Antidepressant-like and Beneficial Effects of a Neoponcirin-Beta-Cyclodextrin Inclusion Complex in Mice Exposed to Prolonged Stress
Abstract
:1. Introduction
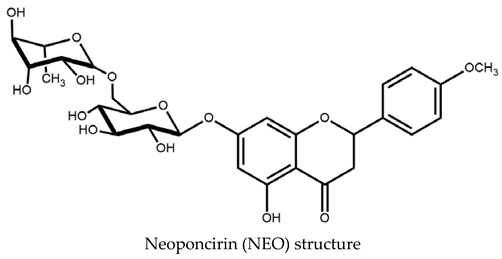
2. Results
2.1. Obtention and Analysis of the NEO/βCD Complex
2.1.1. Analysis by Nuclear Magnetic Resonance (NMR) of NEO/βCD
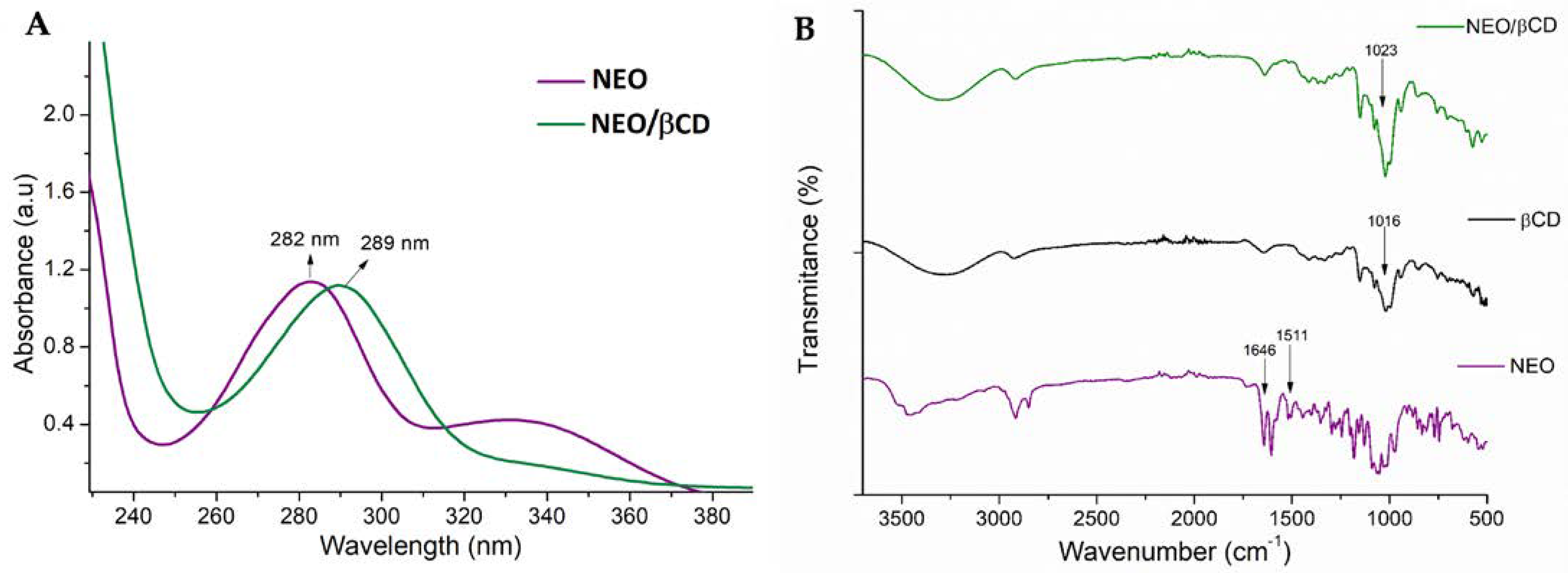
2.1.2. Analysis by Ultraviolet-Visible (UV-Vis) and Fourier-Transform InfraRed (FT-IR) Spectroscopy Methods of NEO/βCD
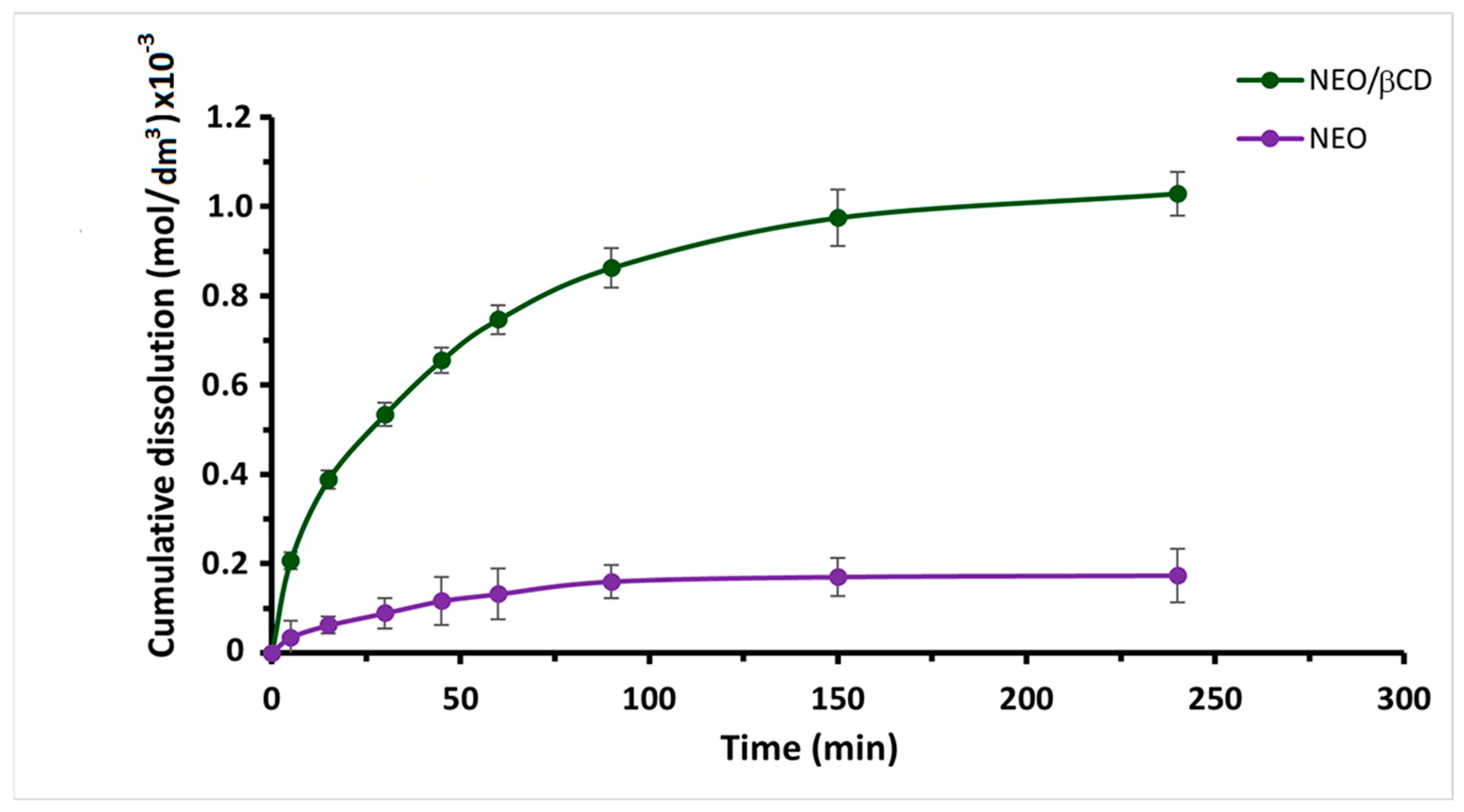
2.1.3. In Vitro Dissolution Study
2.2. Behavioral Evaluation of NEO/βCD
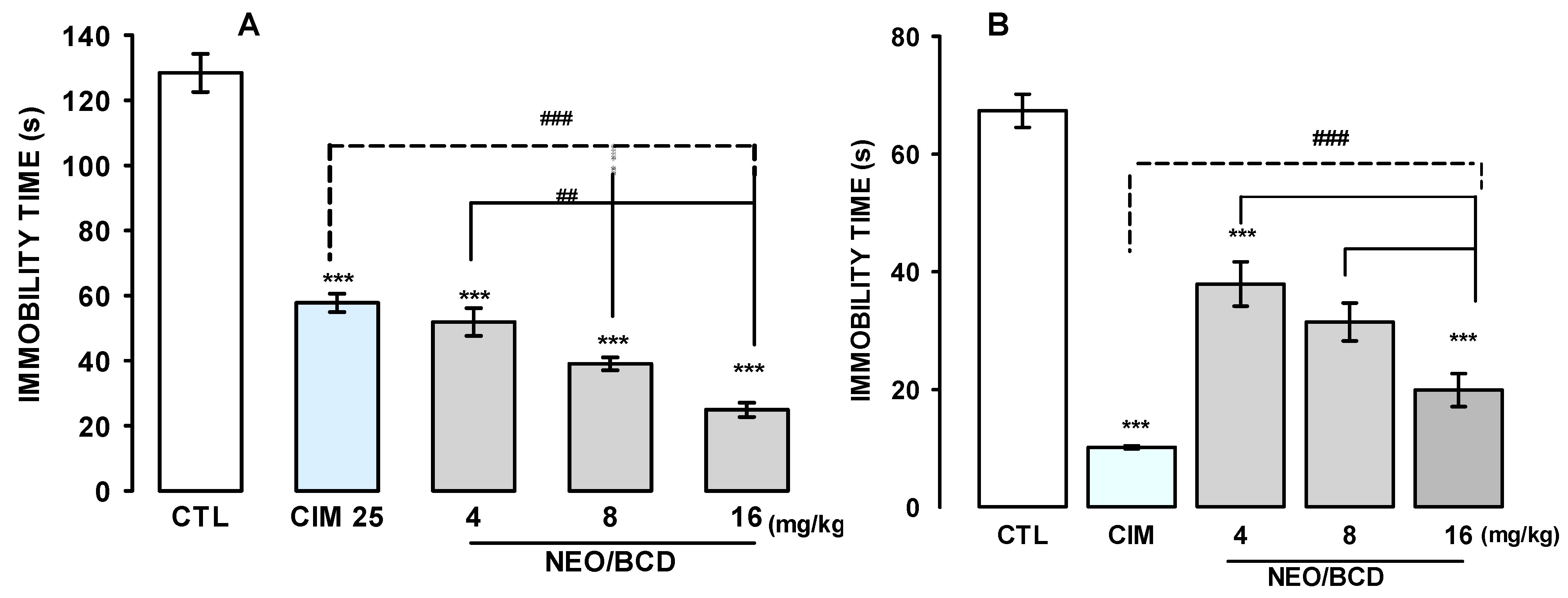
2.2.1. Oral Acute Antidepressant-Like Effects of NEO/βCD in the TST and the FST
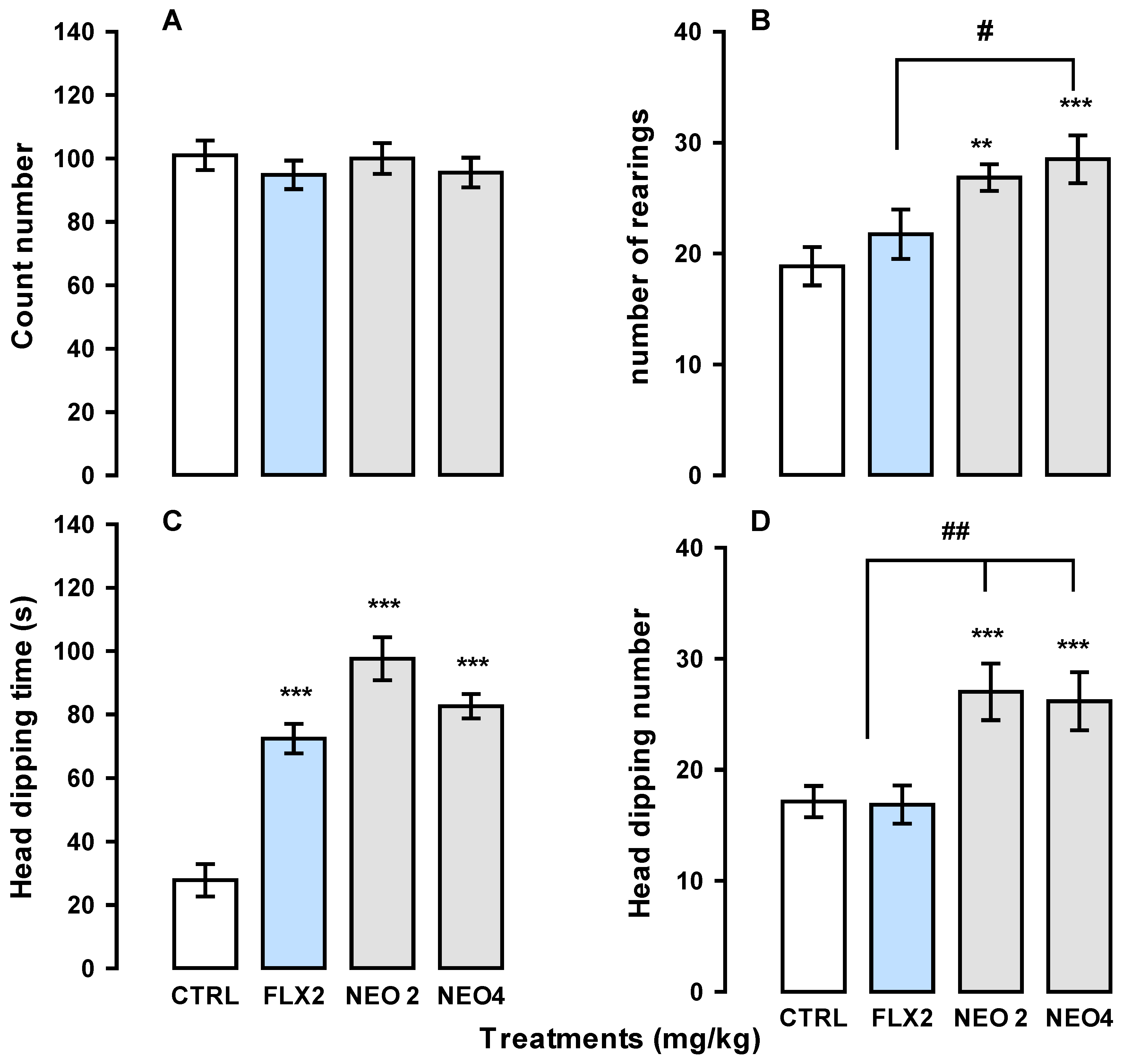
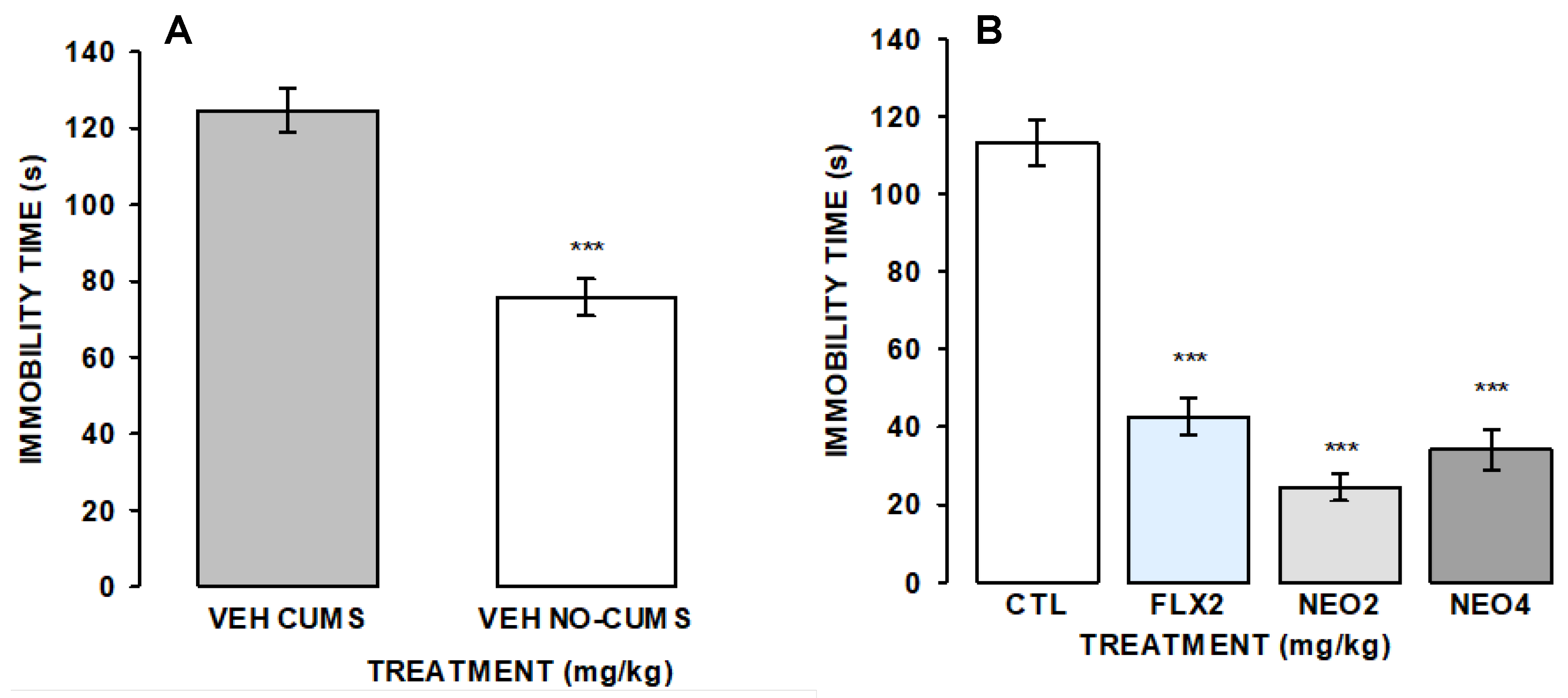
2.2.2. Hole-Board and the FST to Measure the Effects of NEO/βCD on the Mice Subjected to Chronic Unpredictable Mild Stress (CUMS)
2.2.3. Corticosterone and Interleukin Levels in the Serum of Mice Subjected to CUMS
3. Discussion
4. Materials and Methods
4.1. Neoponcirin Isolation, Formation, and Characterization of the NEO/βCD Complex
4.1.1. Formation of the NEO/βCD Complex
4.1.2. NMR Study of the NEO/βCD Complex
4.1.3. Dissolution Study
4.2. Pharmacological Evaluation
4.2.1. Animals
4.2.2. Drugs
4.2.3. Tail Suspension Test (TST)
4.2.4. Forced Swimming Test (FST)
4.2.5. Open Field Test (OFT)
4.2.6. Hole Board Test (HBT)
4.2.7. Evaluation of the Oral Acute Effects of NEO/βCD in Despair Tests
- Experimental Design
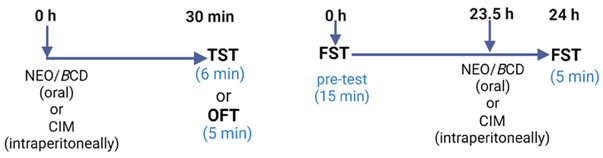
4.2.8. Evaluation of the Chronic Effects of NEO/βCD in Animals Subjected to the Chronic Unpredictable Mild Stress Test (CUMS)

4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalin, N.H. The Critical Relationship Between Anxiety and Depression. Am. J. Psychiatry 2020, 177, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Santomauro, D.F.; Mantilla Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global Prevalence and Burden of Depressive and Anxiety Disorders in 204 Countries and Territories in 2020 Due to the COVID-19 Pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 2020, 11, 595584. [Google Scholar] [CrossRef] [PubMed]
- Leppien, E.; Bystrak, T.; Doughty, B. Antidepressant Medications. In Side Effects of Drugs Annual; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–21. [Google Scholar]
- Cassani, J.; Escalona Araujo, A.G.; Martínez-Vázquez, M.; Manjarrez, N.; Moreno, J.; Estrada-Reyes, R. Anxiolytic-like and Antinociceptive Effects of 2(S)-Neoponcirin in Mice. Molecules 2013, 18, 7584–7599. [Google Scholar] [CrossRef] [PubMed]
- Pannu, A.; Sharma, P.C.; Thakur, V.K.; Goyal, R.K. Emerging Role of Flavonoids as the Treatment of Depression. Biomolecules 2021, 11, 1825. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.; Rupasinghe, H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, R.; Martínez-Vázquez, M.; Gallegos-Solís, A.; Heinze, G.; Moreno, J. Depressant Effects of Clinopodium Mexicanum Benth. Govaerts (Lamiaceae) on the Central Nervous System. J. Ethnopharmacol. 2010, 130, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins Inclusion Complex: Preparation Methods, Analytical Techniques and Food Industry Applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Franzini, R.; Ciogli, A.; Gasparrini, F.; Ismail, O.H.; Villani, C. Recent Developments in Chiral Separations by Supercritical Fluid Chromatography. In Chiral Analysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 607–629. [Google Scholar]
- López-Méndez, L.J.; Rojas-Aguirre, Y.; Vázquez-Lima, H.; Cassani, J.; Enríquez, R.G.; Rojo-Domínguez, A.; Guadarrama, P. On the Conformational Search of a ΒCD Dendritic Derivative: NMR and Theoretical Calculations Working Together Reveal a Donut-like Amphiphilic Structure. J. Mol. Struct. 2020, 1204, 127535. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Piotrowska, H.; Szymanowska-Powałowska, D.; Lewandowska, K.; Goscianska, J.; Pietrzak, R.; Bednarski, W.; Majka, Z.; Cielecka-Piontek, J. Complex of Rutin with β-Cyclodextrin as Potential Delivery System. PLoS ONE 2015, 10, e0120858. [Google Scholar]
- Kapoor, M.P.; Moriwaki, M.; Minoura, K.; Timm, D.; Abe, A.; Kito, K. Structural Investigation of Hesperetin-7-O-Glucoside Inclusion Complex with β-Cyclodextrin: A Spectroscopic Assessment. Molecules 2022, 27, 5395. [Google Scholar] [CrossRef] [PubMed]
- Pessine, T.; Calderini, F.B.; Alexandrino, L.G. Review: Cyclodextrin Inclusion Complexes Probed by NMR Techniques. In Magnetic Resonance Spectroscopy; InTech: London, UK, 2012. [Google Scholar]
- Kanaujia, P.; Poovizhi, P.; Ng, W.K.; Tan, R.B.H. Amorphous Formulations for Dissolution and Bioavailability Enhancement of Poorly Soluble APIs. Powder Technol. 2015, 285, 2–15. [Google Scholar] [CrossRef]
- Khatun, B.; Rather, M.A.; Rohilla, S.; Borah, R.; Mandal, M.; Maji, T.K. Curcumin–Hydroxypropyl-$$\beta$$-Cyclodextrin Complex Preparation Methods: A Comparative Study. Chem. Pap. 2023, 77, 4409–4424. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as Pharmaceutical Solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Pérez, J.J.; Benítez-Coronel, V.; Jiménez-Rubio, G.; Hernández-Hernández, O.T.; Martínez-Mota, L. Corrigendum to “Young-Adult Male Rats’ Vulnerability to Chronic Mild Stress Is Reflected by Anxious-Like Instead of Depressive-Like Behaviors”. Neurosci. J. 2017, 2017, 8952079. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Hernández, A.; González-González, R.; Guzmán-Velázquez, S.; Hernández Hernández, O.T.; Zamudio, S.R.; Martínez-Mota, L. Antidepressant-like Effects of Acupuncture via Modulation of Corticosterone, Sex Hormones, and Hippocampal BDNF Expression in Male Rats. Brain Res. Bull. 2021, 173, 53–65. [Google Scholar] [CrossRef]
- Du Preez, A.; Eum, J.; Eiben, I.; Eiben, P.; Zunszain, P.A.; Pariante, C.M.; Thuret, S.; Fernandes, C. Do Different Types of Stress Differentially Alter Behavioural and Neurobiological Outcomes Associated with Depression in Rodent Models? A Systematic Review. Front. Neuroendocrinol. 2021, 61, 100896. [Google Scholar] [CrossRef]
- McEwen, B.S. Glucocorticoids, Depression, and Mood Disorders: Structural Remodeling in the Brain. Metabolism 2005, 54, 20–23. [Google Scholar] [CrossRef]
- Tafet, G.E.; Nemeroff, C.B. The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. J. Neuropsychiatry Clin. Neurosci. 2016, 28, 77–88. [Google Scholar] [CrossRef]
- Arrieta-Báez, D.; Gómez-Patiño, M.B.; Jurado Hernández, N.; Mayagoitia-Novales, L.; Dorantes-Barrón, A.M.; Estrada-Reyes, R. Antidepressant-like Effects of a Methanol Extract of Leonotis Nepetifolia in Mice. Nat. Prod. Res. 2022, 36, 6170–6176. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Reliability of the Chronic Mild Stress Model of Depression: A User Survey. Neurobiol. Stress 2017, 6, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Lotrich, F.E. Inflammatory Cytokines in Depression: Neurobiological Mechanisms and Therapeutic Implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef]
- Golovatscka, V.; Ennes, H.; Mayer, E.A.; Bradesi, S. Chronic Stress-Induced Changes in Pro-Inflammatory Cytokines and Spinal Glia Markers in the Rat: A Time Course Study. Neuroimmunomodulation 2012, 19, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Rhie, S.J.; Jung, E.-Y.; Shim, I. The Role of Neuroinflammation on Pathogenesis of Affective Disorders. J. Exerc. Rehabil. 2020, 16, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.O.V.; Reiche, E.M.V.; Morimoto, H.K.; Matsuo, T.; Itano, E.N.; Xavier, E.C.D.; Yamashita, C.M.; Vieira, V.R.; Menoli, A.V.; Silva, S.S.; et al. Immune and Hormonal Activity in Adults Suffering from Depression. Brazilian J. Med. Biol. Res. 2002, 35, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Jahnsen, H.; Laursen, A.M. The Effects of a Benzodiazepine on the Hyperpolarizing and the Depolarizing Responses of Hippocampal Cells to GABA. Brain Res. 1981, 207, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Hyman, S.E. Animal Models of Neuropsychiatric Disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef]
- Möhler, H. The GABA System in Anxiety and Depression and Its Therapeutic Potential. Neuropharmacology 2012, 62, 42–53. [Google Scholar] [CrossRef]
- Smith, K.S.; Rudolph, U. Anxiety and Depression: Mouse Genetics and Pharmacological Approaches to the Role of GABAA Receptor Subtypes. Neuropharmacology 2012, 62, 54–62. [Google Scholar] [CrossRef]
- Wilson, M.A.; Biscardi, R. Sex Differences in GABA/Benzodiazepine Receptor Changes and Corticosterone Release after Acute Stress in Rats. Exp. Brain Res. 1994, 101, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.P.; Nguyen, M.; Yow, T.T.; Chu, C.; Johnston, G.A.R.; Hanrahan, J.R.; Chebib, M. The Flavonoid Glycosides, Myricitrin, Gossypin and Naringin Exert Anxiolytic Action in Mice. Neurochem. Res. 2009, 34, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Hyunmyung Kim, H.-W.K.; Aqueous, S.J. Solubility Enhancement of Some Flavones by Complexation with Cyclodextrins. Bull. Korean Chem. Soc. 2008, 29, 590–594. [Google Scholar]
- Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0.
- Estrada-Reyes, R.; Valdés-Tovar, M.; Arrieta-Baez, D.; Dorantes-Barrón, A.; Quero-Chávez, D.; Solís-Chagoyán, H.; Argueta, J.; Dubocovich, M.; Benítez-King, G. The Timing of Melatonin Administration Is Crucial for Its Antidepressant-Like Effect in Mice. Int. J. Mol. Sci. 2018, 19, 2278. [Google Scholar] [CrossRef]
- Martinez-Mota, L.; Cruz-Tavera, A.; Dorantes-Barrón, A.M.; Arrieta-Báez, D.; Ramírez-Salado, I.; Cruz-Aguilar, M.A.; Mayagoitia-Novales, L.; Cassani, J.; Estrada-Reyes, R. Calea zacatechichi Schltdl. (Compositae) Produces Anxiolytic- and Antidepressant-like Effects, and Increases the Hippocampal Activity during REM Sleep in Rodents. J. Ethnopharmacol. 2021, 265, 113316. [Google Scholar] [CrossRef] [PubMed]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of Motor Activity and Anxiety-Related Behaviour in Rodents: Methodological Aspects and Role of Nitric Oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced Swimming Test in Mice: A Review of Antidepressant Activity. Psychopharmacology 2005, 177, 245–255. [Google Scholar] [CrossRef]

| 1H NMR | Chemical Shifts (ppm) | 1H NMR | Chemical Shifts (ppm) | ||||
|---|---|---|---|---|---|---|---|
| βCD | NEO/βCD | Δδ = δIC − δβCD | NEO | NEO/βCD | Δδ = δIC − δNEO | ||
| H1 | 4.824 | 4.823 | −0.001 | H6, H8 | 6.137 | 6.127 | −0.009 |
| H3 | 3.601 | 4.593 | −0.007 | H1′″ | 4.976 | 4.790 | −0.186 |
| H5 | 3.662 | 4.643 | −0.018 | H6′″ | 1.082 | 1.068 | −0.014 |
| Acute treatment (mg/kg) | Count number | Rearing number |
| CTL | 48.37 ± 2.50 | 30.25 ± 1.69 |
| CIM 25 | 55.25 ± 2.25 | 27.75 ± 2.11 |
| NEO/βCD 4 | 51.87 ± 4.90 | 30.25 ± 1.65 |
| NEO/βCD 8 | 45.50 ± 4.27 | 28.25 ± 1.53 |
| NEO/βCD16 | 46.00 ± 4.03 | 30.25 ± 2.05 |
| F(4,35) = 1.20, p = 0.32 | F(4,49) = 0.46, p = 0.76 | |
| Chronic treatment (mg/kg/day) | Count number | Rearing number |
| CTL | 52.12 ± 2.91 | 28.87 ± 1.77 |
| FLX 2 | 47.00 ± 4.90 | 24.12 ± 2.25 |
| NEO/βCD 4 | 49.62 ± 3.07 | 30.12 ± 2.01 |
| NEO/βCD 8 | 50.60 ± 4.46 | 29.62 ± 1.78 |
| F(3,28) = 0.30, p = 0.82 | F(3,28) = 1.96, p = 0.14 |
| Treatment (mg/kg/day) | Corticosterone (ng/mL) | IL-1β (pM/mL) | IL-6 (pM/mL) |
|---|---|---|---|
| VEH | 1159.14 ± 184.83 | 798.83 ± 63.49 | 258.57 ± 20.01 |
| FLX 2 | 139.50 ± 33.06 ** | 567.83 ± 42.16 ** | 291.55 ± 20.48 # |
| NEO/βCD 2 | 303.14 ± 50.54 ** | 624.83 ± 28.63 * | 387.43 ± 35.80 # |
| NEO/βCD 4 | 125.50 ± 32.55 ** | 587.29 ± 25.27 ** | 280.57 ± 17.37 |
| H = 17.48, df = 3, p = 0.002 | F(3,20) = 6.11, p = 0.004 | F(3,20) = 6.27, p = 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Méndez, L.J.; Martínez-Mota, L.; Cassani, J.; Mayagoitia-Novales, L.; Benítez-King, G.; Becerril-Villanueva, L.E.; Dorantes-Barrón, A.M.; Jurado-Hernández, N.; Estrada-Reyes, R. Antidepressant-like and Beneficial Effects of a Neoponcirin-Beta-Cyclodextrin Inclusion Complex in Mice Exposed to Prolonged Stress. Int. J. Mol. Sci. 2024, 25, 8289. https://doi.org/10.3390/ijms25158289
López Méndez LJ, Martínez-Mota L, Cassani J, Mayagoitia-Novales L, Benítez-King G, Becerril-Villanueva LE, Dorantes-Barrón AM, Jurado-Hernández N, Estrada-Reyes R. Antidepressant-like and Beneficial Effects of a Neoponcirin-Beta-Cyclodextrin Inclusion Complex in Mice Exposed to Prolonged Stress. International Journal of Molecular Sciences. 2024; 25(15):8289. https://doi.org/10.3390/ijms25158289
Chicago/Turabian StyleLópez Méndez, Luis José, Lucía Martínez-Mota, Julia Cassani, Lilian Mayagoitia-Novales, Gloria Benítez-King, Luis Enrique Becerril-Villanueva, Ana María Dorantes-Barrón, Noé Jurado-Hernández, and Rosa Estrada-Reyes. 2024. "Antidepressant-like and Beneficial Effects of a Neoponcirin-Beta-Cyclodextrin Inclusion Complex in Mice Exposed to Prolonged Stress" International Journal of Molecular Sciences 25, no. 15: 8289. https://doi.org/10.3390/ijms25158289





