Transcriptomic Identification of Potential C2H2 Zinc Finger Protein Transcription Factors in Pinus massoniana in Response to Biotic and Abiotic Stresses
Abstract
1. Introduction
2. Results
2.1. Transcriptome-Wide Identification and Analysis of C2H2 ZFPs in P. massoniana
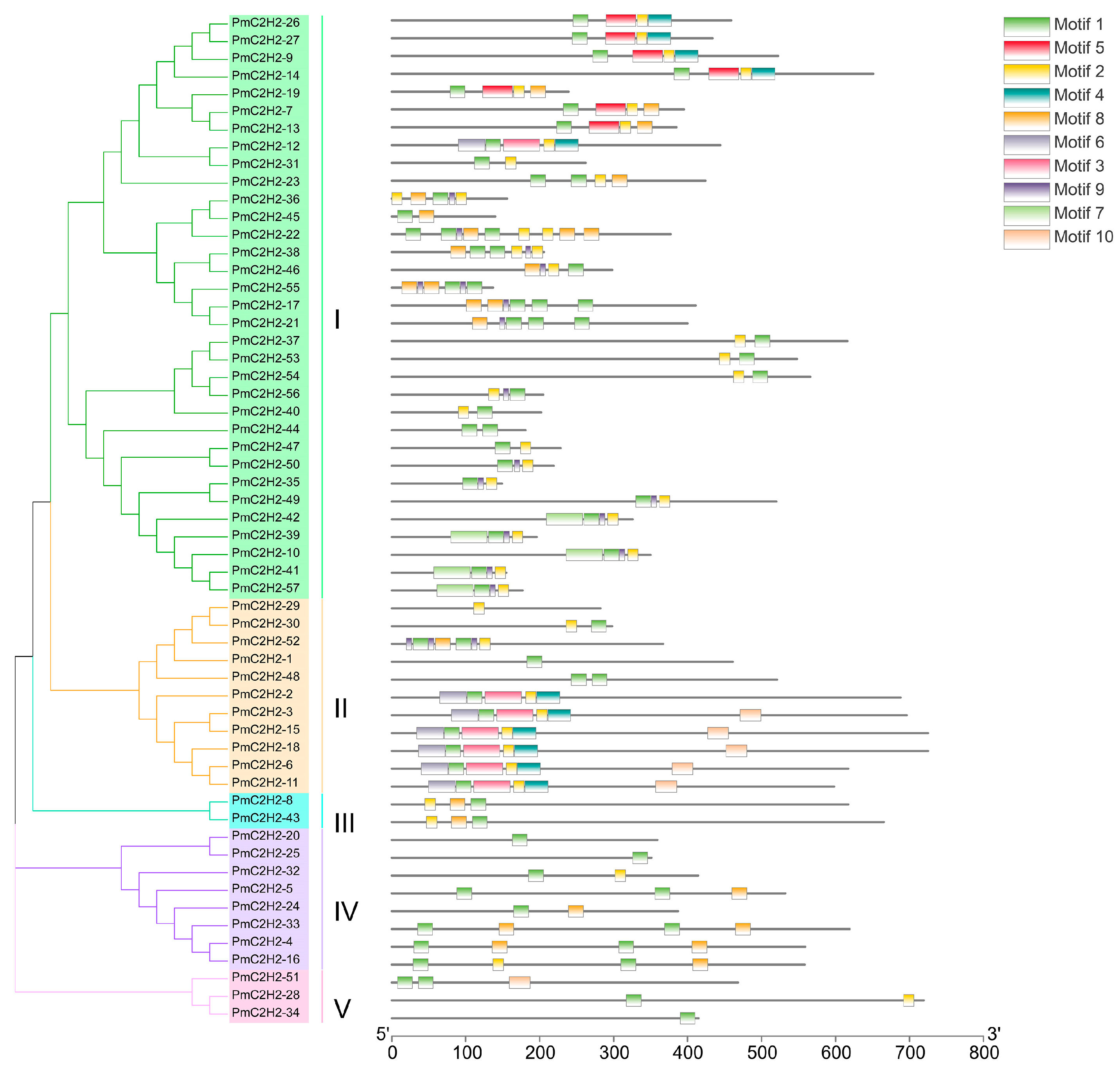
2.2. Phylogenetic Analysis and Domain Analysis of PmC2H2s
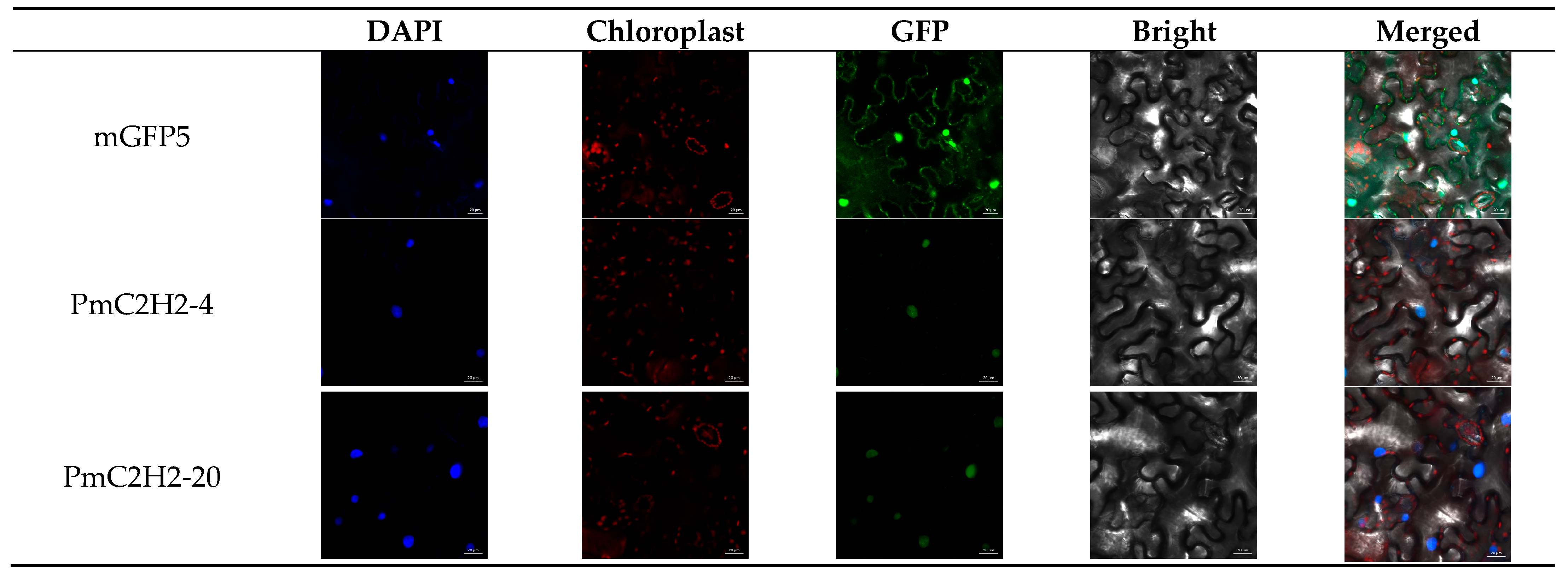
2.3. Subcellular Localization Analysis
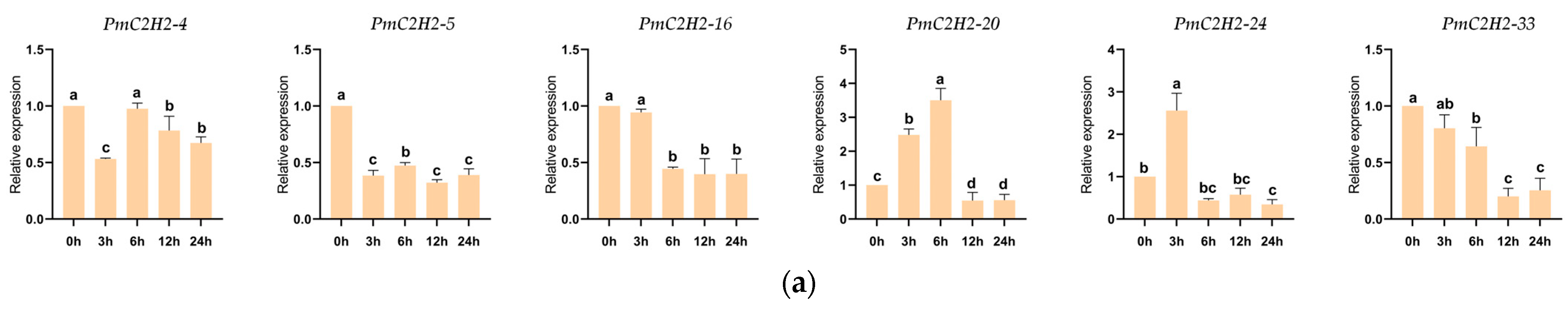
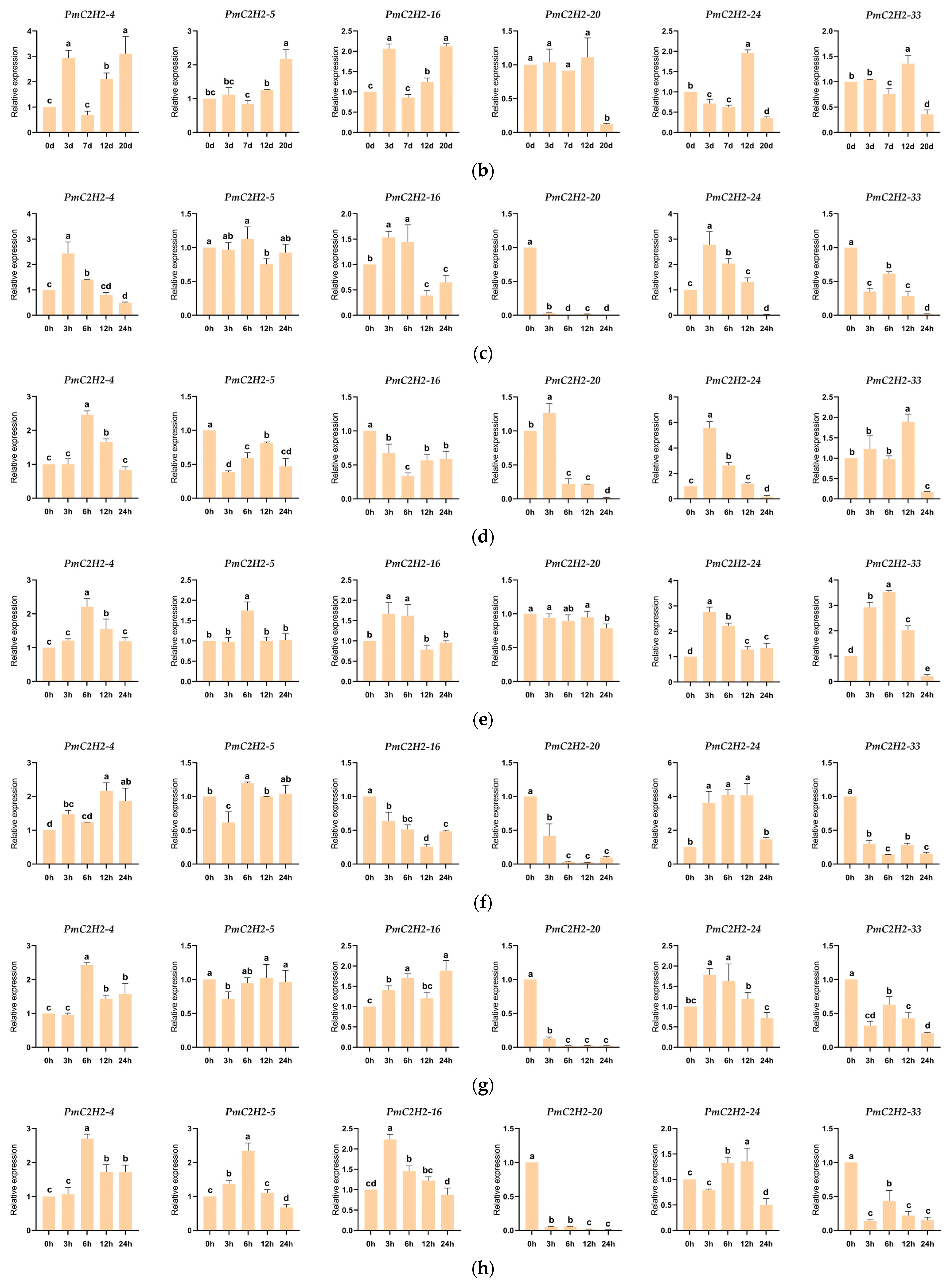
2.4. Expression Patterns of PmC2H2 ZFPs under Nematode and Drought Stress
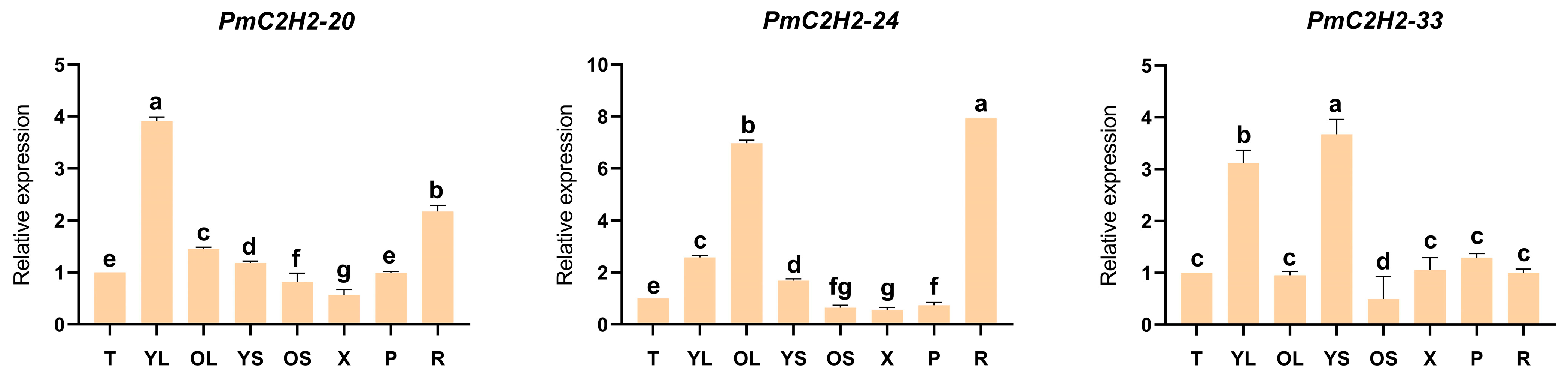
2.5. Expression Patterns of Q-Type C2H2 ZFPs Genes in Different Tissues
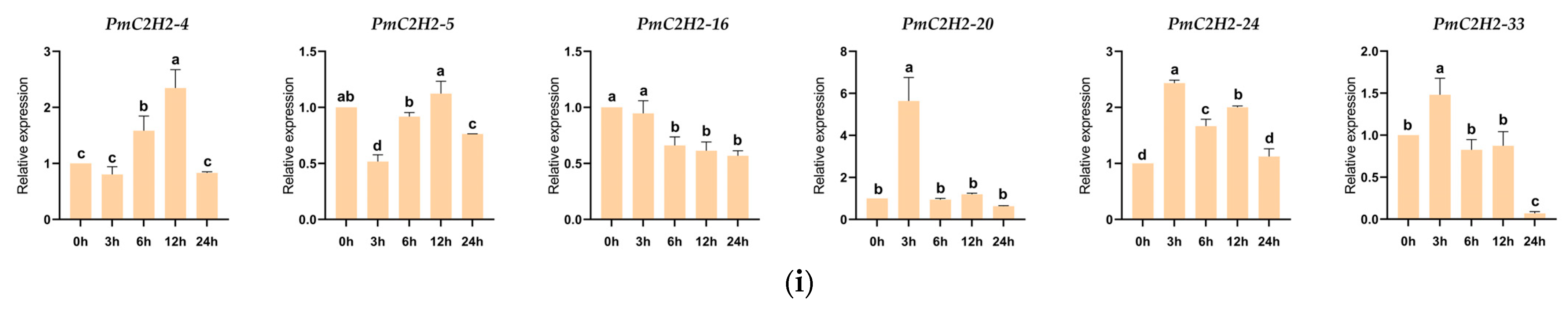
2.6. Expression Levels of Q-Type C2H2 ZFPs Genes under Abiotic Stresses
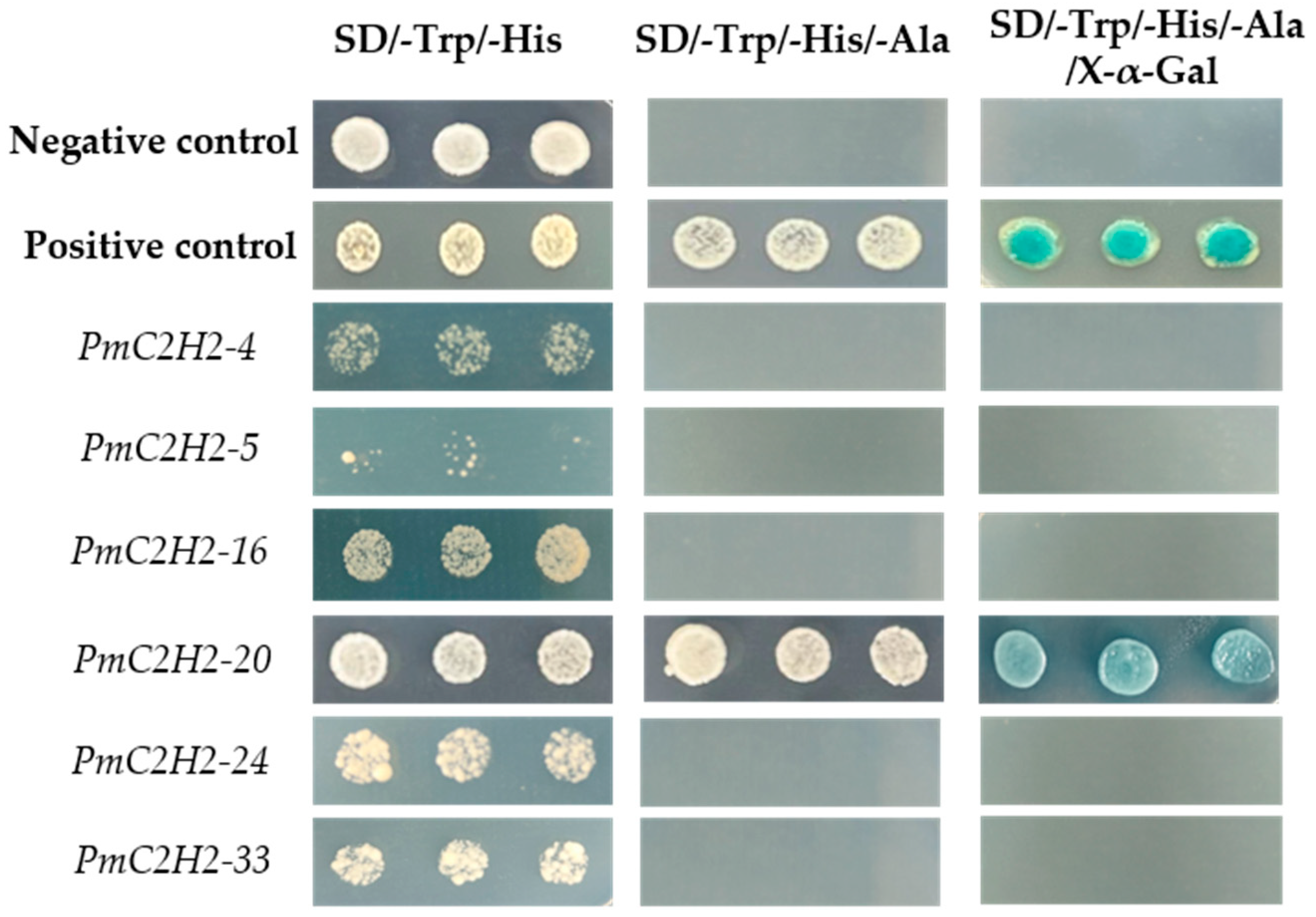
2.7. Transcriptional Activity Analysis
3. Discussion
4. Materials and Methods
4.1. Identification of C2H2 Genes in P. massoniana
4.2. Phylogenetic and Bioinformatics Analysis
4.3. Subcellular Localization Analysis
4.4. RNA-Seq Data Analysis
4.5. Plant Materials and Abiotic Stress Treatments
4.6. RNA Extraction and qRT-PCR Analysis
4.7. Transcriptional-Activation Activity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, W.; Liu, S.; Deng, X.; Shen, A.; Lei, X.; Tian, D.; Zhao, M.; Peng, C. General allometric equations and biomass allocation 469 of Pinus massoniana trees on a regional scale in southern China. Ecol. Res. 2011, 26, 697–711. [Google Scholar] [CrossRef]
- Du, M.; Ding, G.; Cai, Q. The Transcriptomic Responses of Pinus massoniana to Drought Stress. Forests 2018, 9, 326. [Google Scholar] [CrossRef]
- Erpen, L.; Devi, H.S.; Grosser, J.W.; Dutt, M. Potential use of the DREB/ERF, MYB, NAC and WRKY transcription factors to improve abiotic and biotic stress in transgenic plants. Plant Cell Tissue Organ Cult. PCTOC 2017, 132, 1–25. [Google Scholar] [CrossRef]
- Roy, S. Function of MYB domain transcription factors in abiotic stress and epigenetic control of stress response in plant genome. Plant Signal Behav. 2016, 11, e1117723. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple Functions of MYB Transcription Factors in Abiotic Stress Responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, P. The DREB transcription factor, a biomacromolecule, responds to abiotic stress by regulating the expression of stress-related genes. Int. J. Mol. Sci. 2023, 243, 125231. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, L.; Jiang, W. Understanding AP2/ERF transcription factor responses and tolerance to various abiotic stresses in plants: A comprehensive review. Int. J. Mol. Sci. 2024, 25, 893. [Google Scholar] [CrossRef]
- Wang, Z.; Dane, F. NAC (NAM/ATAF/CUC) transcription factors in different stresses and their signaling pathway. Acta Physiol. Plant. 2013, 35, 1397–1408. [Google Scholar] [CrossRef]
- Li, W.; Pang, S.; Lu, Z.; Jin, B. Function and mechanism of WRKY transcription factors in abiotic stress responses of plants. Plants 2020, 9, 1515. [Google Scholar] [CrossRef]
- Radani, Y.; Li, R.; Korboe, H.M.; Ma, H.; Yang, L. Transcriptional and post-translational regulation of plant bHLH transcription factors during the response to environmental stresses. Plants 2023, 12, 2113. [Google Scholar] [CrossRef]
- Liu, H.; Tang, X.; Zhang, N.; Li, S.; Si, H. Role of bZIP transcription factors in plant salt stress. Int. J. Mol. Sci. 2023, 24, 7893. [Google Scholar] [CrossRef]
- Liu, Y.; Khan, A.R.; Gan, Y. C2H2 zinc finger proteins response to abiotic stress in plants. Int. J. Mol. Sci. 2022, 23, 2730. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Ding, Y.; Hu, S.; Ding, L.; Chen, Z.; Zhu, C. The role of HD-Zip class I transcription factors in plant response to abiotic stresses. Physiol. Plant. 2019, 167, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Wang, Q.; Li, H.; Ding, G.; Wen, X. Transcriptome-Wide identification and expression profiles of masson pine wrky transcription factors in response to low phosphorus stress. Plant Mol. Biol. Rep. 2021, 39, 1–9. [Google Scholar] [CrossRef]
- Von Koskull-Döring, P.; Scharf, K.D.; Nover, L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007, 12, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, T.; Chen, H.; Tang, H.; Mu, Y.; Gou, L.; Haibib, A.; Lan, X.; Ma, J. The wheat (Triticum aestiveum L.) MADS-box transcription factor TaMADS32 plays a role in response to abiotic stresses. Biotechnol. Biotechnol. Equip. 2022, 36, 451–461. [Google Scholar] [CrossRef]
- Wang, D.; Yao, S.; Agassin, R.H.; Zhang, M.; Lou, X.; Huang, Z.; Zhang, J.; Ji, K. Transcriptome-Wide identification of CCCH-type zinc finger proteins family in Pinus massoniana and RR-TZF proteins in stress response. Genes 2022, 13, 1639. [Google Scholar] [CrossRef]
- Han, G.; Qiao, Z.; Li, Y.; Wang, C.; Wang, B. The roles of CCCH zinc-finger proteins in plant abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 8327. [Google Scholar] [CrossRef]
- Miller, J.; McLachlan, A.D.; Klug, A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985, 4, 1609–1614. [Google Scholar] [CrossRef]
- Han, G.; Lu, C.; Guo, J.; Qiao, Z.; Sui, N.; Qiu, N.; Wang, B. C2H2 zinc finger proteins: Master regulators of abiotic stress responses in plants. Front. Plant Sci. 2020, 11, 115. [Google Scholar] [CrossRef]
- Sakamoto, H.; Maruyama, K.; Sakuma, Y.; Meshi, T.; Iwabuchi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 2004, 136, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Kielbowicz-Matuk, A. Involvement of plant C2H-type zinc finger transcription factors in stress responses. Plant Sci. 2012, 185, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Faraji, S.; Rasouli, S.H.; Kazemitabar, S.K. Genome-wide exploration of C2H2 zinc finger family in durum wheat (Triticum turgidum ssp Durum): Insights into the roles in biological processes especially stress response. Biometals 2018, 31, 1019–1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sadeghnezhad, E.; Nick, P. Upstream of gene expression: What is the role of microtubules in cold signalling? J. Exp. Bot. 2020, 71, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, S.A.; Nekludova, L.; Pabo, C.O. DNA recognition by Cys2His2 zinc finger proteins. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Ciftci-Yilmaz, S.; Mittler, R. The zinc finger network of plants. Cell. Mol. Life Sci. 2008, 65, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Englbrecht, C.C.; Schoof, H.; Bohm, S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genom. 2004, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Arora, R.; Ray, S.; Singh, A.K.; Singh, V.P.; Takatsuji, H.; Kapoor, S.; Tyagi, A.K. Genome-wide identification of C2H2 zinc-finger gene family in rice and their phylogeny and expression analysis. Plant Mol. Biol. 2007, 65, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Z.; Xu, X.; Zhang, H.; Li, C. Genome-Wide analysis of C2H2 zinc-finger family transcription factors and their responses to abiotic stresses in poplar (Populus trichocarpa). PLoS ONE 2015, 10, e0134753. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Li, R.; Wang, L.; Zhang, C.; Chen, L.; Hao, Q.; Zhang, X.; Chen, H.; Shan, Z.; et al. Genome-Wide identification and classification of soybean C2H2 zinc finger proteins and their expression analysis in legume-rhizobium symbiosis. Front. Microbiol. 2018, 9, 126. [Google Scholar] [CrossRef]
- Liu, K.; Hou, Q.; Yu, R.; Deng, H.; Shen, L.; Wang, Q.; Wen, X. Genome-wide analysis of C2H2 zinc finger family and their response to abiotic stresses in apple. Gene 2024, 904, 148164. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, A.; Houde, M. Genome wide identification of C1-2i zinc finger proteins and their response to abiotic stress in hexaploidy wheat. Mol. Genet. Genom. 2016, 291, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Takatsuji, H.; Mori, M.; Benfey, P.N.; Ren, L.; Chua, N.H. Characterization of a zinc finger DNA-binding protein expressed specifically in Petunia petals and seedlings. EMBO J. 1992, 11, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Minami, M.; Huh, G.H.; Iwabuchi, M. The putative zinc-finger protein WZF1 interacts with a cis-acting element of wheat histone genes. Eur. J. Biochem. 1993, 217, 1049–1056. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.F.; Wang, Q.H.; Zhang, H.S. Identification of a rice zinc finger protein whose expression is transiently induced by drought, cold but not by salinity and abscisic acid. DNA Seq. 2005, 16, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chu, Z.; Luo, J.; Zhou, Y.; Cai, Y.; Lu, Y.; Xia, J.; Kuang, H.; Ye, Z.; Ouyang, B. The C2H2 zinc-finger protein SlZF3 regulates AsA synthesis and salt tolerance by interacting with CSN5B. Plant Biotechnol. J. 2018, 16, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Lee, S.H.; Cheong, Y.H.; Yoo, C.M.; Lee, S.I.; Chun, H.J.; Yun, D.J.; Hong, J.C.; Lee, S.Y.; Lim, C.O.; et al. A novel cold-inducible zinc finger protein from soybean, SCOF-1, enhances cold tolerance in transgenic plants. Plant J. 2001, 25, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, D.; Hu, R.; Hua, C.; Ali, I.; Zhang, A.; Liu, B.; Wu, M.; Huang, L.; Gan, Y. AtGIS, a C2H2 zinc-finger transcription factor from Arabidopsis regulates glandular trichome development through GA signaling in tobacco. Biochem. Biophys. Res. Commun. 2017, 483, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Muthamilarasan, M.; Bonthala, V.S.; Mishra, A.K.; Khandelwal, R.; Khan, Y.; Roy, R.; Prasad, M. C2H2 type of zinc finger transcription factors in foxtail millet define response to abiotic stresses. Funct. Integr. Genom. 2014, 14, 531–543. [Google Scholar] [CrossRef]
- Lyu, T.; Liu, W.; Hu, Z.; Xiang, X.; Liu, T.; Xiong, X.; Cao, J. Molecular characterization and expression analysis reveal the roles of Cys2/His2 zinc-finger transcription factors during flower development of Brassica rapa subsp. chinensis. Plant Mol. Biol. 2020, 102, 123–141. [Google Scholar] [CrossRef]
- Bohm, S.; Frishman, D.; Mewes, H.W. Variations of the C2H2 zinc finger motif in the yeast genome and classification of yeast zinc finger proteins. Nucleic Acids Res. 1997, 25, 2464–2469. [Google Scholar] [CrossRef]
- Kubo, K.I.; Sakamoto, A.; Kobayashi, A.; Rybka, Z.; Kanno, Y.; Nakagawa, H.; Takatsuji, H. Cys2/His2 zinc-finger protein family of petunia: Evolution and general mechanism of target-sequence recognition. Nucleic Acids Res. 1998, 26, 608–615. [Google Scholar] [CrossRef]
- Yoshioka, K.; Fukushima, S.; Yamazaki, T.; Yoshida, M.; Takatsuji, H. The plant zinc finger protein ZPT2-2 has a unique mode of DNA interaction. J. Biol. Chem. 2001, 276, 35802–35807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takatsuji, H.; Matsumoto, T. Target-sequence recognition by separate-type Cys2/His2 zinc finger proteins in plants. J. Biol.Chem. 1996, 271, 23368–23373. [Google Scholar] [CrossRef]
- Ding, Q.; Zhao, H.; Zhu, P.; Jiang, X.; Nie, F.; Li, G. Genome-wide identification and expression analyses of C2H2 zinc finger transcription factors in Pleurotus ostreatus. PeerJ 2022, 10, e12654. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chan, Z. The cysteine2/histidine2-type transcription factor zinc finger of Arabidopsis thaliana 6-activated c-repeat-binding factor pathway is essential for melatonin-mediated freezing stress resistance in Arabidopsis. J. Pineal Res. 2014, 57, 185–191. [Google Scholar] [CrossRef]
- Dong, T.; Park, Y.; Hwang, I. Abscisic acid: Biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 2015, 58, 29–48. [Google Scholar]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. R. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Lippuner, V.; Cyert, M.S.; Gasser, C.S. Two classes of plant cDNA clones differentially complement yeast calcineurin mutants and increase salt tolerance of wild-type yeast. J. Biol. Chem. 1996, 271, 12859–12866. [Google Scholar] [CrossRef]
- Sakamoto, H.; Araki, T.; Meshi, T.; Iwabuchi, M. Expression of a subset of the Arabidopsis Cys2/His2-type zinc-finger protein gene family under water stress. Gene 2000, 248, 23–32. [Google Scholar] [CrossRef]
- Shao, L.; Li, L.; Huang, X.; Fu, Y.; Yang, D.; Li, C.; Yang, J. Identification of C2H2 zinc finger genes through genome-wide association study and functional analyses of LkZFPs in response to stresses in Larix kaempferi. BMC Plant Biol. 2023, 23, 298. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, L.; Zhang, Y.; Xu, L.; Li, N.; Zhang, X.; Pan, Y. Genome-wide identification of C2H2 zinc-finger genes and their expression patterns under heat stress in tomato (Solanum lycopersicum L.). PeerJ 2019, 7, e7929. [Google Scholar] [CrossRef]
- Yin, M.; Wang, Y.; Zhang, L.; Li, J.; Quan, W.; Yang, L.; Wang, Q.; Chan, Z. The Arabidopsis Cys2/His2 zinc fnger transcription factor ZAT18 is a positive regulator of plant tolerance to drought stress. J. Exp. Bot. 2017, 68, 2991–3005. [Google Scholar] [CrossRef]
- Ciftci-Yilmaz, S.; Morsy, M.R.; Song, L.; Coutu, A.; Krizek, B.A.; Lewis, M.W.; Warren, D.; Cushman, J.; Connolly, E.L.; Mittler, R. The EAR-motif of the Cys2/His2-type zinc finger protein Zat7 plays a key role in the defense response of Arabidopsis to salinity stress. J. Biol. Chem. 2007, 282, 9260–9268. [Google Scholar] [CrossRef]
- Wu, F.; Sun, X.; Zou, B.; Zhu, P.; Ji, K. Transcriptional analysis of Masson pine (Pinus massoniana) under high CO2 stress. Genes 2019, 10, 804. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Chen, P.; Zhang, C.; Yao, S.; Hao, Q.; Agassin, R.H.; Ji, K. The transcriptomic analysis of the response of Pinus massoniana to drought stress and a functional study on the ERF1 transcription factor. Int. J. Mol. Sci. 2023, 24, 11103. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.H.; Ma, Y.Y.; Zhu, L.Z.; Chen, Y.; Ji, K.S. Selection of suitable reference genes in Pinus massoniana Lamb. under different abiotic stresses for qPCR normalization. Forests 2019, 10, 632. [Google Scholar] [CrossRef]


| Gene ID | Aa | Mw (kDa) | pI | Subcellular Localization |
|---|---|---|---|---|
| PmC2H2-1 | 461 | 53.28 | 5.68 | Nucleus |
| PmC2H2-2 | 688 | 76.54 | 8.58 | Nucleus |
| PmC2H2-3 | 696 | 72.08 | 9.15 | Nucleus |
| PmC2H2-4 | 559 | 61.93 | 5.47 | Nucleus |
| PmC2H2-5 | 532 | 58.45513 | 6.23 | Nucleus |
| PmC2H2-6 | 617 | 66.9 | 9.1 | Nucleus |
| PmC2H2-7 | 395 | 44.67 | 6.59 | Nucleus |
| PmC2H2-8 | 617 | 66.87 | 5.59 | Nucleus |
| PmC2H2-9 | 522 | 57.31 | 5.49 | Nucleus |
| PmC2H2-10 | 350 | 37.35 | 8.77 | Nucleus |
| PmC2H2-11 | 598 | 65.21 | 8.94 | Nucleus |
| PmC2H2-12 | 444 | 48.93 | 9.22 | Nucleus |
| PmC2H2-13 | 385 | 43.03 | 5.93 | Nucleus |
| PmC2H2-14 | 651 | 68.65 | 6.53 | Nucleus |
| PmC2H2-15 | 725 | 76.17 | 9.19 | Nucleus |
| PmC2H2-16 | 558 | 61.93 | 5.81 | Nucleus |
| PmC2H2-17 | 411 | 46.68 | 5.43 | Nucleus |
| PmC2H2-18 | 725 | 76.67 | 9.31 | Nucleus |
| PmC2H2-19 | 239 | 27.41 | 8.01 | Nucleus |
| PmC2H2-20 | 359 | 39.9 | 5.85 | Nucleus |
| PmC2H2-21 | 400 | 45.44 | 5.47 | Nucleus |
| PmC2H2-22 | 377 | 43.58 | 9.46 | Nucleus |
| PmC2H2-23 | 424 | 46.51 | 6.65 | Nucleus |
| PmC2H2-24 | 387 | 43.52 | 5.89 | Nucleus |
| PmC2H2-25 | 351 | 37.69 | 4.82 | Nucleus |
| PmC2H2-26 | 459 | 50.47 | 7.01 | Nucleus |
| PmC2H2-27 | 434 | 47.89 | 8.84 | Nucleus |
| PmC2H2-28 | 719 | 81.74 | 5.28 | Nucleus |
| PmC2H2-29 | 282 | 30.88 | 8.93 | Chloroplast |
| PmC2H2-30 | 298 | 32.77 | 8.06 | Nucleus |
| PmC2H2-31 | 262 | 30.15 | 7.21 | Nucleus |
| PmC2H2-32 | 414 | 45.99 | 8.84 | Nucleus |
| PmC2H2-33 | 619 | 69.2 | 5.88 | Nucleus |
| PmC2H2-34 | 415 | 46.88 | 6.68 | Nucleus |
| PmC2H2-35 | 149 | 16.2 | 9.42 | Nucleus |
| PmC2H2-36 | 156 | 17.88 | 9.2 | Nucleus |
| PmC2H2-37 | 616 | 67.23 | 5.63 | Nucleus |
| PmC2H2-38 | 206 | 22.94 | 8.53 | Nucleus |
| PmC2H2-39 | 196 | 20.93 | 9.1 | Nucleus |
| PmC2H2-40 | 202 | 22.12 | 9.51 | Nucleus |
| PmC2H2-41 | 155 | 16.99 | 9.33 | Nucleus |
| PmC2H2-42 | 326 | 34.651 | 7.74 | Nucleus |
| PmC2H2-43 | 665 | 69.54 | 5.63 | Nucleus |
| PmC2H2-44 | 181 | 19.99 | 9.71 | Nucleus |
| PmC2H2-45 | 140 | 16.17 | 9.87 | Nucleus |
| PmC2H2-46 | 298 | 32.7 | 8.41 | Nucleus |
| PmC2H2-47 | 228 | 25.1 | 7.1 | Nucleus |
| PmC2H2-48 | 521 | 59.88 | 6.36 | Nucleus |
| PmC2H2-49 | 520 | 57.43 | 8.18 | Nucleus |
| PmC2H2-50 | 219 | 24.31 | 8.79 | Nucleus |
| PmC2H2-51 | 468 | 53.08 | 8.61 | Nucleus |
| PmC2H2-52 | 367 | 40.87 | 8.21 | Nucleus |
| PmC2H2-53 | 548 | 60.01 | 4.96 | Nucleus |
| PmC2H2-54 | 566 | 61.77 | 5.5 | Nucleus |
| PmC2H2-55 | 137 | 16.09 | 9.5 | Nucleus |
| PmC2H2-56 | 205 | 23.6 | 6.38 | Nucleus |
| PmC2H2-57 | 177 | 19.21 | 9.1 | Nucleus |
| Motif | Length | Motif Consensus | Motif Logo |
|---|---|---|---|
| 1 | 21 | CEICGKGFSRPQNLQQHMRTH |  |
| 2 | 15 | GCGKRFSVVSDLKRH |  |
| 3 | 50 | WKLRQRTTKEIRKRVYICPEPTCVHHDPSRALGDLTGIKKHFCRKHGEKK |  |
| 4 | 32 | SKTCGTREYRCDCGTLFSRRDSFITHRAFCDA |  |
| 5 | 41 | YSCPFEGCRRNKBHPKFKPLKSIRSLRNHYKRSHCPKMYTC |  |
| 6 | 37 | QQNTTIKRKRNLPGTPDPDAEVIALSPKTLMATNRFV |  |
| 7 | 50 | YSSASESVDLPNSRTLPRPSALVGGSMPPAPQSMMGQFSSKVSSSSQKKH |  |
| 8 | 21 | CSICGRSFTSKQALKGHIRVH |  |
| 9 | 8 | TGEKPFVC |  |
| 10 | 29 | QQQQHTSTPQMSATALLQKAAQMGATASN |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Qiu, Z.; Xu, T.; Yao, S.; Chen, M.; Li, Q.; Agassin, R.H.; Ji, K. Transcriptomic Identification of Potential C2H2 Zinc Finger Protein Transcription Factors in Pinus massoniana in Response to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2024, 25, 8361. https://doi.org/10.3390/ijms25158361
Wang D, Qiu Z, Xu T, Yao S, Chen M, Li Q, Agassin RH, Ji K. Transcriptomic Identification of Potential C2H2 Zinc Finger Protein Transcription Factors in Pinus massoniana in Response to Biotic and Abiotic Stresses. International Journal of Molecular Sciences. 2024; 25(15):8361. https://doi.org/10.3390/ijms25158361
Chicago/Turabian StyleWang, Dengbao, Zimo Qiu, Tao Xu, Sheng Yao, Meijing Chen, Qianzi Li, Romaric Hippolyte Agassin, and Kongshu Ji. 2024. "Transcriptomic Identification of Potential C2H2 Zinc Finger Protein Transcription Factors in Pinus massoniana in Response to Biotic and Abiotic Stresses" International Journal of Molecular Sciences 25, no. 15: 8361. https://doi.org/10.3390/ijms25158361
APA StyleWang, D., Qiu, Z., Xu, T., Yao, S., Chen, M., Li, Q., Agassin, R. H., & Ji, K. (2024). Transcriptomic Identification of Potential C2H2 Zinc Finger Protein Transcription Factors in Pinus massoniana in Response to Biotic and Abiotic Stresses. International Journal of Molecular Sciences, 25(15), 8361. https://doi.org/10.3390/ijms25158361







