Proteomic Analysis Reveals Physiological Activities of Aβ Peptide for Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
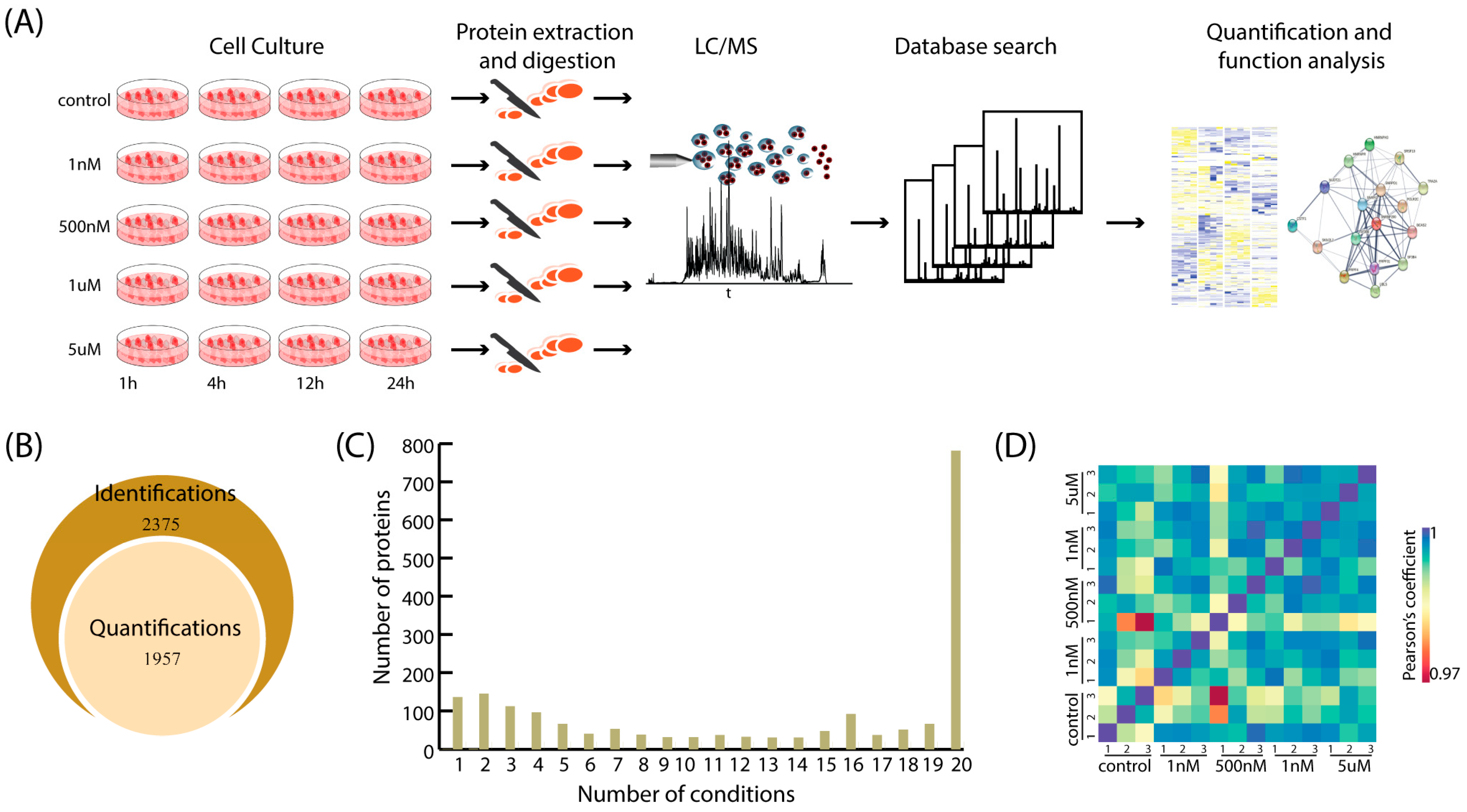
2.1. Overview of Proteomic Analysis for Aβ-Induced SHY5Y Cells
2.2. General Insights into Differential Expressed Proteins Identified with MS
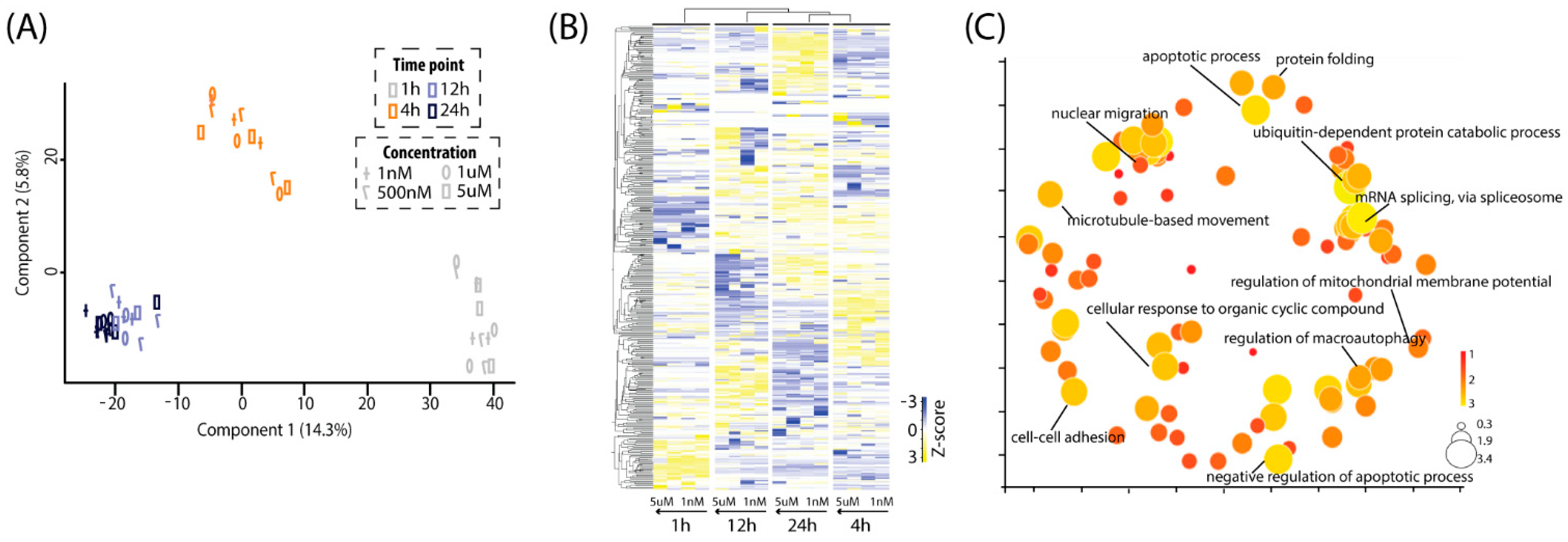
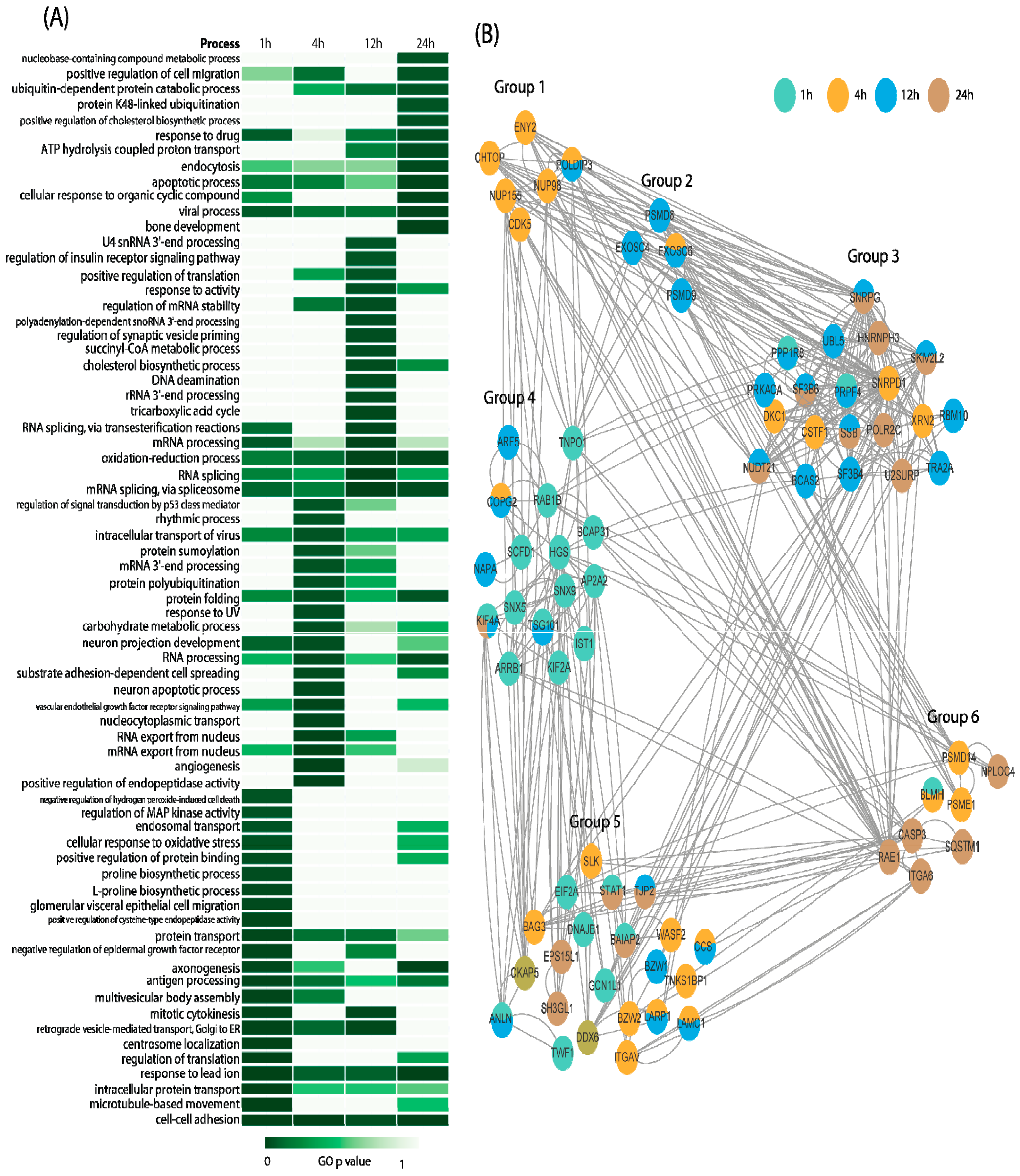
2.3. Time–Course Pattern Analysis of Aβ-Induced Proteomic Events
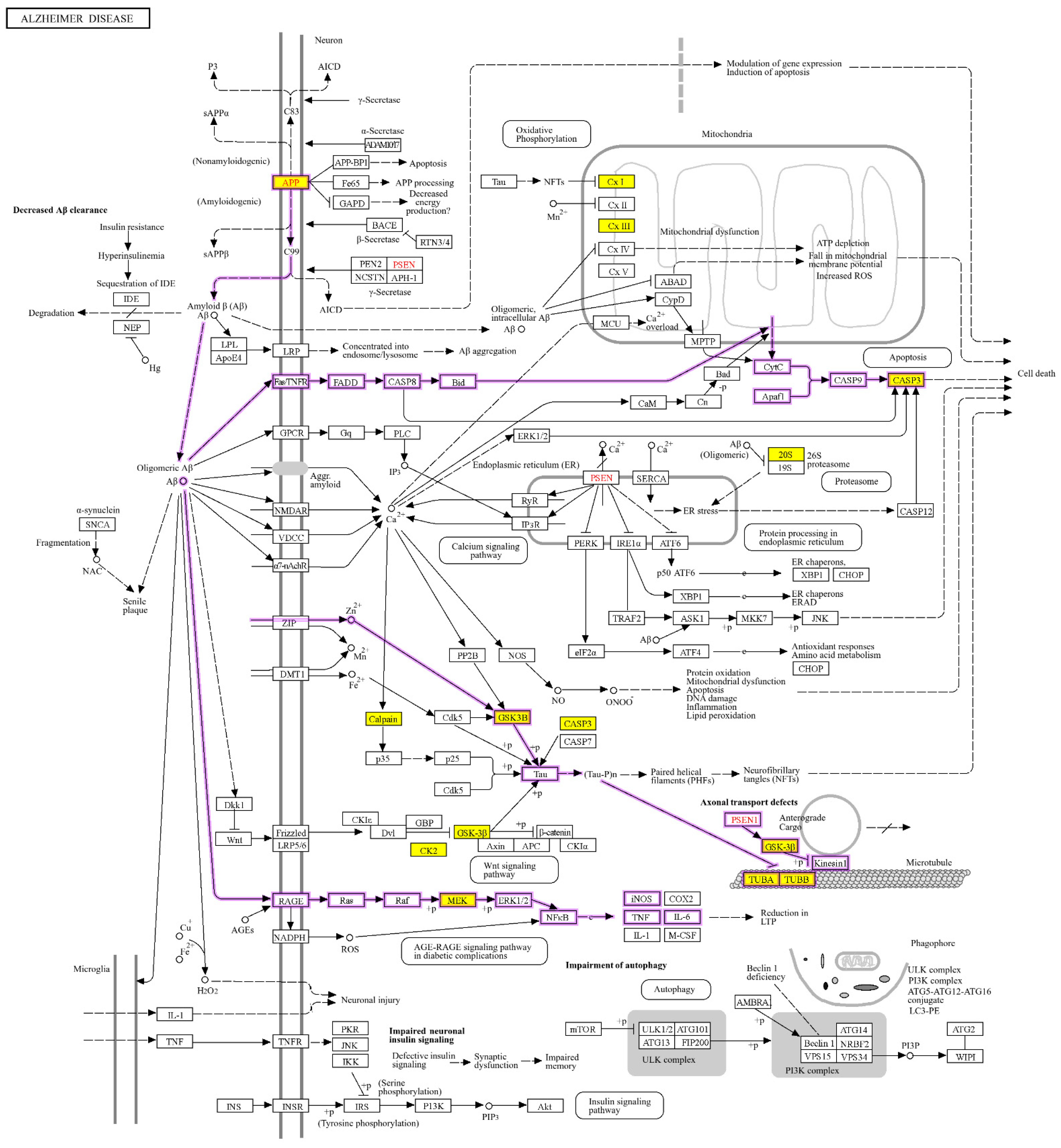
2.4. Physiological Pathways Stimulated by Aβ Monomers in Neuronal Cells
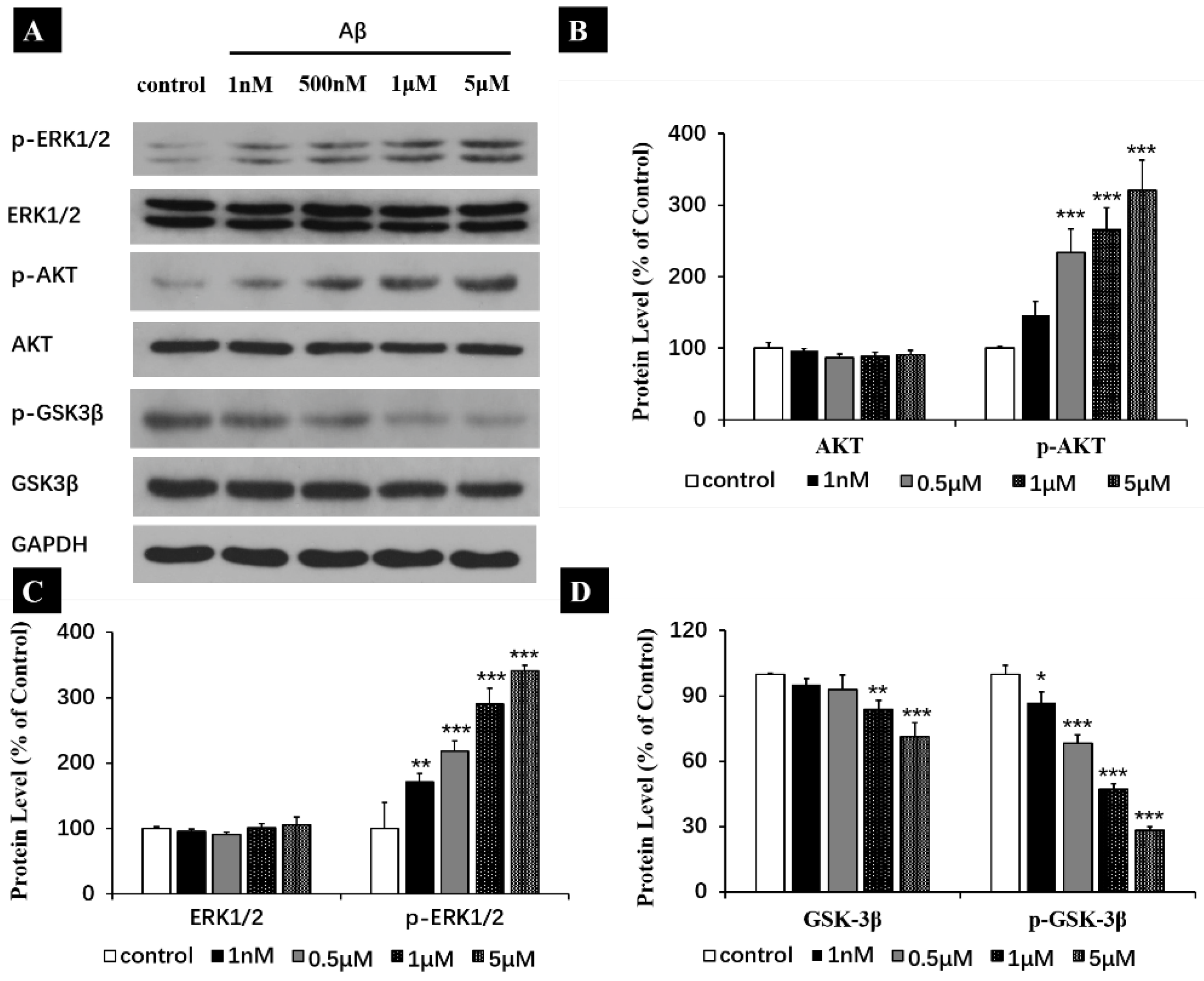
2.5. Aβ Regulating ERK, AKT and GSK-3β through Their Phosphorylation
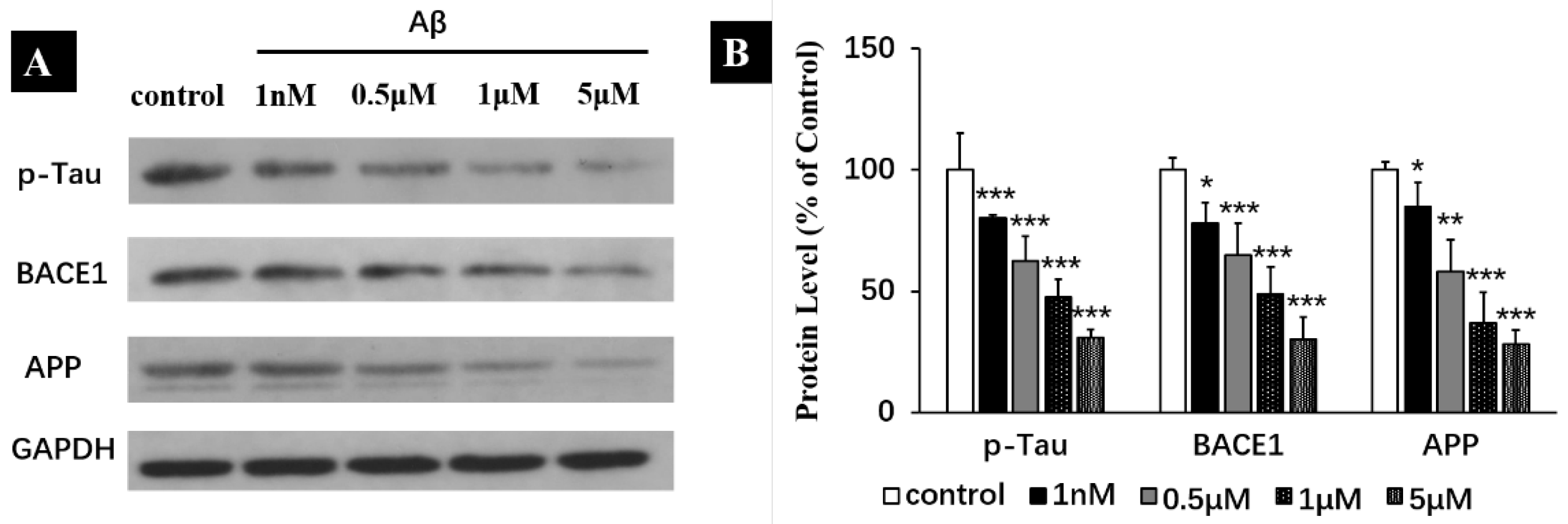
2.6. Aβ Monomers Inhibiting the Expression of APP, BACE1 and p-Tau to Achieve Their Physiological Functions
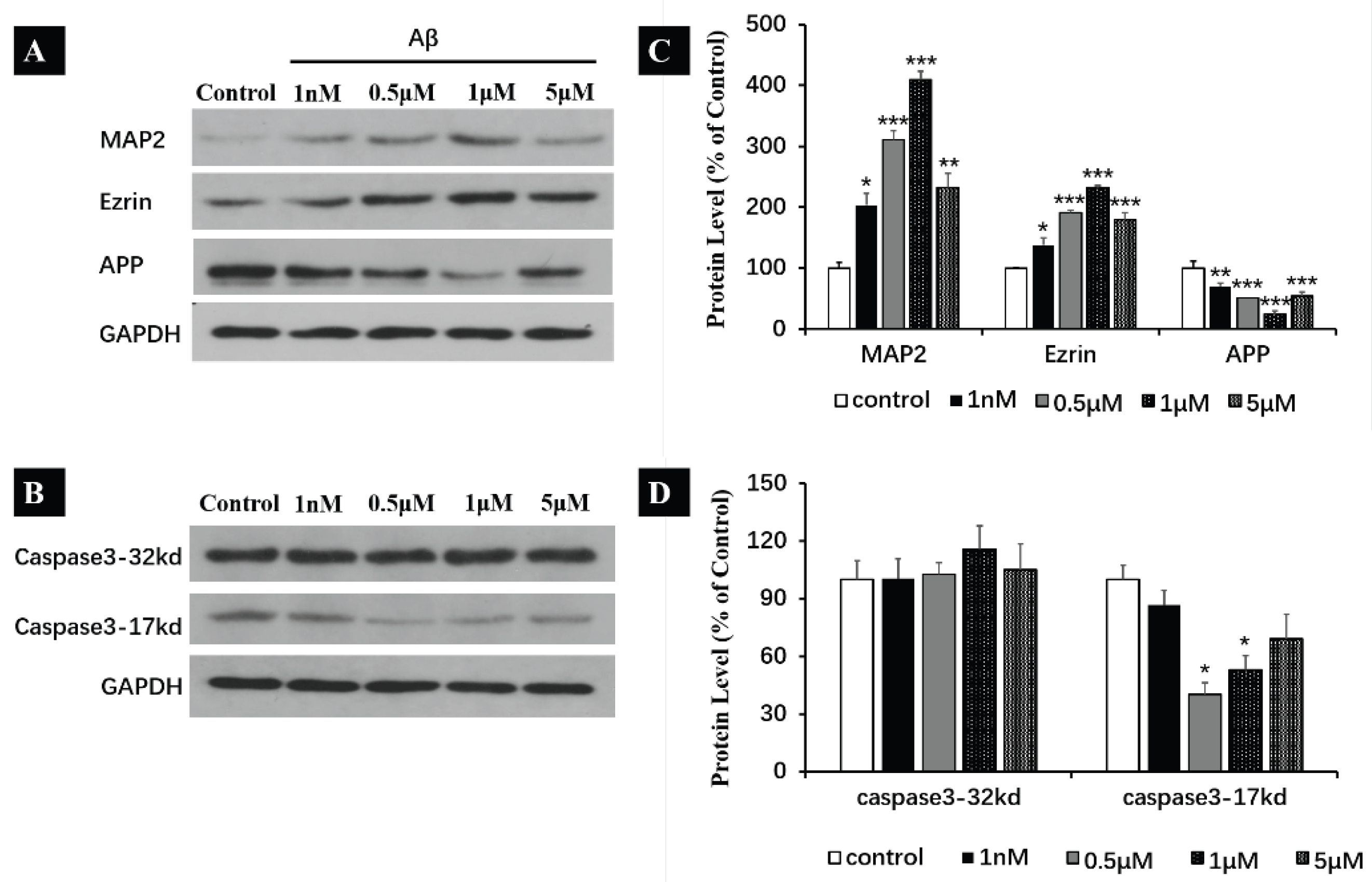
2.7. Prolonged Exposure of Aβ Monomers Increasing the Expression of MAP2, Ezrin and APP
3. Discussion
4. Materials and Methods
4.1. Sample Preparation of Aβ40 Monomers Solution
4.2. Cell Culture and Aβ Treatment
4.3. Protein Extraction and Digestion
4.4. MS Analysis
4.5. MS Data Analysis
4.6. Western Blot Validation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, S.; Muller-Schiffmann, A.; Erichsen, L.; Bohndorf, M.; Wruck, W.; Sleegers, K.; Van Broeckhoven, C.; Korth, C.; Adjaye, J. Ipsc-derived neuronal cultures carrying the alzheimer’s disease associated trem2 r47h variant enables the construction of an abeta-induced gene regulatory network. Int. J. Mol. Sci. 2020, 21, 4516. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Hyman, B.T.; Flory, J.; Damasio, A.R.; Van Hoesen, G.W. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with alzheimer’s disease. Cereb. Cortex 1991, 1, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Iwatsubo, T.; Odaka, A.; Suzuki, N.; Mizusawa, H.; Nukina, N.; Ihara, Y. Visualization of a beta 42(43) and a beta 40 in senile plaques with end-specific a beta monoclonals: Evidence that an initially deposited species is a beta 42(43). Neuron 1994, 13, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Roy, S. The physical approximation of app and bace-1: A key event in alzheimer’s disease pathogenesis. Dev. Neurobiol. 2018, 78, 340–347. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B. Lessons from a failed γ-secretase alzheimer trial. Cell 2014, 159, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Willem, M.; Tahirovic, S.; Busche, M.A.; Ovsepian, S.V.; Chafai, M.; Kootar, S.; Hornburg, D.; Evans, L.D.; Moore, S.; Daria, A.; et al. Eta-secretase processing of app inhibits neuronal activity in the hippocampus. Nature 2015, 526, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.L.; Wyttenbach, T.; Baumketner, A.; Shea, J.E.; Bitan, G.; Teplow, D.B.; Bowers, M.T. Amyloid beta-protein: Monomer structure and early aggregation states of abeta42 and its pro19 alloform. J. Am. Chem. Soc. 2005, 127, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.L.; Krafft, G.A.; Finch, C.E. Targeting small abeta oligomers: The solution to an alzheimer’s disease conundrum? Trends Neurosci. 2001, 24, 219–224. [Google Scholar] [CrossRef]
- Lu, J.X.; Qiang, W.; Yau, W.M.; Schwieters, C.D.; Meredith, S.C.; Tycko, R. Molecular structure of β-amyloid fibrils in alzheimer’s disease brain tissue. Cell 2013, 154, 1257–1268. [Google Scholar] [CrossRef]
- Pepeu, G.; Grazia Giovannini, M. The fate of the brain cholinergic neurons in neurodegenerative diseases. Brain Res. 2017, 1670, 173–184. [Google Scholar] [CrossRef]
- Guo, Q.; Sopher, B.L.; Furukawa, K.; Pham, D.G.; Robinson, N.; Martin, G.M.; Mattson, M.P. Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: Involvement of calcium and oxyradicals. J. Neurosci. 1997, 17, 4212–4222. [Google Scholar] [CrossRef]
- Huang, L.; Mcclatchy, D.B.; Maher, P.; Liang, Z.; Diedrich, J.K.; Soriano-Castell, D.; Goldberg, J.; Shokhirev, M.; Yates, J.R., 3rd; Schubert, D.; et al. Intracellular amyloid toxicity induces oxytosis/ferroptosis regulated cell death. Cell Death Dis. 2020, 11, 828. [Google Scholar] [CrossRef]
- Brown, G.C.; Heneka, M.T. The endotoxin hypothesis of alzheimer’s disease. Mol. Neurodegener. 2024, 19, 30. [Google Scholar] [CrossRef]
- Plant, L.D.; Boyle, J.P.; Smith, I.F.; Peers, C.; Pearson, H.A. The production of amyloid beta peptide is a critical requirement for the viability of central neurons. J. Neurosci. 2003, 23, 5531–5535. [Google Scholar] [CrossRef]
- Soucek, T.; Cumming, R.; Dargusch, R.; Maher, P.; Schubert, D. The regulation of glucose metabolism by hif-1 mediates a neuroprotective response to amyloid beta peptide. Neuron 2003, 39, 43–56. [Google Scholar] [CrossRef]
- Whitson, J.S.; Selkoe, D.J.; Cotman, C.W. Amyloid beta protein enhances the survival of hippocampal neurons in vitro. Science 1989, 243, 1488–1490. [Google Scholar] [CrossRef]
- Richards, A.L.; Eckhardt, M.; Krogan, N.J. Mass spectrometry-based protein-protein interaction networks for the study of human diseases. Mol. Syst. Biol. 2021, 17, e8792. [Google Scholar] [CrossRef]
- Guo, Y.; You, J.; Zhang, Y.; Liu, W.S.; Huang, Y.Y.; Zhang, Y.R.; Zhang, W.; Dong, Q.; Feng, J.F.; Cheng, W.; et al. Plasma proteomic profiles predict future dementia in healthy adults. Nat. Aging 2024, 4, 247–260. [Google Scholar] [CrossRef]
- La Manno, G.; Soldatov, R.; Zeisel, A.; Braun, E.; Hochgerner, H.; Petukhov, V.; Lidschreiber, K.; Kastriti, M.E.; Lonnerberg, P.; Furlan, A.; et al. Rna velocity of single cells. Nature 2018, 560, 494–498. [Google Scholar] [CrossRef]
- Supek, F.; Bosnjak, M.; Skunca, N.; Smuc, T. Revigo summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, J.; Jiang, C.; Qin, J.; Ke, K.; Ding, F. Involvement of erk1/2 pathway in neuroprotective effects of pyrroloquinoline quinine against rotenone-induced sh-sy5y cell injury. Neuroscience 2014, 270, 183–191. [Google Scholar] [CrossRef]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the akt and erk1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef]
- Bhat, R.V.; Shanley, J.; Correll, M.P.; Fieles, W.E.; Keith, R.A.; Scott, C.W.; Lee, C.M. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc. Natl. Acad. Sci. USA 2000, 97, 11074–11079. [Google Scholar] [CrossRef] [PubMed]
- Seimi, S.K.; Seinosuke, K.; Tsuyoshi, S.; Tomomi, U.; Tetsuaki, H.; Miki, K.; Ryuji, T.; Kenji, I.; Mitsuhiro, Y. Glycogen synthase kinase-3beta is involved in the process of myocardial hypertrophy stimulated by insulin-like growth factor-1. Circ. J. 2004, 68, 247–253. [Google Scholar]
- Giuffrida, M.L.; Tomasello, F.; Caraci, F.; Chiechio, S.; Nicoletti, F.; Copani, A. Beta-amyloid monomer and insulin/igf-1 signaling in alzheimer’s disease. Mol. Neurobiol. 2012, 46, 605–613. [Google Scholar] [CrossRef]
- Nisa, F.Y.; Rahman, M.A.; Hossen, M.A.; Khan, M.F.; Khan, M.A.N.; Majid, M.; Sultana, F.; Haque, M.A. Role of neurotoxicants in the pathogenesis of alzheimer’s disease: A mechanistic insight. Ann. Med. 2021, 53, 1476–1501. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, L.; Laird, F.; Wong, P.C.; Lee, H.K. Bace1 knock-outs display deficits in activity-dependent potentiation of synaptic transmission at mossy fiber to ca3 synapses in the hippocampus. J. Neurosci. 2008, 28, 8677–8681. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, D.; Privitera, L.; Leznik, E.; Fà, M.; Staniszewski, A.; Palmeri, A.; Arancio, O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 14537–14545. [Google Scholar] [CrossRef]
- Ittner, L.M.; Ke, Y.D.; Delerue, F.; Bi, M.; Gladbach, A.; Van Eersel, J.; Wölfing, H.; Chieng, B.C.; Christie, M.J.; Napier, I.A.; et al. Dendritic function of tau mediates amyloid-beta toxicity in alzheimer’s disease mouse models. Cell 2010, 142, 387–397. [Google Scholar] [CrossRef]
- Yin, X.; Zhao, C.; Qiu, Y.; Zhou, Z.; Bao, J.; Qian, W. Dendritic/post-synaptic tau and early pathology of alzheimer’s disease. Front. Mol. Neurosci. 2021, 14, 671779. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.B.C.; Emptage, N.J.; Jeans, A.F. Long-term depression links amyloid-β to the pathological hyperphosphorylation of tau. Cell Rep. 2021, 36, 109638. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.H.; Capetillo-Zarate, E.; Lin, M.T.; Milner, T.A.; Gouras, G.K. Accumulation of intraneuronal β-amyloid 42 peptides is associated with early changes in microtubule-associated protein 2 in neurites and synapses. PLoS ONE 2013, 8, e51965. [Google Scholar] [CrossRef] [PubMed]
- Korzhevskiĭ, D.E.; Karpenko, M.N.; Kirik, O.V. Microtubule-associated proteins as markers of nerve cell differentiation and functional status. Morfologiia 2011, 139, 13–21. [Google Scholar] [PubMed]
- Ognibene, M.; Pezzolo, A. Ezrin interacts with the tumor suppressor chl1 and promotes neuronal differentiation of human neuroblastoma. PLoS ONE 2020, 15, e0244069. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Nhan, H.S.; Tyan, S.H.; Kawakatsu, Y.; Zhang, C.; Navarro, M.; Koo, E.H. Caspase activation and caspase-mediated cleavage of app is associated with amyloid β-protein-induced synapse loss in alzheimer’s disease. Cell Rep. 2020, 31, 107839. [Google Scholar] [CrossRef] [PubMed]
- Dorandish, S.; Williams, A.; Atali, S.; Sendo, S.; Price, D.; Thompson, C.; Guthrie, J.; Heyl, D.; Evans, H.G. Regulation of amyloid-β levels by matrix metalloproteinase-2/9 (mmp2/9) in the media of lung cancer cells. Sci. Rep. 2021, 11, 9708. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.L.; Caraci, F.; Pignataro, B.; Cataldo, S.; De Bona, P.; Bruno, V.; Molinaro, G.; Pappalardo, G.; Messina, A.; Palmigiano, A.; et al. Beta-amyloid monomers are neuroprotective. J. Neurosci. 2009, 29, 10582–10587. [Google Scholar] [CrossRef] [PubMed]
- Mandelkow, E.M.; Mandelkow, E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2012, 2, a006247. [Google Scholar] [CrossRef]
- Santa-Maria, I.; Varghese, M.; Ksiezak-Reding, H.; Dzhun, A.; Wang, J.; Pasinetti, G.M. Paired helical filaments from alzheimer disease brain induce intracellular accumulation of tau protein in aggresomes. J. Biol. Chem. 2012, 287, 20522–20533. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The string database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, X.; Cao, Z.; Ma, Z.; Liu, Q.; Huang, W.; Sun, T.; Li, J.; Yang, C. Proteomic Analysis Reveals Physiological Activities of Aβ Peptide for Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 8336. https://doi.org/10.3390/ijms25158336
Ai X, Cao Z, Ma Z, Liu Q, Huang W, Sun T, Li J, Yang C. Proteomic Analysis Reveals Physiological Activities of Aβ Peptide for Alzheimer’s Disease. International Journal of Molecular Sciences. 2024; 25(15):8336. https://doi.org/10.3390/ijms25158336
Chicago/Turabian StyleAi, Xiaorui, Zeyu Cao, Zhaoru Ma, Qinghuan Liu, Wei Huang, Taolei Sun, Jing Li, and Chenxi Yang. 2024. "Proteomic Analysis Reveals Physiological Activities of Aβ Peptide for Alzheimer’s Disease" International Journal of Molecular Sciences 25, no. 15: 8336. https://doi.org/10.3390/ijms25158336





