Which Constituents Determine the Antioxidant Activity and Cytotoxicity of Garlic? Role of Organosulfur Compounds and Phenolics
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents, Disposables and Equipment
4.2. Estimation of the Antioxidant Activity
4.2.1. ABTS● Reduction Assay
4.2.2. DPPH● Reduction Assay
4.2.3. The Ferric Reducing Antioxidant Power (FRAP) Assay
4.2.4. Calculation of the Antioxidant Activity
4.3. Cell Culture
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Espinoza, T.; Valencia, E.; Albarrán, M.; Díaz, D.; Quevedo, R.A.; Díaz, O.; Bastías, J. Garlic (Allium sativum L.) and its beneficial properties for health: A review. Agroind. Sci. 2020, 10, 103–115. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; García-Recio, E.; Ruiz, C.; De Luna-Bertos, E.; Illescas-Montes, R.; Costela-Ruiz, V.J. Biological properties and therapeutic applications of garlic and its components. Food Funct. 2022, 13, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Ekşi, G.; Özkan, A.M.G.; Koyuncu, M. Garlic and onions: An eastern tale. J. Ethnopharmacol. 2020, 253, 112675. [Google Scholar] [CrossRef] [PubMed]
- Aydın, S.; Tahmas Kahyaoğlu, D. Antioxidant effect potential of garlic in vitro and real food system: Effects of garlic supplementation on oxidation stability and sensory properties of butter. Eur. J. Lipid Sci. Technol. 2020, 122, 1900261. [Google Scholar] [CrossRef]
- Farhat, Z.; Hershberger, P.A.; Freudenheim, J.L.; Mammen, M.J.; Hageman Blair, R.; Aga, D.S.; Mu, L. Types of garlic and their anticancer and antioxidant activity: A review of the epidemiologic and experimental evidence. Eur. J. Nutr. 2021, 60, 3585–3609. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Soare, R.; Băbeanu, C.; Botu, M. Evaluation of productivity components and antioxidant activity of different types of garlic. Horticulturae 2023, 9, 1039. [Google Scholar] [CrossRef]
- Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Anti-Cancer and anti-inflammatory properties of black garlic. Int. J. Mol. Sci. 2024, 25, 1801. [Google Scholar] [CrossRef] [PubMed]
- Saikat, A.S.M.; Hossain, R.; Mina, F.B.; Das, S.; Khan, I.N.; Mubarak, M.S.; Islam, M.T. Antidiabetic effect of garlic. Rev. Farm. 2021, 32, 1–11. [Google Scholar] [CrossRef]
- Li, M.; Yun, W.; Wang, G.; Li, A.; Gao, J.; He, Q. Roles and mechanisms of garlic and its extracts on atherosclerosis: A review. Front. Pharmacol. 2022, 13, 954938. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- De Greef, D.; Barton, E.M.; Sandberg, E.N.; Croley, C.R.; Pumarol, J.; Wong, T.L.; Das, N.; Bishayee, A. Anticancer potential of garlic and its bioactive constituents: A systematic and comprehensive review. Sem. Cancer Biol. 2021, 73, 219–264. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Canistro, D.; Pellicioni, V.; Vivarelli, F.; Fimognari, C. Anticancer potential of allicin: A review. Pharmacol. Res. 2022, 177, 106118. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Banerjee, S.; Bose, S.; Mazumder, S.; Haber, R.A.; Farzaei, M.H.; Bishayee, A. Garlic constituents for cancer prevention and therapy: From phytochemistry to novel formulations. Pharmacol. Res. 2022, 175, 105837. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, M.; Shah, M.; Ranginwala, A.; Agrawal, P.; Acharya, D.; Thakkar, S. Antifungal effects of tulsi, garlic, cinnamon and lemongrass in powder and oil form on Candida albicans: An in vitro study. J. Oral Maxillofac. Pathol. 2021, 25, 306–312. [Google Scholar] [CrossRef] [PubMed]
- El-Bayoumy, K.; Sinha, R.; Pinto, J.T.; Rivlin, R.S. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J. Nutr. 2006, 136, 864S–869S. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.K.; Mukherjee, P.K.; Maulik, S.K. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res. 2003, 17, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Fei, M.L.; Tong, L.I.; Wei, L.I.; De Yang, L. Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage. Ind. Crops Prod. 2015, 69, 137–142. [Google Scholar] [CrossRef]
- Li, C.; Jing, H.; Ma, G.; Liang, P. Allicin induces apoptosis through activation of both intrinsic and extrinsic pathways in glioma cells. Mol. Med. Rep. 2018, 17, 5976–5981. [Google Scholar] [CrossRef]
- Furdak, P.; Pieńkowska, N.; Bartosz, G.; Sadowska-Bartosz, I. Extracts of common vegetables inhibit the growth of ovary cancer cells. Foods 2022, 11, 2518. [Google Scholar] [CrossRef]
- Fleischauer, A.T.; Arab, L. Garlic and cancer: A critical review of the epidemiologic literature. J. Nutr. 2001, 131, 1032s–1040s. [Google Scholar] [CrossRef]
- Mathew, B.; Biju, R. Neuroprotective effects of garlic a review. Libyan J. Med. 2008, 3, 23–33. [Google Scholar] [PubMed]
- El-Saber Batiha, G.; Beshbishy, A.M.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Devkota, H.P. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Ruan, J.; Zhuang, X.; Zhang, X.; Li, Z. Phytochemicals of garlic: Promising candidates for cancer therapy. Biomed. Pharmacother. 2020, 123, 109730. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Foerster Née Reiter, J.; Kappler, U.; Antelmann, H.; Noll, U.; Gruhlke, M.C.H.; Slusarenko, A.J. Allicin, the odor of freshly crushed garlic: A review of recent progress in understanding allicin’s effects on cells. Molecules 2021, 26, 1505. [Google Scholar] [CrossRef]
- Block, E. The organosulfur chemistry of the genus Allium—implications for the organic chemistry of sulfur. Angew. Chemie Int. Edit. 1992, 31, 1135–1178. [Google Scholar] [CrossRef]
- Han, J.; Lawson, L.; Han, G.; Han, P. A spectrophotometric method for quantitative determination of allicin and total garlic thiosulfinates. Anal Biochem. 1995, 225, 157–160. [Google Scholar] [CrossRef]
- Trio, P.Z.; You, S.; He, X.; He, J.; Sakao, K.; Hou, D.-X. Chemopreventive functions and molecular mechanisms of garlic organosulfur compounds. Food Funct. 2014, 5, 833–844. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic role of functional components in Alliums for preventive chronic disease in human being. Evid. Based Complement. Altern. Med. 2017, 2017, 9402849. [Google Scholar] [CrossRef]
- Hirsch, K.; Danilenko, M.; Giat, J.; Miron, T.; Rabinkov, A.; Wilchek, M.; Mirelman, D.; Levy, J.; Sharoni, Y. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr. Cancer 2000, 38, 245–254. [Google Scholar] [CrossRef]
- Oommen, S.; Anto, R.J.; Srinivas, G.; Karunagaran, D. Allicin (from garlic) induces caspase-mediated apoptosis in cancer cells. Eur. J. Pharmacol. 2004, 485, 97–103. [Google Scholar] [CrossRef]
- Haghi, A.; Azimi, H.; Rahimi, R. A comprehensive review on pharmacotherapeutics of three phytochemicals, curcumin, quercetin, and allicin, in the treatment of gastric cancer. J. Gastrointest. Cancer 2017, 48, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Fang, D.; Hang, H.; Tang, Z. The mechanism in gastric cancer chemoprevention by allicin. Anti-Cancer Agents Med. Chem. 2016, 16, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Chu, Q.; Ling, M.T.; Feng, H.; Cheung, H.W.; Tsao, S.W.; Wang, X.; Wong, Y.C. A novel anticancer effect of garlic derivatives: Inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis 2006, 27, 2180–2189. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.S.; Feng, J.G.; Zhang, D.; Zhang, B.; Luo, M.; Su, D.; Lin, N.M. S-allylcysteine, a garlic derivative, suppresses proliferation and induces apoptosis in human ovarian cancer cells in vitro. Acta Pharmacol. Sin. 2014, 35, 267–274. [Google Scholar] [CrossRef]
- Soto, V.C.; Gonzalez, R.E.; Sance, M.M.; Galmarini, C.R. Organosulfur and phenolic content of garlic (Allium sativum L.) and onion (Allium cepa L.) and its relationship with antioxidant activity. In Proceedings of the VII International Symposium on Edible Alliaceae, Nigde, Turkey, 21–25 May 2015; pp. 277–290. [Google Scholar]
- Prasad, K.; Laxdal, V.A.; Yu, M.; Raney, B.L. Antioxidant activity of allicin, an active principle in garlic. Mol. Cell. Biochem. 1995, 148, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Tanaka, K.; Sato, E.; Okajima, H. Kinetic and mechanistic studies of allicin as an antioxidant. Org. Biomol. Chem. 2006, 4, 4113–4117. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.Y. The antioxidant properties of garlic compounds: Allyl cysteine, alliin, allicin, and allyl disulfide. J. Med. Food 2006, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yan, Y.-X.; Yu, Q.-T.; Deng, Y.; Wu, D.-T.; Wang, Y.; Zhao, J. Comparison of immunomodulatory effects of fresh garlic and black garlic polysaccharides on RAW 264.7 macrophages. J. Food Sci. 2017, 82, 765–771. [Google Scholar] [CrossRef]
- Sharma, N. Efficacy of garlic and onion against virus. Int. J. Res. Pharm. Sci. 2019, 10, 3578–3586. [Google Scholar] [CrossRef]
- Kovarovič, J.; Bystricka, J.; Vollmannová, A.; Tóth, T.; Brindza, J. Biologically valuable substances in garlic (Allium sativum L.)—A review. J. Centr. Eur. Agric. 2019, 20, 292–304. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Propert. 2017, 20 (Suppl. S2), 1700–1741. [Google Scholar] [CrossRef]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Translat. Cancer Res. 2020, 9, 7619. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.U.; Suhail, M.; Khan, M.K.; Zughaibi, T.A.; Alserihi, R.F.; Zaidi, S.K.; Tabrez, S. Polyphenols as anticancer agents: Toxicological concern to healthy cells. Phytother. Res. 2021, 35, 6063–6079. [Google Scholar] [CrossRef] [PubMed]
- Mahmutovic, O.; Tahirovic, I.; Copra, A.; Memic, M.; Ibragic, S.; Karic, L. Correlation of total secondary sulfur compounds, total phenols and antioxidant capacity in the Ramsons and Garlic. Br. J. Pharm. Res. 2014, 4, 2662–2669. [Google Scholar] [CrossRef]
- Furdak, P.; Pieńkowska, N.; Kapusta, I.; Bartosz, G.; Sadowska-Bartosz, I. Comparison of antioxidant and antiproliferative effects of various forms of garlic and ramsons. Molecules 2023, 28, 6512. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shen, X.; Cheng, S.; Li, P.; Du, J.; Chang, Y.; Meng, H. Evaluation of garlic cultivars for polyphenolic content and antioxidant properties. PLoS ONE 2013, 8, e79730. [Google Scholar] [CrossRef] [PubMed]
- Škrovánková, S.; Mlček, J.; Snopek, L.; Planetová, T. Polyphenols and antioxidant capacity in different types of garlic. Potrav. Slovak J. Food Sci. 2018, 12, 267–272. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; Cozzolino, A.; Riccardi, R.; Spigno, P.; Tremonte, P.; Coppola, R.; Nazzaro, F. Phenolic constituents, antioxidant, antimicrobial and anti-proliferative activities of different endemic Italian varieties of garlic (Allium sativum L.). J. Funct. Foods 2016, 21, 240–248. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, D. Allicin suppresses the migration and invasion in cervical cancer cells mainly by inhibiting NRF2. Exp. Ther. Med. 2019, 17, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Cho, S.J.; Kwon, H.C.; Lee, K.R.; Rhee, D.K.; Pyo, S. Caspase-independent cell death by allicin in human epithelial carcinoma cells: Involvement of PKA. Cancer Lett. 2005, 224, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Maitisha, G.; Aimaiti, M.; An, Z.; Li, X. Allicin induces cell cycle arrest and apoptosis of breast cancer cells in vitro via modulating the p53 pathway. Mol. Biol. Rep. 2021, 48, 7261–7272. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, J.; Zhai, D.; Zhang, D.; Shen, W.; Bai, L.; Cai, Z.; Yu, C. Role of JNK activation and mitochondrial bax translocation in allicin-induced apoptosis in human ovarian cancer SKOV3 cells. Evid. Based Complement. Alternat. Med. 2014, 2014, 378684. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Choi, Y.J.; Cha, S.H.; Choi, C.H.; Cho, W.H. Allicin inhibits cell growth and induces apoptosis in U87MG human glioblastoma cells through an ERK-dependent pathway. Oncol. Rep. 2012, 28, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ha, M.; Gong, Y.; Xu, Y.; Dong, N.; Yuan, Y. Allicin induces apoptosis in gastric cancer cells through activation of both extrinsic and intrinsic pathways. Oncol. Rep. 2010, 24, 1585–1592. [Google Scholar] [PubMed]
- Rosas-González, V.C.; Téllez-Bañuelos, M.C.; Hernández-Flores, G.; Bravo-Cuellar, A.; Aguilar-Lemarroy, A.; Jave-Suárez, L.F.; Haramati, J.; Solorzano-Ibarra, F.; Ortiz-Lazareno, P.C. Differential effects of alliin and allicin on apoptosis and senescence in luminal A and triple-negative breast cancer: Caspase, ΔΨm, and pro-apoptotic gene involvement. Fundam. Clin. Pharmacol. 2020, 34, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Yang, J.S.; Lin, S.Y.; Tan, T.W.; Ho, C.C.; Hsia, T.C.; Chiu, T.H.; Yu, C.S.; Lu, H.F.; Weng, Y.S.; et al. Diallyl disulfide (DADS) induces apoptosis in human cervical cancer Ca Ski cells via reactive oxygen species and Ca2+-dependent mitochondria-dependent pathway. Anticancer. Res. 2008, 28, 2791–2799. [Google Scholar] [PubMed]
- Lei, X.Y.; Yao, S.Q.; Zu, X.Y.; Huang, Z.X.; Liu, L.J.; Zhong, M.; Zhu, B.Y.; Tang, S.S.; Liao, D.F. Apoptosis induced by diallyl disulfide in human breast cancer cell line MCF-7. Acta Pharmacol. Sin. 2008, 29, 1233–1239. [Google Scholar] [CrossRef]
- Gunadharini, D.N.; Arunkumar, A.; Krishnamoorthy, G.; Muthuvel, R.; Vijayababu, M.R.; Kanagaraj, P.; Srinivasan, N.; Aruldhas, M.M.; Arunakaran, J. Antiproliferative effect of diallyl disulfide (DADS) on prostate cancer cell line LNCaP. Cell Biochem. Funct. 2006, 24, 407–412. [Google Scholar]
- Wu, X.J.; Kassie, F.; Mersch-Sundermann, V. The role of reactive oxygen species (ROS) production on diallyl disulfide (DADS) induced apoptosis and cell cycle arrest in human A549 lung carcinoma cells. Mutat. Res. 2005, 579, 115–124. [Google Scholar] [CrossRef]
- Song, J.D.; Lee, S.K.; Kim, K.M.; Park, S.E.; Park, S.J.; Kim, K.H.; Ahn, S.C.; Park, Y.C. Molecular mechanism of diallyl disulfide in cell cycle arrest and apoptosis in HCT-116 colon cancer cells. J. Biochem. Mol. Toxicol. 2009, 23, 71–79. [Google Scholar] [CrossRef]
- Sujatha, P.; Anantharaju, P.G.; Veeresh, P.M.; Dey, S.; Bovilla, V.R.; Madhunapantula, S.R.V. Diallyl disulfide (DADS) retards the growth of breast cancer cells in vitro and in vivo through apoptosis induction. Biomed. Pharmacol. J. 2017, 10, 1619–1630. [Google Scholar]
- Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M.; Glozman, S.; Yavin, E.; Weiner, L. S-Allylmercaptoglutathione: The reaction product of allicin with glutathione possesses SH-modifying and antioxidant properties. Biochim. Biophys. Acta Mol. Cell Res. 2000, 1499, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C. Thiol-modification as important mode of action for allicin from garlic (Allium sativum). Multidisc. Digit. Publ. Inst. Proc. 2019, 11, 27. [Google Scholar]
- Furdak, P.; Bartosz, G.; Stefaniuk, I.; Cieniek, B.; Bieszczad-Bedrejczuk, E.; Soszyński, M.; Sadowska-Bartosz, I. Effect of garlic extract on the erythrocyte as a simple model cell. Int. J. Mol. Sci. 2024, 25, 5115. [Google Scholar] [CrossRef] [PubMed]
- Shailasree, S.; Venkataramana, M.; Niranjana, S.R.; Prakash, H.S. Cytotoxic effect of p-Coumaric acid on neuroblastoma, N2a cell via generation of reactive oxygen species leading to dysfunction of mitochondria inducing apoptosis and autophagy. Mol. Neurobiol. 2015, 51, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, Z.; Liu, W.; Pan, Z.; Zhang, X.; Li, M.; Liu, X.; Zheng, Q.; Li, D. The anti-tumor effects of p-coumaric acid on melanoma A375 and B16 cells. Front. Oncol. 2020, 10, 558414. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. World J. Gastroenterol. 2013, 19, 7726–7734. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef]
- Eroğlu, C.; Seçme, M.; Bağcı, G.; Dodurga, Y. Assessment of the anticancer mechanism of ferulic acid via cell cycle and apoptotic pathways in human prostate cancer cell lines. Tumour Biol. 2015, 36, 9437–9446. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gong, X.; Jiang, R.; Li, H.; Du, W.; Kuang, G. Ferulic acid inhibits proliferation and promotes apoptosis via blockage of PI3K/Akt pathway in osteosarcoma cell. Am. J. Transl. Res. 2016, 8, 968–980. [Google Scholar] [PubMed]
- Rajendra Prasad, N.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Jastrzębska-Stojko, Ż.; Stojko, R.; Wojtyczka, R.D.; Stojko, J. Migration rate inhibition of breast cancer cells treated by caffeic acid and caffeic acid phenethyl ester: An in vitro comparison study. Nutrients 2017, 9, 1144. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Hsieh, C.H.; Hsiao, M.W.; Lin, W.C.; Hung, Y.C.; Ye, J.C. Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwan J. Obstet. Gynecol. 2010, 49, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pei, J.; Zheng, Y.; Miao, Y.J.; Duan, B.Z.; Huang, L.F. Gallic acid: A potential anti-cancer agent. Chin. J. Integr. Med. 2022, 28, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Suzuki, R.; Sakaguchi, N.; Li, Z.; Takeda, T.; Ogihara, Y.; Jiang, B.Y.; Chen, Y. Selective induction of cell death in cancer cells by gallic acid. Biol. Pharm. Bull. 1995, 18, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol. Lett. 2013, 6, 1749–1755. [Google Scholar] [CrossRef]
- Chen, H.M.; Wu, Y.C.; Chia, Y.C.; Chang, F.R.; Hsu, H.K.; Hsieh, Y.C.; Chen, C.C.; Yuan, S.S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009, 286, 161–171. [Google Scholar] [CrossRef]
- Yoshida, M.; Sakai, T.; Hosokawa, N.; Marui, N.; Matsumoto, K.; Fujioka, A.; Nishino, H.; Aoike, A. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett. 1990, 260, 10–13. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tran, E.; Nguyen, T.H.; Do, P.T.; Huynh, T.H.; Huynh, H. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis 2004, 25, 647–659. [Google Scholar] [CrossRef]
- Zhang, X.A.; Zhang, S.; Yin, Q.; Zhang, J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharmacogn. Mag. 2015, 11, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.H.; Song, H.Y.; Zhou, Y.F.; Yuan, G.Y.; Zheng, F.J. Effects of quercetin on the proliferation of breast cancer cells and expression of survivin in vitro. Exp. Ther. Med. 2013, 6, 1155–1158. [Google Scholar] [CrossRef]
- Gulati, N.; Laudet, B.; Zohrabian, V.M.; Murali, R.A.J.; Jhanwar-Uniyal, M.E.E.N.A. The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006, 26, 1177–1181. [Google Scholar]
- Moreno-Ortega, A.; Di Pede, G.; Pereira-Caro, G.; Calani, L.; Mena, P.; Del Rio, D.; Moreno-Rojas, J.M. In vitro colonic fermentation of (poly)phenols and organosulfur compounds of fresh and black garlic. J. Agric. Food Chem. 2022, 70, 3666–3677. [Google Scholar] [CrossRef] [PubMed]
- Shirzad, H.; Taji, F.; Rafieian-Kopaei, M. Correlation between antioxidant activity of garlic extracts and WEHI-164 fibrosarcoma tumor growth in BALB/c mice. J. Med. Food 2011, 14, 969–974. [Google Scholar] [CrossRef]
- Kut, K.; Cieniek, B.; Stefaniuk, I.; Bartosz, G.; Sadowska-Bartosz, I. A modification of the ABTS• decolorization method and an insight into its mechanism. Processes 2022, 10, 1288. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kuczera, K.; Naparło, K.; Soszyński, M.; Bartosz, G.; Sadowska-Bartosz, I. Capsaicin toxicity to the yeast Saccharomyces cerevisiae is not due to oxidative stress but to disruption of membrane structure. Chem. Biol. Interact. 2023, 374, 110407. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
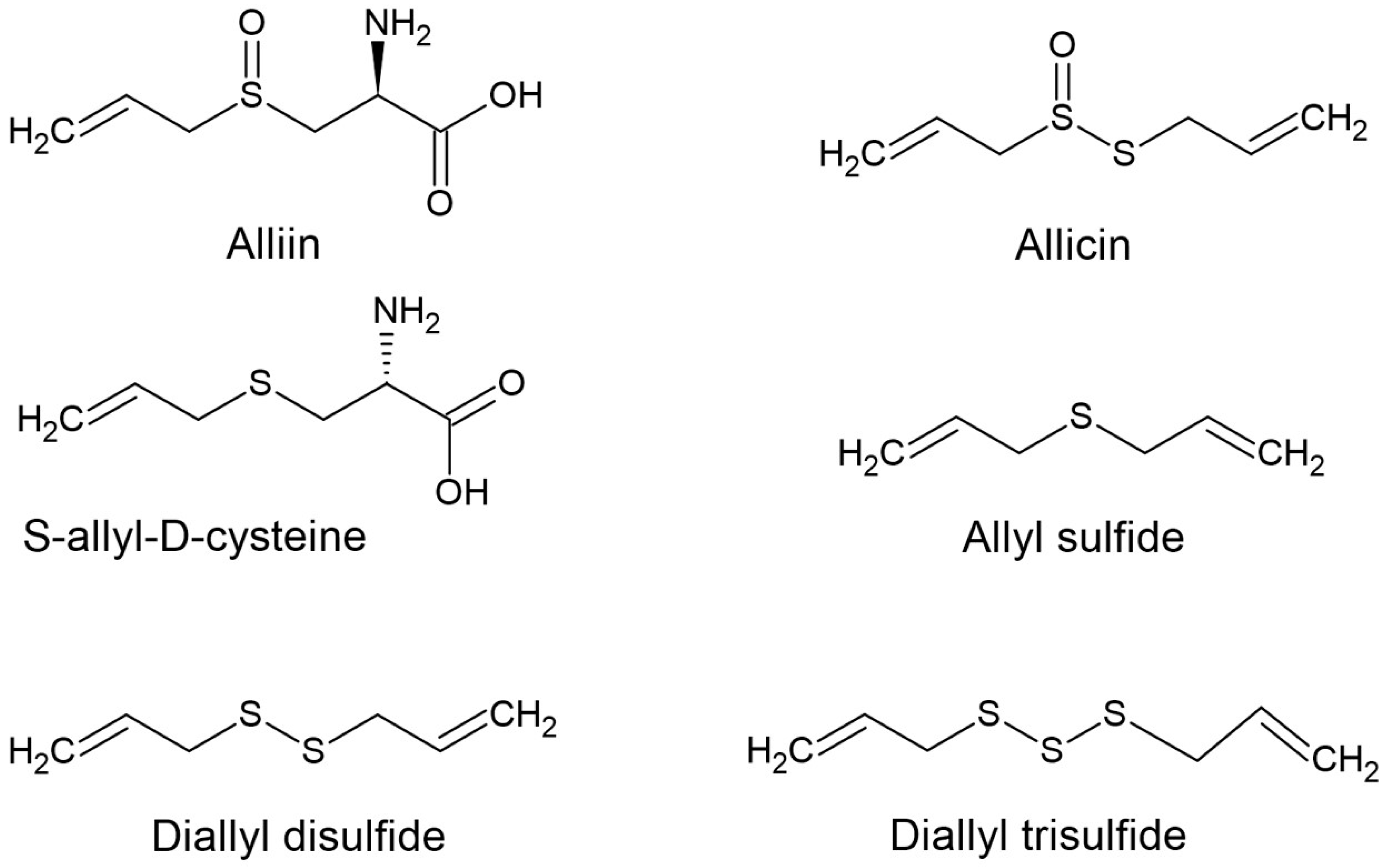
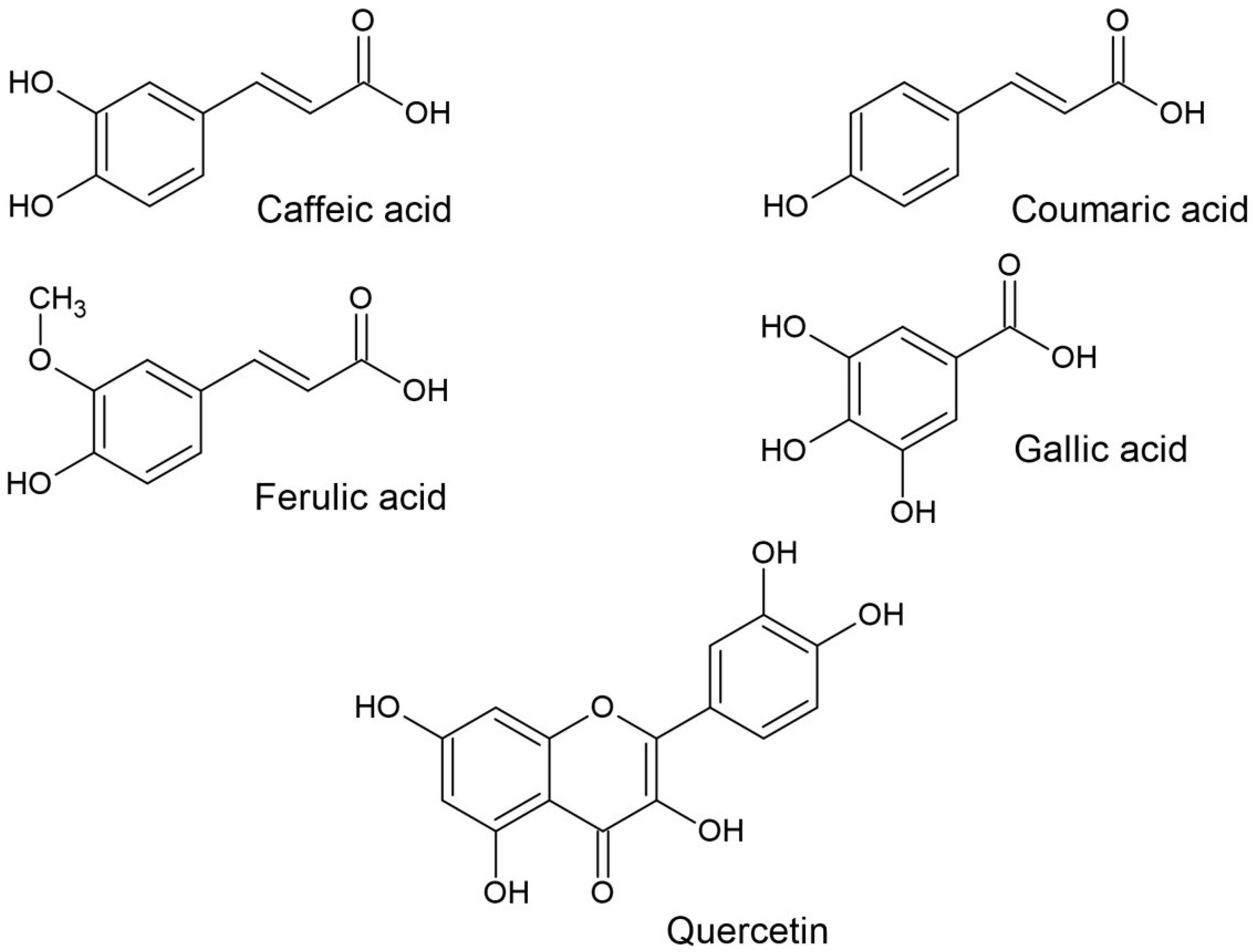
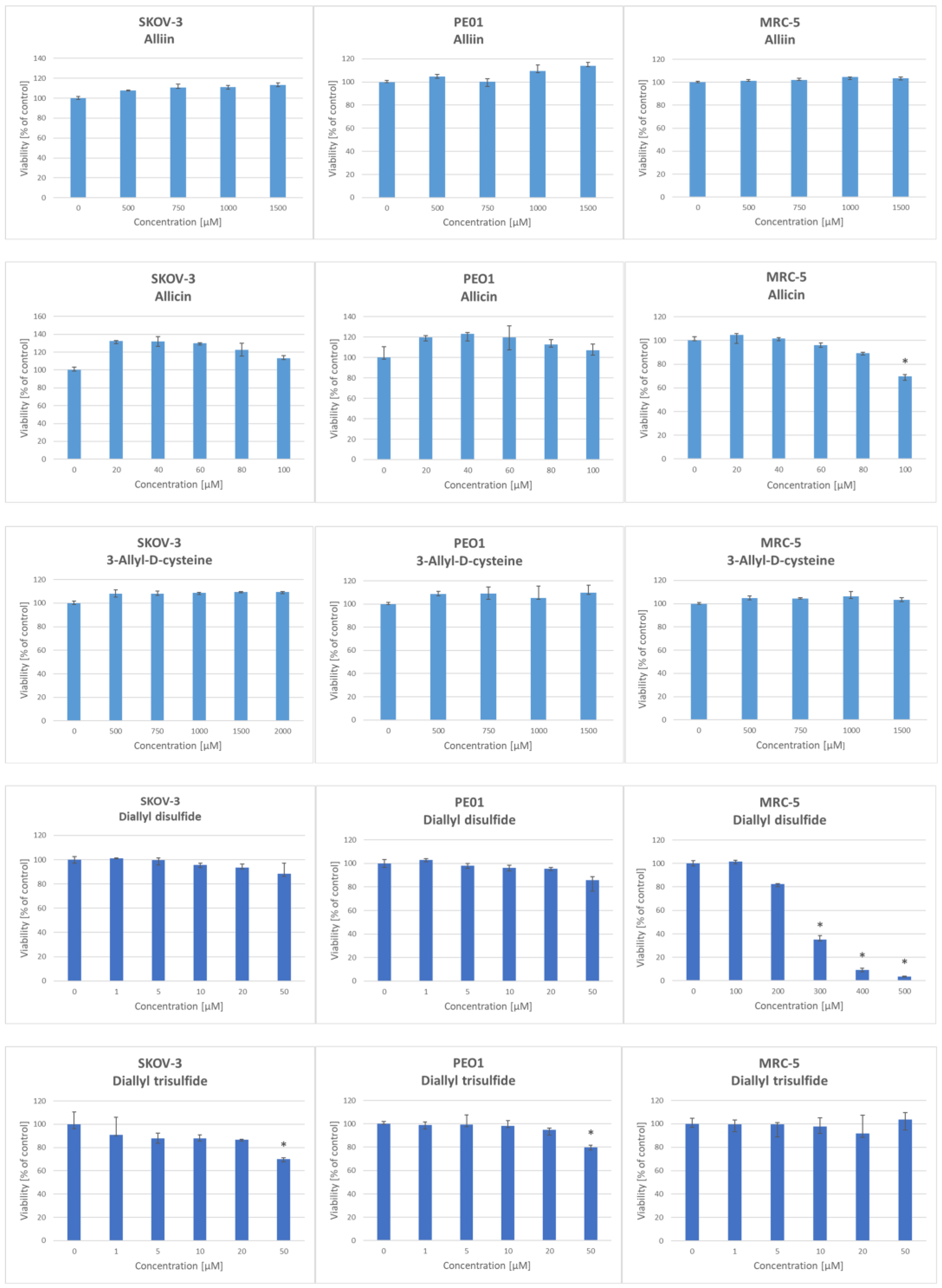

| Compound | ABTS● Decolorization | DPPH● Decolorization | FRAP |
|---|---|---|---|
| Organosulfur compounds | |||
| Alliin | 0.035 ± 0.012 | 0.004 ± 0.001 | 0.0006 ± 0.0007 |
| Allicin | −0.038 ± 0.020 | −0.023 ± 0.009 | −0.0014 ± 0.0016 |
| S-Allyl-D-cysteine | 0.070 ± 0.059 | −0.020 ± 0.004 | 0.0003 ± 0.0002 |
| Allyl sulfide | 0.016 ± 0.010 | −0.006 ± 0.011 | 0.0026 ± 0.0010 |
| Diallyl disulfide | 0.013 ± 0.006 | 0.049 ± 0.014 | 0.0025 ± 0.0011 |
| Diallyl trisulfide | 0.030 ± 0.006 | 0.005 ± 0.0003 | 0.0022 ± 0.0004 |
| Phenolics | |||
| Caffeic acid | 0.771 ± 0.066 | 0.001 ± 0.006 | 0.019 ± 0.002 |
| Coumaric acid | 0.585 ± 0.082 | 0.076 ± 0.005 | 0.153 ± 0.003 |
| Ferulic acid | 1.409 ± 0.096 | 0.185 ± 0.070 | 0.554 ± 0.017 |
| Gallic acid | 0.972 ± 0.028 | 0.178 ± 0.023 | 0.332 ± 0.001 |
| Quercetin | 1.138 ± 0.029 | 0.384 ± 0.040 | 1.350 ± 0.201 |
| Compound | IC50 [μM] | ||
|---|---|---|---|
| SKOV-3 | PEO1 | MRC-5 | |
| Organosulfur compounds | |||
| Alliin | nd | 263 | 321 |
| Allicin | 236 | 282 | 120 |
| S-Allyl-D-cysteine | 421 | nd | 578 |
| Allyl sulfide | 243 | 257 | 163 |
| Diallyl disulfide | nd | 265 | 268 |
| Diallyl trisulfide | 154 | 721 | nd |
| Phenolics | |||
| Caffeic acid | 1317 | 245 | nd |
| Coumaric acid | nd | 1427 | nd |
| Ferulic acid | nd | nd | nd |
| Gallic acid | 115 | 19 | 243 |
| Quercetin | 272 | 100 | 111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furdak, P.; Bartosz, G.; Sadowska-Bartosz, I. Which Constituents Determine the Antioxidant Activity and Cytotoxicity of Garlic? Role of Organosulfur Compounds and Phenolics. Int. J. Mol. Sci. 2024, 25, 8391. https://doi.org/10.3390/ijms25158391
Furdak P, Bartosz G, Sadowska-Bartosz I. Which Constituents Determine the Antioxidant Activity and Cytotoxicity of Garlic? Role of Organosulfur Compounds and Phenolics. International Journal of Molecular Sciences. 2024; 25(15):8391. https://doi.org/10.3390/ijms25158391
Chicago/Turabian StyleFurdak, Paulina, Grzegorz Bartosz, and Izabela Sadowska-Bartosz. 2024. "Which Constituents Determine the Antioxidant Activity and Cytotoxicity of Garlic? Role of Organosulfur Compounds and Phenolics" International Journal of Molecular Sciences 25, no. 15: 8391. https://doi.org/10.3390/ijms25158391






