A Transcriptomic Analysis of Bottle Gourd-Type Rootstock Roots Identifies Novel Transcription Factors Responsive to Low Root Zone Temperature Stress
Abstract
1. Introduction
2. Results
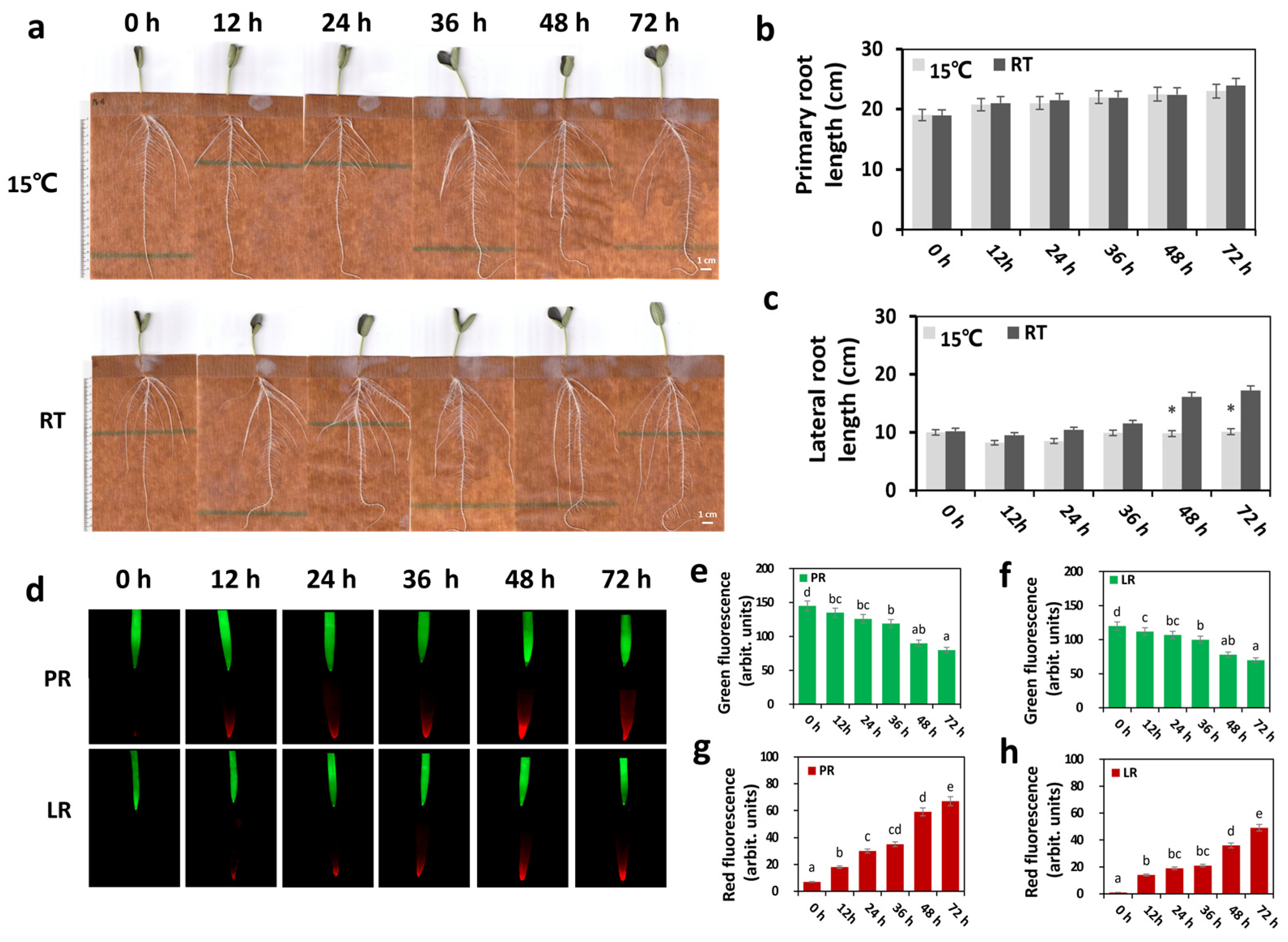
2.1. LRT Stress Has a Major Impact on the Bottle Gourd Root
2.2. LRT Affects Bottle Gourd Roots’ Physiological Traits
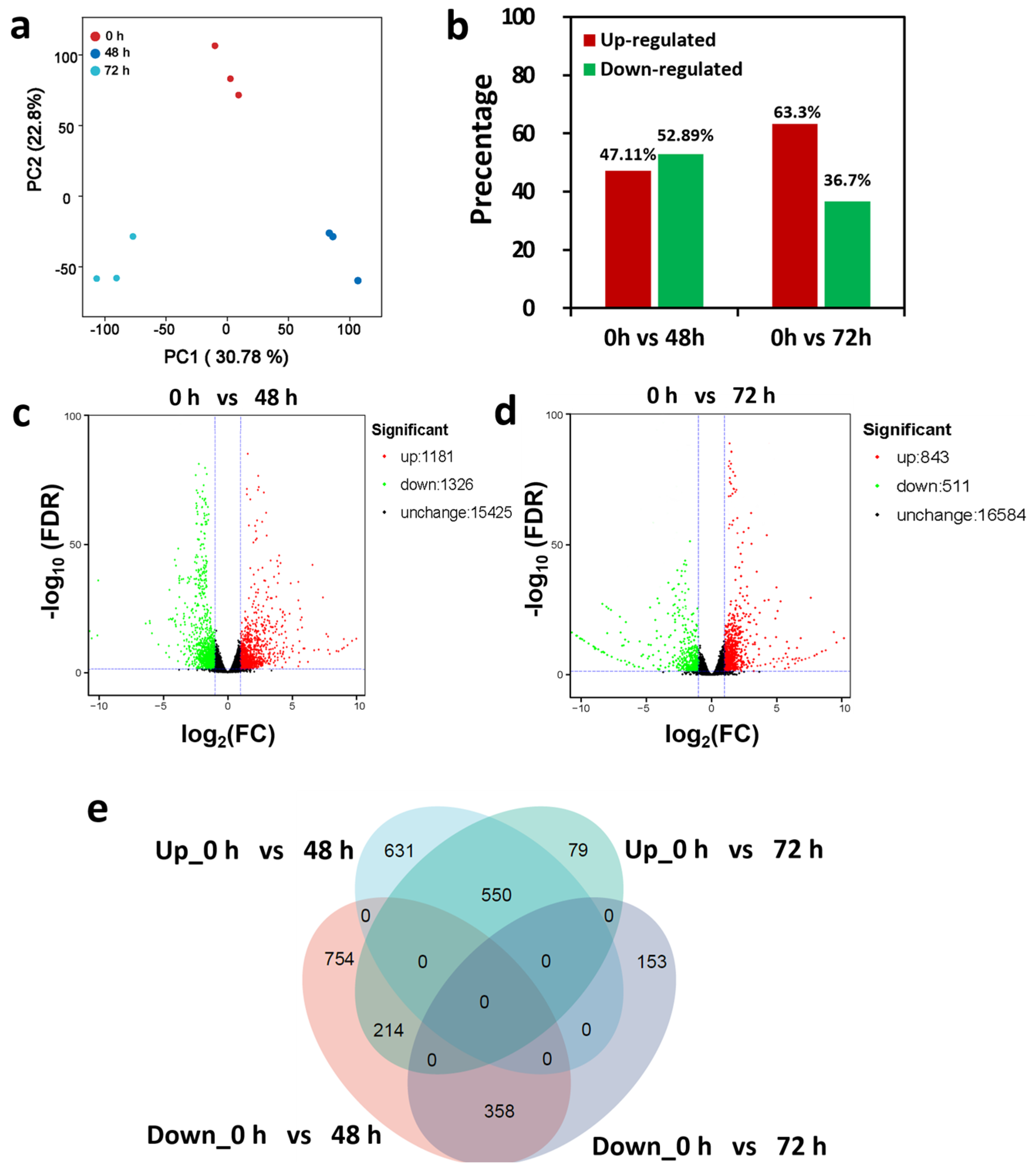
2.3. Transcriptomic Analysis Reveals DEGs in Response to LRT Stress
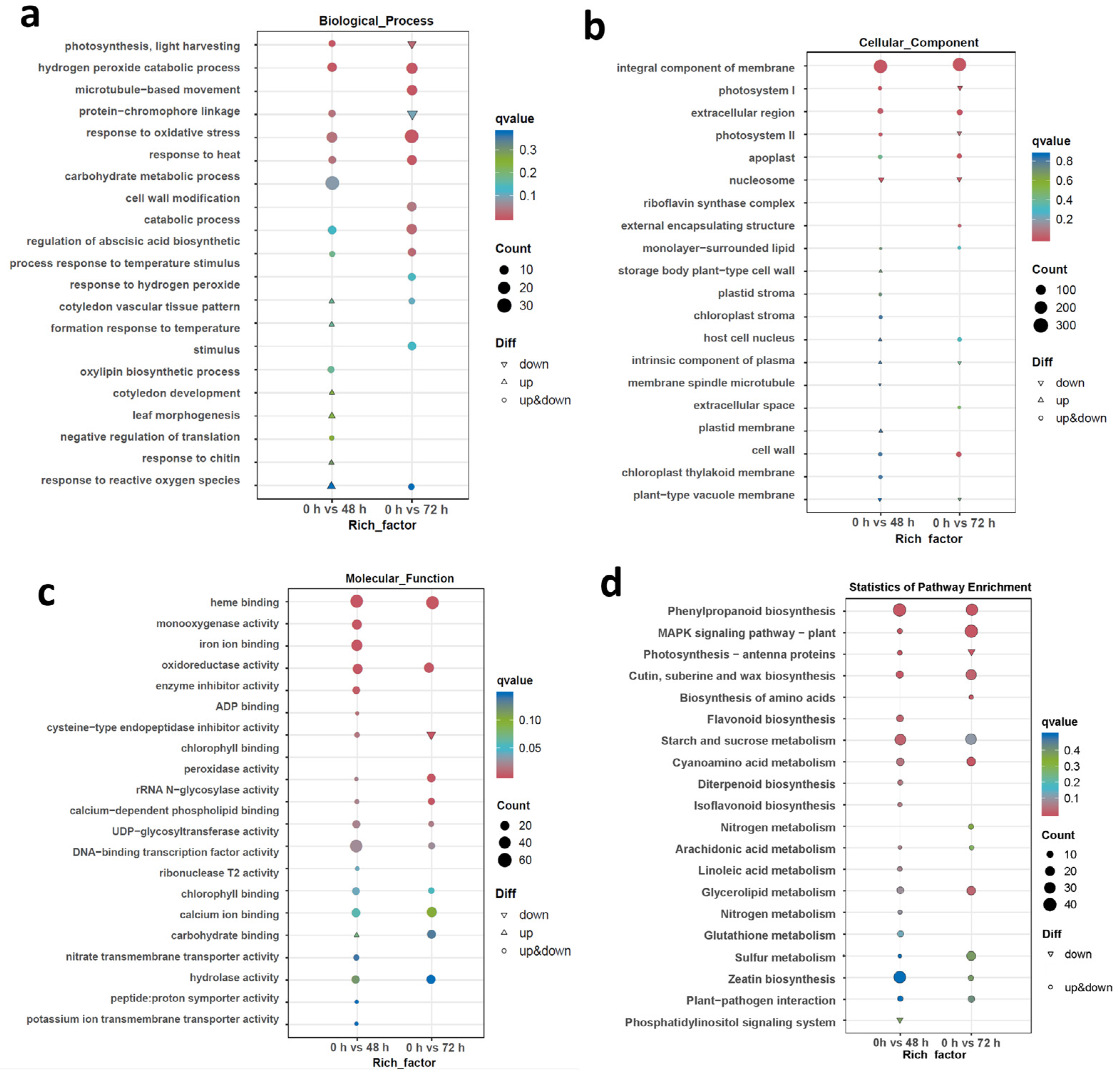
2.4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis among DEGs Involved in Translation and the Stress Response
2.5. TF Families Respond to LRT Differentially
2.6. qRT-PCR Analysis Validates the Expression Patterns of DEGs
3. Discussion
3.1. LRT Stress Affected the Phenotype and Physiology of Bottle Gourd Roots
3.2. Dynamic Changes in ROS Levels Occurred under LRT Conditions
3.3. Differentially Expressed TFs of the Bottle Gourd Root in Response to LRT Stress
4. Materials and Methods
4.1. Plant Materials, 15 °C LRT Treatment and Sample Preparation
4.2. Cell Viability Staining
4.3. Determination of Relative Electrolyte Leakage
4.4. Determination of Malondialdehyde (MDA) and Peroxide Enzyme Activity
4.5. Total RNA Extraction
4.6. Library Construction and RNA-Seq Data Analysis
4.7. Quantitative Real-Time PCR (qRT-PCR) for RNA-Seq Validation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Foyer, C.H.; Vanacker, H.; Gomez, L.D.; Harbinson, J. Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: Review. Plant Physiol. Biochem. 2002, 40, 659–668. [Google Scholar] [CrossRef]
- Singh, T.; Bisht, N.; Ansari, M.M.; Chauhan, P.S. The hidden harmony: Exploring ROS-phytohormone nexus for shaping plant root architecture in response to environmental cues. Plant Physiol. Biochem. 2024, 206, 108273. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.N.; Ren, J.L.; Zhang, Y.; Chen, X.M.; Qi, M.F.; Li, T.L.; Zhang, G.Z.; Liu, Y.F. Effect of low root-zone temperature on photosynthesis, root structure and mineral element absorption of tomato seedlings. Sci. Hortic. 2023, 315, 111956. [Google Scholar]
- Zhao, Y.; Zhang, Q.; Li, J.; Yan, X.; He, H.; Gao, X.; Jia, G. High temperature in the root zone repressed flowering in Lilium× fromology by disturbing the photoperiodic pathway and reconfiguring hormones and primary metabolism. Environ. Exp. Bot. 2021, 192, 104644. [Google Scholar] [CrossRef]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root plasticity under abiotic stress. Plant Physiol. 2021, 187, 1057–1070. [Google Scholar] [CrossRef]
- Ahamed, A.; Murai-Hatano, M.; Ishikawa-Sakurai, J.; Hayashi, H.; Kawamura, Y.; Uemura, M. Cold stress-induced acclimation in rice is mediated by root-specific aquaporins. Plant Cell Physiol. 2012, 53, 1445–1456. [Google Scholar] [CrossRef]
- Hsu, C.H.; Hsu, Y.T. Biochemical responses of rice roots to cold stress. Bot. Stud. 2019, 60, 14. [Google Scholar] [CrossRef]
- Erickson, D.L.; Smith, B.D.; Clarke, A.C.; Sandweiss, D.H.; Tuross, N. An Asian origin for a 10,000-year-old domesticated plant in the Americas. Proc. Natl. Acad. Sci. USA 2005, 102, 18315–18320. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021, 10, 57. [Google Scholar] [CrossRef]
- Morales, C.; Riveros-Burgos, C.; Espinoza, S.F.; Maldonado, C.; Mashilo, J.; Pinto, C.; Contreras-Soto, R.I. Rootstocks comparison in grafted watermelon under water deficit: Effects on the fruit quality and yield. Plants 2023, 12, 509. [Google Scholar] [CrossRef]
- Lee, J.M. Cultivation of grafted vegetables I. current status, grafting methods, and benefits. HortScience 1994, 29, 235–239. [Google Scholar] [CrossRef]
- Davis, A.R.; Perkins-Veazie, P.; Sakata, Y.; López-Galarza, S.; Maroto, J.V.; Lee, S. Cucurbit grafting. CRC Crit. Rev. Plant Sci. 2008, 27, 50–74. [Google Scholar] [CrossRef]
- King, S.R.; Davis, A.R.; Liu, W.; Levi, A.; King, S.R.; Davis, A.R. Grafting for disease resistance. HortScience 2008, 43, 1673–1676. [Google Scholar] [CrossRef]
- Oumouloud, A.; El-Otmani, M.; Chikh-Rouhou, H.; Garcés Claver, A.; González Torres, R.; Perl-Treves, R. Breeding melon for resistance to Fusarium wilt: Recent developments. Euphytica 2013, 192, 155–169. [Google Scholar] [CrossRef]
- Decker-Walters, D.S.; Wilkins-Ellert, M.; Chung, S.-M.; Staub, J.E. Discovery and genetic assessment of wild bottle gourd [Lagenaria siceraria (Mol.) Standley; Cucurbitaceae] from Zimbabwe. Econ. Bot. 2004, 58, 501–508. [Google Scholar] [CrossRef]
- Fernández-García, N.; Martínez, V.; Carvajal, M. Effect of salinity on growth, mineral composition, and water relations of grafted tomato plants. J. Plant Nutr. Soil. Sci. 2004, 167, 616–622. [Google Scholar] [CrossRef]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 42–50. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Rahman, A. Cold stress response in Arabidopsis thaliana is mediated by GNOM ARF-GEF. Plant J. 2019, 97, 500–516. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Chen, G.; Luo, G.; Shen, X.; Ouyang, B.; Bie, Z.L. Transcription factor SlWRKY50 enhances cold tolerance in tomato by activating the jasmonic acid signaling. Plant Physiol. 2024, 194, 1075–1090. [Google Scholar] [CrossRef]
- Huang, X.; Cao, L.; Fan, J.; Ma, G.; Chen, L. CdWRKY2-mediated sucrose biosynthesis and CBF-signaling pathways coordinately contribute to cold tolerance in bermudagrass. Plant Biotechnol. J. 2022, 20, 660–675. [Google Scholar] [CrossRef]
- Bianchimano, L.; De Luca, M.B.; Borniego, M.B.; Iglesias, M.J.; Casal, J.J. Temperature regulation of auxin-related gene expression and its implications for plant growth. J. Exp. Bot. 2023, 74, 7015–7033. [Google Scholar] [CrossRef]
- Wachsman, G.; Zhang, J.; Moreno-Risueno, M.A.; Anderson, C.T.; Benfey, P.N. Cell wall remodeling and vesicle trafficking mediate the root clock in Arabidopsis. Science 2020, 370, 819–823. [Google Scholar] [CrossRef]
- Holt, D.B.; Gupta, V.; Meyer, D.; Abel, N.B.; Andersen, S.U.; Stougaard, J.; Markmann, K. micro-RNA 172 (miR172) signals epidermal infection and is expressed in cells primed for bacterial invasion in Lotus japonicus roots and nodules. New Phytol. 2015, 208, 241–256. [Google Scholar] [CrossRef]
- Sexauer, M.; Bhasin, H.; Schön, M.; Roitsch, E.; Wall, C.; Herzog, U.; Markmann, K. A micro-RNA mediates shoot control of root branching. Nat. Commun. 2023, 6, 8083–8094. [Google Scholar] [CrossRef]
- Moison, M.; Pacheco, J.M.; Lucero, L.; Fonouni-Farde, C.; Rodríguez-Melo, J.; Mansilla, N.; Christ, A.; Bazin, J.; Benhamed, M.; Ibañez, F.; et al. The lncRNA APOLO interacts with the transcription factor WRKY42 to trigger root hair cell expansion in response to cold. Mol. Plant 2021, 7, 937–948. [Google Scholar] [CrossRef]
- Aidoo, M.K.; Sherman, T.; Lazarovitch, N.; Fait, A.; Rachmilevitch, S. Physiology and metabolism of grafted bell pepper in response to low root-zone temperature. Funct. Plant Biol. 2019, 46, 339–349. [Google Scholar] [CrossRef]
- Fu, X.; Zhong, L.; Wang, H.; He, H.; Chen, X. Elucidation of the Mechanism of Rapid Growth Recovery in Rice Seedlings after Exposure to Low-Temperature Low-Light Stress: Analysis of Rice Root Transcriptome, Metabolome, and Physiology. Int. J. Mol. Sci. 2023, 11, 17359. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Xu, J.; Fu, X.; Gao, J.; Wang, B.; Han, H.; Wang, L.; Peng, R.; Yao, Q. A tomato ERF transcription factor, SlERF84, confers enhanced tolerance to drought and salt stress but negatively regulates immunity against Pseudomonas syringae pv. Tomato DC3000. Plant Physiol. Biochem. 2018, 132, 683–695. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Zhang, Y.; Wang, B.; Ran, Q.; Zhang, J. The bHLH family member ZmPTF1 regulates drought tolerance in maize by promoting root development and abscisic acid synthesis. J. Exp. Bot. 2019, 70, 5471–5486. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Chu, X.; Wang, J.G.; Li, M.; Zhang, S.; Gao, Y.; Fan, M.; Han, C.; Xiang, F.; Li, G.; Wang, Y.; et al. HBI transcription factor-mediated ROS homeostasis regulates nitrate signal transduction. Plant Cell 2021, 33, 3004–3021. [Google Scholar] [CrossRef]
- Perez-Garcia, P.; Pucciariello, O.; Sanchez-Corrionero, A.; Cabrera, J.; Del Barrio, C.; Del Pozo, J.C.; Perales, M.; Wabnik, K.; Moreno-Risueno, M.A. The cold-induced factor CBF3 mediates root stem cell activity, regeneration, and developmental responses to cold. Plant Commun. 2023, 13, 100737–100743. [Google Scholar] [CrossRef]
- Yu, S.W.; Huang, A.N.; Li, J.; Gao, L.; Feng, Y.N.; Pemberton, E.; Chen, C.L. OsNAC45 plays complex roles by mediating POD activity and the expression of development-related genes under various abiotic stresses in rice root. Plant Growth Regul. 2018, 84, 519–531. [Google Scholar] [CrossRef]
- Yarra, R.; Wei, W. The NAC-type transcription factor GmNAC20 improves cold, salinity tolerance, and lateral root formation in transgenic rice plants. Funct. Integr. Genomics 2021, 21, 473–487. [Google Scholar] [CrossRef]
- Hao, Y.J.; Wei, W.; Song, Q.X.; Chen, H.W.; Zhang, Y.Q.; Wang, F.; Zou, H.F.; Lei, G.; Tian, A.G.; Zhang, W.K.; et al. Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J. 2011, 68, 302–313. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Xu, Z.; Chen, M.; Yu, D. Functions of WRKYs in plant growth and development. Trends Plant Sci. 2023, 28, 630–645. [Google Scholar] [CrossRef]
- Yao, C.; Li, X.; Li, Y.; Yang, G.; Liu, W.; Shao, B.; Zhong, J.; Huang, P.; Han, D. Overexpression of a Malus baccata MYB Transcription Factor Gene MbMYB4 Increases Cold and Drought Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 4, 1794. [Google Scholar] [CrossRef]
- Vergara, A.; Haas, J.C.; Aro, T.; Stachula, P.; Street, N.R.; Hurry, V. Norway spruce deploys tissue-specific responses during acclimation to cold. Plant Cell Environ. 2022, 45, 427–445. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Yang, B.; Xu, S.; Wei, X.; Zhao, P.; Niu, F.; Sun, M.; Wang, C.; Cheng, H.; et al. WRKY55 transcription factor positively regulates leaf senescence and the defense response by modulating the transcription of genes implicated in the biosynthesis of reactive oxygen species and salicylic acid in Arabidopsis. Development 2020, 18, 147–158. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, H.; Sui, Y.; Li, S.; Wu, F.; Sun, X.; Sui, N. SbWRKY55 regulates sorghum response to saline environment by its dual role in abscisic acid signaling. Theor. Appl. Genet. 2022, 135, 2609–2625. [Google Scholar] [CrossRef]
- Lu, K.K.; Song, R.F.; Guo, J.X.; Zhang, Y.; Zuo, J.X.; Chen, H.H.; Liao, C.Y.; Hu, X.Y.; Ren, F.; Lu, Y.T.; et al. CycC1;1-WRKY75 complex-mediated transcriptional regulation of SOS1 controls salt stress tolerance in Arabidopsis. Plant Cell 2023, 26, 2570–2591. [Google Scholar] [CrossRef]
- Xie, C.; Li, C.; Wang, F.; Zhang, F.; Liu, J.; Wang, J.; Zhang, X.; Kong, X.; Ding, Z. NAC1 regulates root ground tissue maturation by coordinating with the SCR/SHR-CYCD6;1 module in Arabidopsis. Mol. Plant 2023, 3, 709–725. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.; Liu, Q.; Ahammed, G.J.; Lin, R.; Wang, L.; Shao, S.; Yu, J.; Zhou, Y. The HY5 and MYB15 transcription factors positively regulate cold tolerance in tomato via the CBF pathway. Plant Cell Environ. 2020, 43, 2712–2726. [Google Scholar] [CrossRef]
- Wan, J.; Wang, R.; Zhang, P.; Sun, L.; Ju, Q.; Huang, H.; Lü, S.; Tran, L.S.; Xu, J. MYB70 modulates seed germination and root system development in Arabidopsis. iScience 2021, 7, 103228. [Google Scholar] [CrossRef]
- Jeon, J.; Cho, C.; Lee, M.R.; Van Binh, N.; Kim, J. CYTOKININ RESPONSE FACTOR2 (CRF2) and CRF3 regulate lateral root development in response to cold stress in Arabidopsis. Plant Cell 2016, 28, 1828–1843. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, K.X.; Wang, W.S.; Gong, W.; Liu, W.C.; Chen, H.G.; Xu, H.H.; Lu, Y.T. Low temperature inhibits root growth by reducing auxin accumulation via ARR1/12. Plant Cell Physiol. 2015, 56, 727–736. [Google Scholar] [CrossRef]
- Aroca, R.; Amodeo, G.; Fernández-Illescas, S.; Herman, E.M.; Chaumont, F.; Chrispeels, M.J. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol. 2005, 137, 341–353. [Google Scholar] [CrossRef]
- Fei, J.; Wang, Y.S.; Cheng, H.; Su, Y.B.; Zhong, Y.J.; Zheng, L. The Kandelia obovata transcription factor KoWRKY40 enhances cold tolerance in transgenic Arabidopsis. BMC Plant Biol. 2022, 22, 274–281. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Hu, H.; Chen, L.; Zhang, H.; Chen, R. CabHLH79 acts upstream of CaNAC035 to regulate cold stress in pepper. Int. J. Mol. Sci. 2022, 25, 2537. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, R.; Xiang, C.; Zhang, R.; Wang, Q.; Wang, T.; Li, X.; Lu, X.; Gao, S.; Liu, Z.; et al. Transcriptomic and physiological analysis reveal that α-Linolenic acid biosynthesis responds to early chilling tolerance in pumpkin rootstock varieties. Front. Plant Sci. 2021, 23, 669565. [Google Scholar] [CrossRef]
- Trevisan, S.; Trentin, A.R.; Ghisi, R.; Masi, A.; Quaggiotti, S. Nitrate affects transcriptional regulation of UPBEAT1 and ROS localisation in roots of Zea mays L. Physiol. Plant. 2019, 166, 794–811. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, H.; Yang, L.; Chen, C.; Shabala, L.; Xiong, M.; Niu, M.; Liu, J.; Zheng, Z.; Zhou, L.; et al. Tissue-specific respiratory burst oxidase homolog-dependent H2O2 signaling to the plasma membrane H+-ATPase confers potassium uptake and salinity tolerance in Cucurbitaceae. J. Exp. Bot. 2019, 70, 5879–5893. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Yu, J.; Lyv, J.; Zhang, J.; Ding, D.; Li, N.; Zhang, J.; Bakpa, E.P.; Yang, Y.; et al. Melatonin enhanced low-temperature combined with low-light tolerance of pepper (Capsicum annuum L.) seedlings by regulating root growth, antioxidant defense system, and osmotic adjustment. Front. Plant Sci. 2022, 28, 998293. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.; Al, F.M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Cui, P.; Li, Y.; Cui, C.; Huo, Y.; Lu, G.; Yang, H. Proteomic and metabolic profile analysis of low-temperature storage responses in Ipomoea batata Lam. tuberous roots. BMC Plant Biol. 2020, 20, 435–447. [Google Scholar] [CrossRef]
- Joshi, M.; Fogelman, E.; Belausov, E.; Ginzberg, I. Potato root system development and factors that determine its architecture. J. Plant Physiol. 2016, 20, 113–123. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Feng, S.; Zhang, J.; Zhang, F.; Wang, W.; Hu, H.; Zhang, W.; Bao, M. The C2H2-type zinc finger protein PhZFP1 regulates cold stress tolerance by modulating galactinol synthesis in Petunia hybrida. J. Exp. Bot. 2022, 18, 6434–6448. [Google Scholar] [CrossRef]
- Agarwal, M.; Hao, Y.; Kapoor, A.; Dong, C.H.; Fujii, H.; Zheng, X.; Zhu, J.K. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 2006, 281, 37636–37645. [Google Scholar] [CrossRef]
- Motte, H.; Vanneste, S.; Beeckman, T. Molecular and Environmental Regulation of Root Development. Annu. Rev. Plant Biol. 2019, 29, 465–488. [Google Scholar] [CrossRef]
- Guo, M.; Yang, F.; Liu, C.; Zou, J.; Qi, Z.; Fotopoulos, V.; Lu, G.; Yu, J.; Zhou, J. A single-nucleotide polymorphism in WRKY33 promoter is associated with the cold sensitivity in cultivated tomato. New Phytol. 2022, 236, 989–1005. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; He, S.P.; Gao, Y.; Wang, N.N.; Lu, R.; Li, X.B. A cotton (Gossypium hirsutum) WRKY transcription factor (GhWRKY22) participates in regulating anther/pollen development. Plant Physiol. Biochem. 2019, 141, 231–239. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Nvsvrot, T.; Huang, L.; Cai, G.; Ding, Y.; Ren, W.; Wang, N. The transcription factor WRKY75 regulates the development of adventitious roots, lateral buds and callus by modulating hydrogen peroxide content in poplar. J. Exp. Bot. 2022, 2, 1483–1498. [Google Scholar] [CrossRef]
- Rishmawi, L.; Pesch, M.; Juengst, C.; Schauss, A.C.; Schrader, A.; Hülskamp, M. Non-cell-autonomous regulation of root hair patterning genes by WRKY75 in Arabidopsis. Plant Physiol. 2014, 165, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Bose, J.; Shabala, L.; Shabala, S. Difference in root K+ retention ability and reduced sensitivity of K+-permeable channels to reactive oxygen species confer differential salt tolerance in three Brassica species. J. Exp. Bot. 2016, 67, 4611–4625. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2004, 15, 550. [Google Scholar] [CrossRef]
- Liu, J.Q.; Ai, Y.X.; Wang, Y.H.; Lu, Q.; Li, T.; Wu, L.; Sun, L.; Shen, H.L. Fine mapping of the Ca3GT gene controlling anthocyanin biosynthesis in mature unripe fruit of Capsicum annuum L. Theor. Appl. Genet. 2020, 133, 2729–2742. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, M.; Xu, J.; Yao, X.; Lou, L.; Hou, Q.; Zhu, L.; Yang, X.; Liu, G.; Xu, J. A Transcriptomic Analysis of Bottle Gourd-Type Rootstock Roots Identifies Novel Transcription Factors Responsive to Low Root Zone Temperature Stress. Int. J. Mol. Sci. 2024, 25, 8288. https://doi.org/10.3390/ijms25158288
Liu J, Zhang M, Xu J, Yao X, Lou L, Hou Q, Zhu L, Yang X, Liu G, Xu J. A Transcriptomic Analysis of Bottle Gourd-Type Rootstock Roots Identifies Novel Transcription Factors Responsive to Low Root Zone Temperature Stress. International Journal of Molecular Sciences. 2024; 25(15):8288. https://doi.org/10.3390/ijms25158288
Chicago/Turabian StyleLiu, Jinqiu, Man Zhang, Jian Xu, Xiefeng Yao, Lina Lou, Qian Hou, Lingli Zhu, Xingping Yang, Guang Liu, and Jinhua Xu. 2024. "A Transcriptomic Analysis of Bottle Gourd-Type Rootstock Roots Identifies Novel Transcription Factors Responsive to Low Root Zone Temperature Stress" International Journal of Molecular Sciences 25, no. 15: 8288. https://doi.org/10.3390/ijms25158288
APA StyleLiu, J., Zhang, M., Xu, J., Yao, X., Lou, L., Hou, Q., Zhu, L., Yang, X., Liu, G., & Xu, J. (2024). A Transcriptomic Analysis of Bottle Gourd-Type Rootstock Roots Identifies Novel Transcription Factors Responsive to Low Root Zone Temperature Stress. International Journal of Molecular Sciences, 25(15), 8288. https://doi.org/10.3390/ijms25158288




