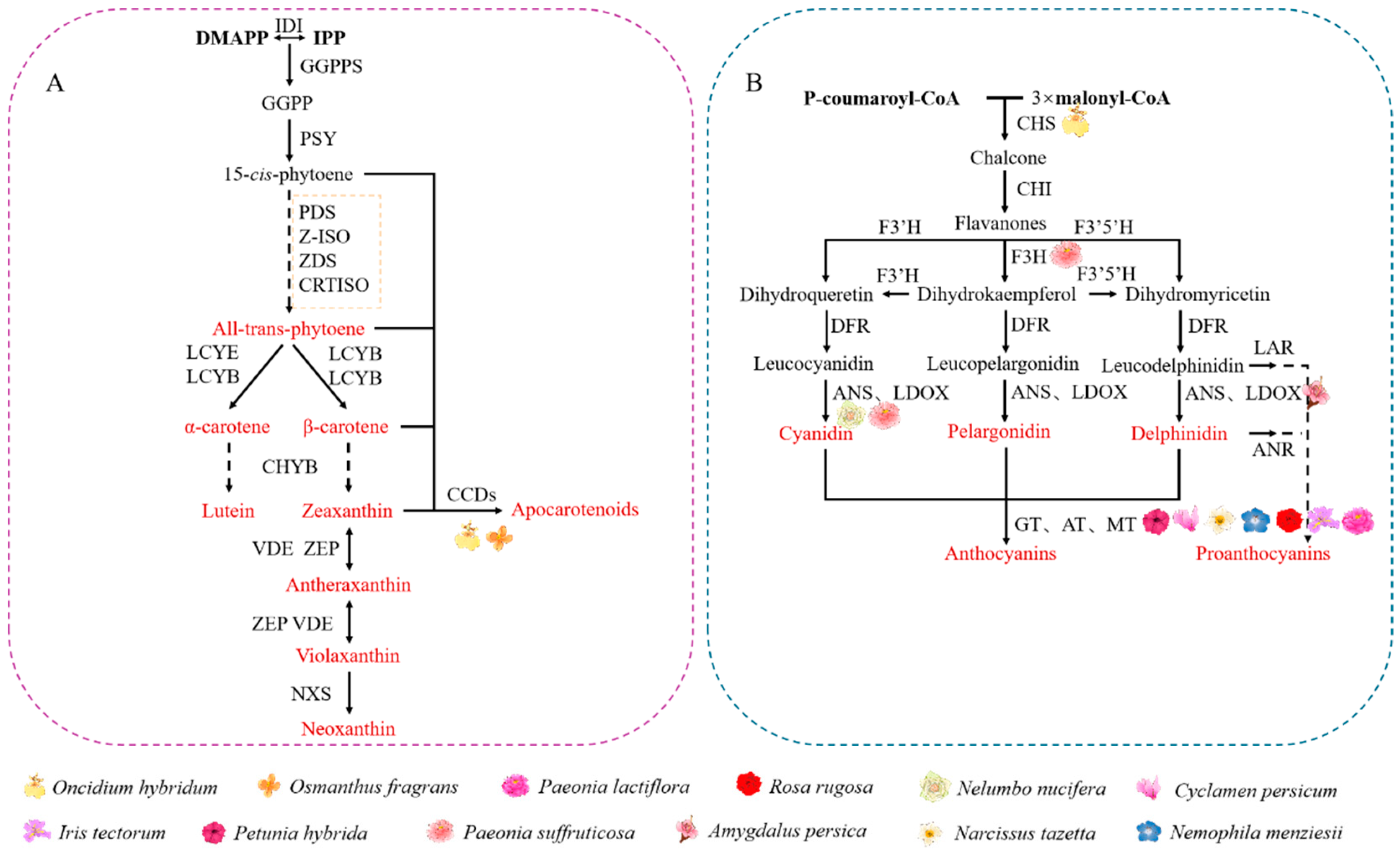
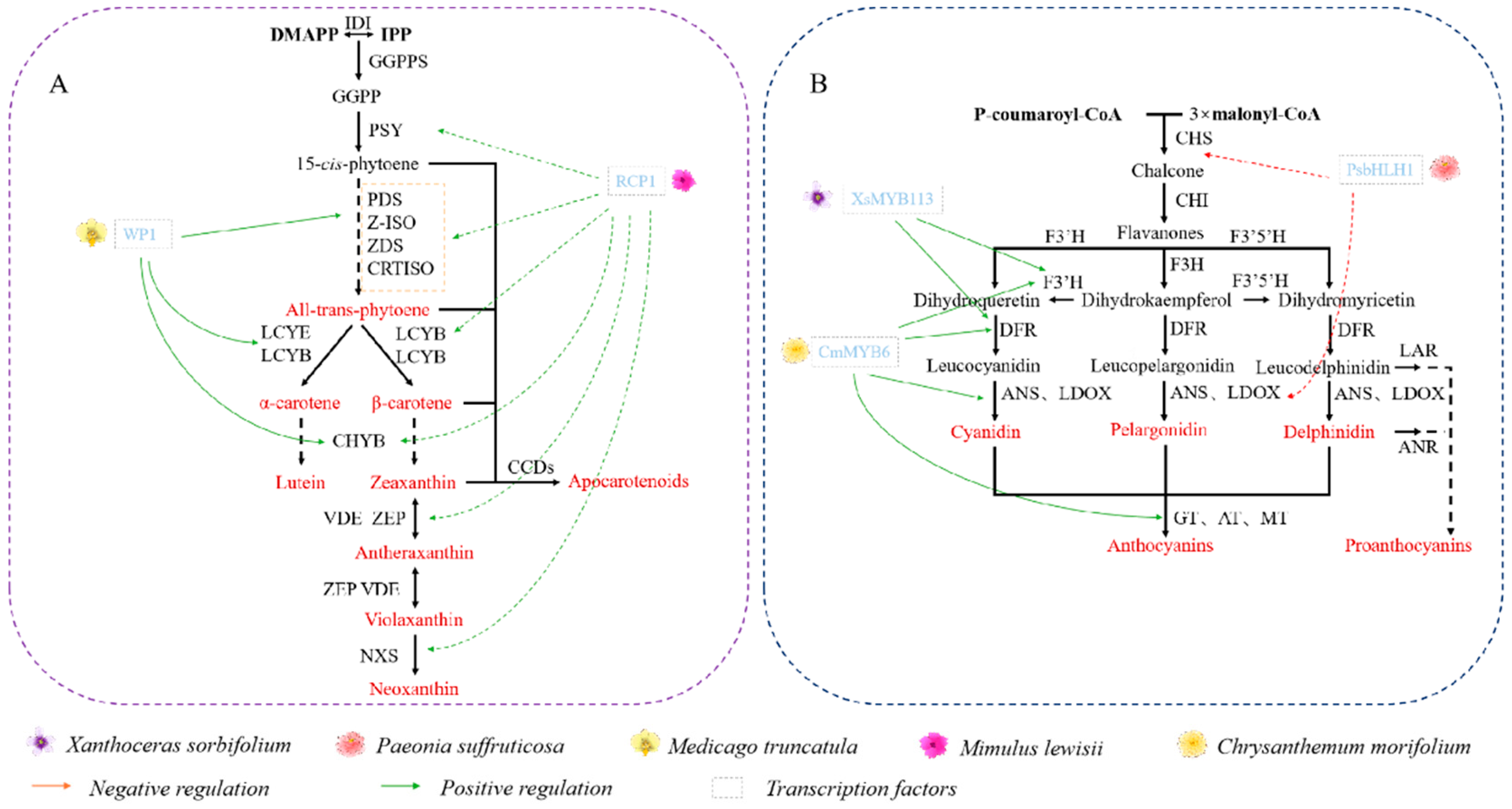
4.1. Carotenoids
4.2. Flavonoids
4.2.1. Effect on Pigment Deposition in Plant Petals
4.2.2. Effect on Spot Formation in Plant Petals
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Attieh, J.; Djiana, R.; Koonjul, P.; Etienne, C.; Sparace, S.A.; Saini, H.S. Cloning and functional expression of two plant thiol methyltransferases: A new class of enzymes involved in the biosynthesis of sulfur volatiles. Plant Mol. Biol. 2002, 50, 511–521. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Muzac, I. The Methyltransferase Gene Superfamily: A Tree with Multiple Branches. Recent Adv. Phytochem. 2000, 34, 349–384. [Google Scholar]
- Struck, A.; Thompson, M.L.; Wong, L.S.; Micklefield, J. S-Adenosyl-Methionine-Dependent Methyltransferases: Highly Versatile Enzymes in Biocatalysis, Biosynthesis and Other Biotechnological Applications. ChemBioChem 2012, 13, 2642–2655. [Google Scholar] [CrossRef]
- Li, J.; Sun, C.; Cai, W.; Li, J.; Rosen, B.P.; Chen, J. Insights into S-adenosyl-L-methionine (SAM)-dependent methyltransferase related diseases and genetic polymorphisms. Mutat. Res.-Rev. Mutat. 2021, 788, 108396. [Google Scholar] [CrossRef]
- Majetic, C.J.; Raguso, R.A.; Ashman, T. The sweet smell of success: Floral scent affects pollinator attraction and seed fitness in Hesperis matronalis. Funct. Ecol. 2009, 23, 480–487. [Google Scholar] [CrossRef]
- Lalko, J.; Lapczynski, A.; McGinty, D.; Bhatia, S.; Letizia, C.S.; Api, A.M. Fragrance material review on ionone. Food Chem. Toxicol. 2007, 45, S251–S257. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Bio. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Wolffe, A.P.; Matzke, M.A. Epigenetics: Regulation Through Repression. Science 1999, 286, 481–486. [Google Scholar] [CrossRef]
- Chen, T.; Li, E. Structure and function of eukaryotic DNA methyltransferases. Curr. Top. Dev. Biol. 2004, 60, 55. [Google Scholar]
- Bartels, A.; Han, Q.; Nair, P.; Stacey, L.; Gaynier, H.; Mosley, M.; Huang, Q.Q.; Pearson, J.K.; Hsieh, T.-F.; An, Y.-Q.C.; et al. Dynamic DNA Methylation in Plant Growth and Development. Int. J. Mol. Sci. 2018, 19, 2144. [Google Scholar] [CrossRef]
- Lindroth, A.M.; Cao, X.F.; Jackson, J.P.; Zilberman, D.; McCallum, C.M.; Henikoff, S.; Jacobsen, S.E. Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science 2001, 292, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, A.M.; Saarikoski, P.; Flygh, G.; Clapham, D.; Grönroos, R.; Thelander, M.; Ronne, H.; von Arnold, S. Two S-adenosylmethionine synthetase-encoding genes differentially expressed during adventitious root development in Pinus contorta. Plant Mol. Biol. 2001, 46, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Saze, H.; Tsugane, K.; Kanno, T.; Nishimura, T. DNA Methylation in Plants: Relationship to Small RNAs and Histone Modifications, and Functions in Transposon Inactivation. Plant Cell Physiol. 2012, 53, 766–784. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Bai, J.; Zheng, S. The Regulation and Function of Histone Methylation. J. Plant Biol. 2018, 61, 347–357. [Google Scholar] [CrossRef]
- Zhang, Y.; Reinberg, D. Transcription regulation by histone methylation: Interplay between different covalent modifications of the core histone tails. Gene Dev. 2001, 15, 2343–2360. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.C.; Allis, C.D. Histone methylation versus histone acetylation: New insights into epigenetic regulation. Curr. Opin. Cell Biol. 2001, 13, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Mellor, J.; Dudek, P.; Clynes, D. A glimpse into the epigenetic landscape of gene regulation. Curr. Opin. Genet. Dev. 2008, 18, 116–122. [Google Scholar] [CrossRef]
- Sendinc, E.; Shi, Y. RNA m6A methylation across the transcriptome. Mol. Cell 2023, 83, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Open Biol. 2017, 169, 1187–1200. [Google Scholar] [CrossRef]
- Quan, Y.; Zhang, X.; Ping, H. Construction of a risk prediction model using m6A RNA methylation regulators in prostate cancer: Comprehensive bioinformatic analysis and histological validation. Cancer Cell Int. 2022, 22, 33. [Google Scholar] [CrossRef]
- Wu, J.; Frazier, K.; Zhang, J.; Gan, Z.; Wang, T.; Zhong, X. Emerging role of m6A RNA methylation in nutritional physiology and metabolism. Obes. Rev. 2020, 21, e12942. [Google Scholar] [CrossRef] [PubMed]
- Zubieta, C.; He, X.Z.; Dixon, R.A.; Noel, J.P. Structures of two natural product methyltransferases reveal the basis for substrate specificity in plant O-methyltransferases. Nat. Struct. Biol. 2001, 8, 271–279. [Google Scholar] [CrossRef]
- Abdelraheem, E.; Thair, B.; Varela, R.F.; Jockmann, E.; Popadic, D.; Hailes, H.C.; Ward, J.M.; Iribarren, A.M.; Lewkowicz, E.S.; Andexer, J.N.; et al. Methyltransferases: Functions and Applications. ChemBioChem 2022, 23, e202200212. [Google Scholar] [CrossRef]
- Zubieta, C.; Kota, P.; Ferrer, J.L.; Dixon, R.A.; Noel, J.P. Structural basis for the modulation of lignin monomer methylation by caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase. Plant Cell 2002, 14, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Sawada, Y.; Ochiai, K.; Sato, M.; Inaba, J.; Hirai, M.Y. Identification of a Unique Type of Isoflavone O-Methyltransferase, GmIOMT1, Based on Multi-Omics Analysis of Soybean under Biotic Stress. Plant Cell Physiol. 2020, 61, 1974–1985. [Google Scholar] [CrossRef]
- Ferrer, J.L.; Zubieta, H.; Dixon, R.A.; Noel, J.P. Crystal structures of alfalfa caffeoyl coenzyme A 3-O-methyltransferase. Plant Physiol. 2005, 137, 1009–1017. [Google Scholar] [CrossRef]
- Wang, B.; Li, M.; Yuan, Y.; Liu, S. Genome-Wide Comprehensive Analysis of the SABATH Gene Family in Arabidopsis and Rice. Evol. Bioinform. 2019, 15. [Google Scholar] [CrossRef]
- Jette, T.K.; Lars, T.L.; Gunnar, B. Floral scents-a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Dudareva, N.; Martin, D.; Kish, C.M.; Kolosova, N.; Gorenstein, N.; Fäldt, J.; Miller, B.; Bohlmann, J. (E)-beta-ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: Function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 2003, 15, 1227–1241. [Google Scholar] [CrossRef]
- Hsieh, M.; Chang, C.; Hsu, S.; Chen, J. Chloroplast localization of methylerythritol 4-phosphate pathway enzymes and regulation of mitochondrial genes in ispD and ispE albino mutants in Arabidopsis. Plant Mol. Biol. 2008, 66, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Pulido, P.; Perello, C.; Rodriguez-Concepcion, M. New Insights into Plant Isoprenoid Metabolism. Mol. Plant 2012, 5, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, J.; He, Z.; Wang, F.; Yang, H.; Yan, Y.; Gao, M.; Gruber, M.Y.; Wan, X.; Wei, S. Implementation of CsLIS/NES in linalool biosynthesis involves transcript splicing regulation in Camellia sinensis. Plant Cell Environ. 2018, 41, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E. Metabolic engineering of plant volatiles. Curr. Opin. Biotech. 2008, 19, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lu, M.; Yue, S.; Li, K.; Dong, M.; Liu, L.; Wang, H.; Shang, F. Comparative methylomics and chromatin accessibility analysis in Osmanthus fragrans uncovers regulation of genic transcription and mechanisms of key floral scent production. Hortic. Res. 2022, 9, uhac096. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Bai, X.; Zhang, H.; Qiu, X.; Jian, H.; Wang, Q.; Wang, H.; Feng, D.; Tang, K.; Yan, H. Loss of Rose Fragrance under Chilling Stress Is Associated with Changes in DNA Methylation and Volatile Biosynthesis. Genes 2023, 14, 692. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Koeduka, T.; Louie, G.V.; Orlova, I.; Kish, C.M.; Ibdah, M.; Wilkerson, C.G.; Bowman, M.E.; Baiga, T.J.; Noel, J.P.; Dudareva, N.; et al. The multiple phenylpropene synthases in both Clarkia breweri and Petunia hybrida represent two distinct protein lineages. Plant J. 2008, 54, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, J.R.; Dudareva, N. A Familiar Ring to It: Biosynthesis of Plant Benzoic Acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef]
- Qualley, A.V.; Widhalm, J.R.; Adebesin, F.; Kish, C.M.; Dudareva, N. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 16383–16388. [Google Scholar] [CrossRef]
- Long, M.C.; Nagegowda, D.A.; Kaminaga, Y.; Ho, K.K.; Kish, C.M.; Schnepp, J.; Sherman, D.; Weiner, H.; Rhodes, D.; Dudareva, N. Involvement of snapdragon benzaldehyde dehydrogenase in benzoic acid biosynthesis. Plant J. 2009, 59, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wang, L.; Li, M.; Liu, F.; Yin, J.; Huang, L.; Zhou, B.; Li, X.; Yu, Y.; Chen, F.; et al. A BAHD acyltransferase contributes to the biosynthesis of both ethyl benzoate and methyl benzoate in the flowers of Lilium oriental hybrid ‘Siberia’. Front. Plant Sci. 2023, 14, 1275960. [Google Scholar] [CrossRef] [PubMed]
- Barkman, T.J. Evidence for positive selection on the floral scent gene isoeugenol-O-methyltransferase. Mol. Biol. Evol. 2003, 20, 168–172. [Google Scholar] [CrossRef]
- Shuiqin Wu, N.W.S.M. Two O-Methyltransferases isolated from flower petals of Rosa chinensis var. spontanea involved in scent biosynthesis. J. Biosci. Bioeng. 2003, 96, 119–128. [Google Scholar]
- Scalliet, G.; Piola, F.; Douady, C.J.; Rety, S.; Raymond, O.; Baudino, S.; Bordji, K.; Bendahmane, M.; Dumas, C.; Cock, J.M.; et al. Scent evolution in Chinese roses. Proc. Natl. Acad. Sci. USA 2008, 105, 5927–5932. [Google Scholar] [CrossRef]
- Wu, S.Q.; Watanabe, N.; Mita, S.; Dohra, H.; Ueda, Y.; Shibuya, M.; Ebizuka, Y. The key role of phloroglucinol O-methyltransferase in the biosynthesis of Rosa chinensis volatile 1,3,5-trimethoxybenzene. Plant Physiol. 2004, 135, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Scalliet, G.; Lionnet, C.; Le Bechec, M.; Dutron, L.; Magnard, J.L.; Baudino, S.; Bergougnoux, V.; Jullien, F.; Chambrier, P.; Vergne, P.; et al. Role of petal-specific orcinol O-methyltransferases in the evolution of rose scent. Plant Physiol. 2006, 140, 18–29. [Google Scholar] [CrossRef]
- Koeduka, T.; Kajiyama, M.; Furuta, T.; Suzuki, H.; Tsuge, T.; Matsui, K. Characterization of an O-methyltransferase specific to guaiacol-type benzenoids from the flowers of loquat (Eriobotrya japonica). J. Biosci. Bioeng. 2016, 122, 679–684. [Google Scholar] [CrossRef]
- Pott, M.B.; Hippauf, F.; Saschenbrecker, S.; Chen, F.; Ross, J.; Kiefer, I.; Slusarenko, A.; Noel, J.P.; Pichersky, E.; Effmert, U.; et al. Biochemical and structural characterization of benzenoid carboxyl methyltransferases involved in floral scent production in Stephanotis floribunda and Nicotiana suaveolens. Plant Physiol. 2004, 135, 1946–1955. [Google Scholar] [CrossRef]
- Rohrbeck, D.; Buss, D.; Effmert, U.; Piechulla, B. Localization of methyl benzoate synthesis and emission in Stephanotis floribunda and Nicotiana suaveolens flowers. Plant Biol. 2006, 8, 615–626. [Google Scholar] [CrossRef]
- Wang, H.; Sun, M.; Li, L.L.; Xie, X.H.; Zhang, Q.X. Cloning and characterization of a benzoic acid/salicylic acid carboxyl methyltransferase gene involved in floral scent production from lily (Lilium ‘Yelloween’). Genet. Mol. Res. 2015, 14, 14510–14521. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wang, L.; Yu, R.; Chen, F.; He, J.; Li, X.; Yu, Y.; Fan, Y. Coordinated and High-Level Expression of Biosynthetic Pathway Genes Is Responsible for the Production of a Major Floral Scent Compound Methyl Benzoate in Hedychium coronarium. Front. Plant Sci. 2021, 12, 650582. [Google Scholar] [CrossRef] [PubMed]
- Koeduka, T.; Kajiyama, M.; Suzuki, H.; Furuta, T.; Tsuge, T.; Matsui, K. Benzenoid biosynthesis in the flowers of Eriobotrya japonica: Molecular cloning and functional characterization of p-methoxybenzoic acid carboxyl methyltransferase. Planta 2016, 244, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Ma, K.; Zhang, M.; Wang, J.; Zhang, Q. Integration of Transcriptome and Methylome Analyses Provides Insight into the Pathway of Floral Scent Biosynthesis in Prunus mume. Front. Genet. 2021, 12, 779557. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Cheng, L.; Mei, Y.; Huang, L.; Zhu, J.; Mi, X.; Yu, Y.; Wei, C. Alternative Splicing of Key Genes in LOX Pathway Involves Biosynthesis of Volatile Fatty Acid Derivatives in Tea Plant (Camellia sinensis). J. Agric. Food Chem. 2019, 67, 13021–13032. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Suchet, C.; Dormont, L.; Schatz, B.; Giurfa, M.; Simon, V.; Raynaud, C.; Chave, J. Floral scent variation in two Antirrhinum majus subspecies influences the choice of naïve bumblebees. Behav. Ecol. Sociobiol. 2011, 65, 1015–1027. [Google Scholar] [CrossRef]
- Seo, H.S.; Song, J.T.; Cheong, J.J.; Lee, Y.H.; Lee, Y.W.; Hwang, I.; Lee, J.S.; Choi, Y.D. Jasmonic acid carboxyl methyltransferase: A key enzyme for jasmonate-regulated plant responses. Proc. Natl. Acad. Sci. USA 2001, 98, 4788–4793. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Chen, X.; Zhou, J.; Wang, S.; Xu, Y. Functional characterization of the CfAOC and CfJMT gene promoters related to MeJA biosynthesis in Cymbidium faberi. Plant Biotechnol. Rep. 2023, 17, 243–253. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, G.; Zhang, W.; Zhang, C.; Chen, X.; Chen, Y.; Yu, C.; Yu, D.; Fu, J.; Chen, F. Biosynthesis and emission of methyl hexanoate, the major constituent of floral scent of a night-blooming water lily Victoria cruziana. Phytochemistry 2021, 191, 112899. [Google Scholar] [CrossRef]
- Ng, J.; Smith, S.D. How to make a red flower: The combinatorial effect of pigments. AoB Plants 2016, 8, plw013. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Bai, C.; Sanahuja, G.; Yuan, D.; Farre, G.; Naqvi, S.; Shi, L.; Capell, T.; Christou, P. The regulation of carotenoid pigmentation in flowers. Arch. Biochem. Biophys. 2010, 504, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Ohmiya, A. Carotenoid Isomerase Is Key Determinant of Petal Color of Calendula officinalis. J. Biol. Chem. 2012, 287, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Fan, J.; Liu, F.; Li, H.; Pu, Y.; Huang, H.; Dai, S. R2R3-MYB transcription factor PhMYB2 positively regulates anthocyanin biosynthesis in Pericallis hybrida. Sci. Hortic. 2023, 322, 112446. [Google Scholar] [CrossRef]
- Sun, X.; He, L.; Guo, Z.; Xiao, Z.; Su, J.; Liu, X.; Zhou, H.; Li, C.; Gao, H. Comparative transcriptome analyses reveal genes related to pigmentation in the petals of a flower color variation cultivar of Rhododendron obtusum. Mol. Biol. Rep. 2022, 49, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.K.; Anzellotti, D. The enzymatic basis of flavonoid biodiversity. In Recent Advances in Phytochemistry; Elsevier: Amsterdam, The Netherlands, 2003; Volume 37, pp. 1–36. [Google Scholar]
- Singh, G.; Sinha, S.; Bandyopadhyay, K.K.; Lawrence, M.; Prasad, R.; Paul, D. Triauxic growth of an oleaginous red yeast Rhodosporidium toruloides on waste ‘extract’ for enhanced and concomitant lipid and β-carotene production. Microb. Cell Fact. 2019, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N. Silencing Phytoene Desaturase Causes Alteration in Monoterpene Volatiles Belonging to the Methylerythritol Phosphate Pathway. Plants 2022, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Guzman, I.; Hamby, S.; Romero, J.; Bosland, P.W.; O’Connell, M.A. Variability of carotenoid biosynthesis in orange colored Capsicum spp. Plant Sci. 2010, 179, 49–59. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, Y.; Al-Babili, S. Exploring the Diversity and Regulation of Apocarotenoid Metabolic Pathways in Plants. Front. Plant Sci. 2021, 12, 787049. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Yang, F.; Wang, X.; Dong, M.; Zhou, P.; Shang, F. cDNA-AFLP analysis on 2 Osmanthus fragrans cultivars with different flower color and molecular characteristics of OfMYB1 gene. Trees 2015, 29, 931–940. [Google Scholar] [CrossRef]
- Chiou, C.; Pan, H.; Chuang, Y.; Yeh, K. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in floral tissues of Oncidium cultivars. Planta 2010, 232, 937–948. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, Z.; Wang, Y.; Wang, C.; Zhu, B.; Liu, H.; Ji, W.; Wen, J.; Chu, C.; Tadege, M.; et al. The MYB Activator WHITE PETAL1 Associates with MtTT8 and MtWD40-1 to Regulate Carotenoid-Derived Flower Pigmentation in Medicago truncatula. Plant Cell 2019, 31, 2751–2767. [Google Scholar] [CrossRef]
- Sagawa, J.M.; Stanley, L.E.; LaFountain, A.M.; Frank, H.A.; Liu, C.; Yuan, Y. An R2R3-MYB transcription factor regulates carotenoid pigmentation in Mimulus lewisii flowers. New Phytol. 2016, 209, 1049–1057. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The Origin and Evolution of Plant Flavonoid Metabolism. Front. Plant Sci. 2019, 10, 00943. [Google Scholar] [CrossRef]
- Kong, J.; Chia, L.; Goh, N.; Chia, T.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2008, 69, 1939–1940. [Google Scholar] [CrossRef]
- Deng, J.; Fu, Z.; Chen, S.; Damaris, R.N.; Wang, K.; Li, T.; Yang, P. Proteomic and Epigenetic Analyses of Lotus (Nelumbo nucifera) Petals Between Red and White cultivars. Plant Cell Physiol. 2015, 56, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Ohmiya, A. Seeing is believing: Engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotech. 2008, 19, 190–197. [Google Scholar] [CrossRef]
- Provenzano, S.; Spelt, C.; Hosokawa, S.; Nakamura, N.; Brugliera, F.; Demelis, L.; Geerke, D.P.; Schubert, A.; Tanaka, Y.; Quattrocchio, F.; et al. Genetic Control and Evolution of Anthocyanin Methylation. Plant Physiol. 2014, 165, 962–977. [Google Scholar] [CrossRef]
- Kita, Y.; Kitamura, S.; Hase, Y.; Narumi, I.; Ishizaka, H.; Kondo, E.; Kameari, N.; Nakayama, M.; Tanikawa, N.; Morita, Y.; et al. Isolation and characterization of the fragrant cyclamen O-methyltransferase involved in flower coloration. Planta 2011, 234, 1127–1136. [Google Scholar]
- Kondo, E.; Nakayama, M.; Kameari, N.; Tanikawa, N.; Morita, Y.; Akita, Y.; Hase, Y.; Tanaka, A.; Ishizaka, H. Red-purple flower due to delphinidin 3,5-diglucoside, a novel pigment for Cyclamen spp., generated by ion-beam irradiation. Plant Biotechnol. 2009, 26, 565–569. [Google Scholar] [CrossRef]
- Du, H.; Wu, J.; Ji, K.; Zeng, Q.; Bhuiya, M.; Su, S.; Shu, Q.; Ren, H.; Liu, Z.; Wang, L. Methylation mediated by an anthocyanin, O-methyltransferase, is involved in purple flower coloration in Paeonia. J. Exp. Bot. 2015, 66, 6563–6577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Z.; Tang, T.; Zhang, H.; Zhao, L. Analysis of Anthocyanins Related Compounds and Their Biosynthesis Pathways in Rosa rugosa ‘Zi zhi’ at Blooming Stage. Sci. Agric. Sin. 2015, 48, 2600–2611. [Google Scholar]
- Zhang, M.; Pan, T.; Yu, L.; Yang, H.; Pan, D. Cloning and Ananysis of Flavonoid O-methyltransferase Gene and Promoter in Narcissus tazetta. var chinensis. Chin. J. Trop. Crop. 2018, 39, 726–732. [Google Scholar]
- Okitsu, N.; Mizuno, T.; Matsu, K.; Choi, S.H.; Tanaka, Y. Molecular cloning of flavonoid biosynthetic genes and biochemical characterization of anthocyanin O-methyltransferase of Nemophila menziesii Hook. and Arn. Plant Biotechnol. 2018, 35, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, H.; Liu, Z.; Zhang, T.; Li, Z.; Cao, L.; Wu, S.; Liu, Y.; Yu, S.; Zhang, Q.; et al. A naturally-occurring phenomenon of flower color change during flower development in Xanthoceras sorbifolium. Front. Plant Sci. 2022, 13, 1072185. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Xue, W.; Li, X.; Wang, L.; Wang, M.; Wang, W.; Yin, X.; Chen, B.; Qu, X.; Li, J.; et al. Mitotically heritable epigenetic modifications of CmMYB6 control anthocyanin biosynthesis in chrysanthemum. New Phytol. 2022, 236, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chuang, Y.; Chiou, C.; Chin, D.; Shen, F.; Yeh, K. Methylation effect on chalcone synthase gene expression determines anthocyanin pigmentation in floral tissues of two Oncidium orchid cultivars. Planta 2012, 236, 401–409. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Xu, Z.; Qi, S.; Yu, X.; Han, X. MSAP analysis of epigenetic changes reveals the mechanism of bicolor petal formation in Paeonia suffruticosa ‘Shima Nishiki’. 3 Biotech 2019, 9, 313. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Y.; Yao, D.; Iqbal, S.; Gao, Z.; Zhang, Z. DNA methylation of LDOX gene contributes to the floral color variegation in peach. J. Plant Physiol. 2020, 246, 153116. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Wang, Q.; Li, B.; Wang, X.; Zhou, X.; Zhang, H.; Xu, W.; Li, S.; Wang, L. The combination of DNA methylation and positive regulation of anthocyanin biosynthesis by MYB and bHLH transcription factors contributes to the petal blotch formation in Xibei tree peony. Hortic. Res. 2023, 10, uhad100. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, S.; Lv, S.; Chen, Q.; Zhou, Z.; Moorthy, M.D.S.; Sathish, D.; Moola, A.K. A systematic review of the O-methyltransferase gene expression. Plant Gene 2021, 27, 100295. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Vanyushin, B.F. Plant DNA methyltransferase genes: Multiplicity, expression, methylation patterns. Biochemistry 2016, 81, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, R.; Xu, Y.; Zhang, C.; Niu, Q.; Lang, Z. DNA cytosine methylation dynamics and functional roles in horticultural crops. Hortic. Res. 2023, 10, uhad170. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, C.C.; Schiestl, F.P. Odour and colour polymorphism in the food-deceptive orchid Dactylorhiza romana. Plant Syst. Evol. 2007, 267, 37–45. [Google Scholar] [CrossRef]
- Li, Y.; Bao, T.; Zhang, J.; Li, H.; Shan, X.; Yan, H.; Kimani, S.; Zhang, L.; Gao, X. The coordinated interaction or regulation between floral pigments and volatile organic compounds. Hortic. Plant J. 2024, 549, 002. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Tian, Q.; Qiu, H.; Wang, R.; Wang, L.; Yue, Y.; Yang, X. Methylation Modification in Ornamental Plants: Impact on Floral Aroma and Color. Int. J. Mol. Sci. 2024, 25, 8267. https://doi.org/10.3390/ijms25158267
Xie C, Tian Q, Qiu H, Wang R, Wang L, Yue Y, Yang X. Methylation Modification in Ornamental Plants: Impact on Floral Aroma and Color. International Journal of Molecular Sciences. 2024; 25(15):8267. https://doi.org/10.3390/ijms25158267
Chicago/Turabian StyleXie, Chenchen, Qingyin Tian, Hanruo Qiu, Rui Wang, Lianggui Wang, Yuanzheng Yue, and Xiulian Yang. 2024. "Methylation Modification in Ornamental Plants: Impact on Floral Aroma and Color" International Journal of Molecular Sciences 25, no. 15: 8267. https://doi.org/10.3390/ijms25158267





