An In-Depth Approach to the Associations between MicroRNAs and Viral Load in Patients with Chronic Hepatitis B—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. The Article Extraction Process
2.2. The Selection Method
2.3. Data Acquisition and Quality Assessment
2.4. A Bioinformatics Analysis on the Retrieved microRNAs
2.5. Statistical Methods
3. Results
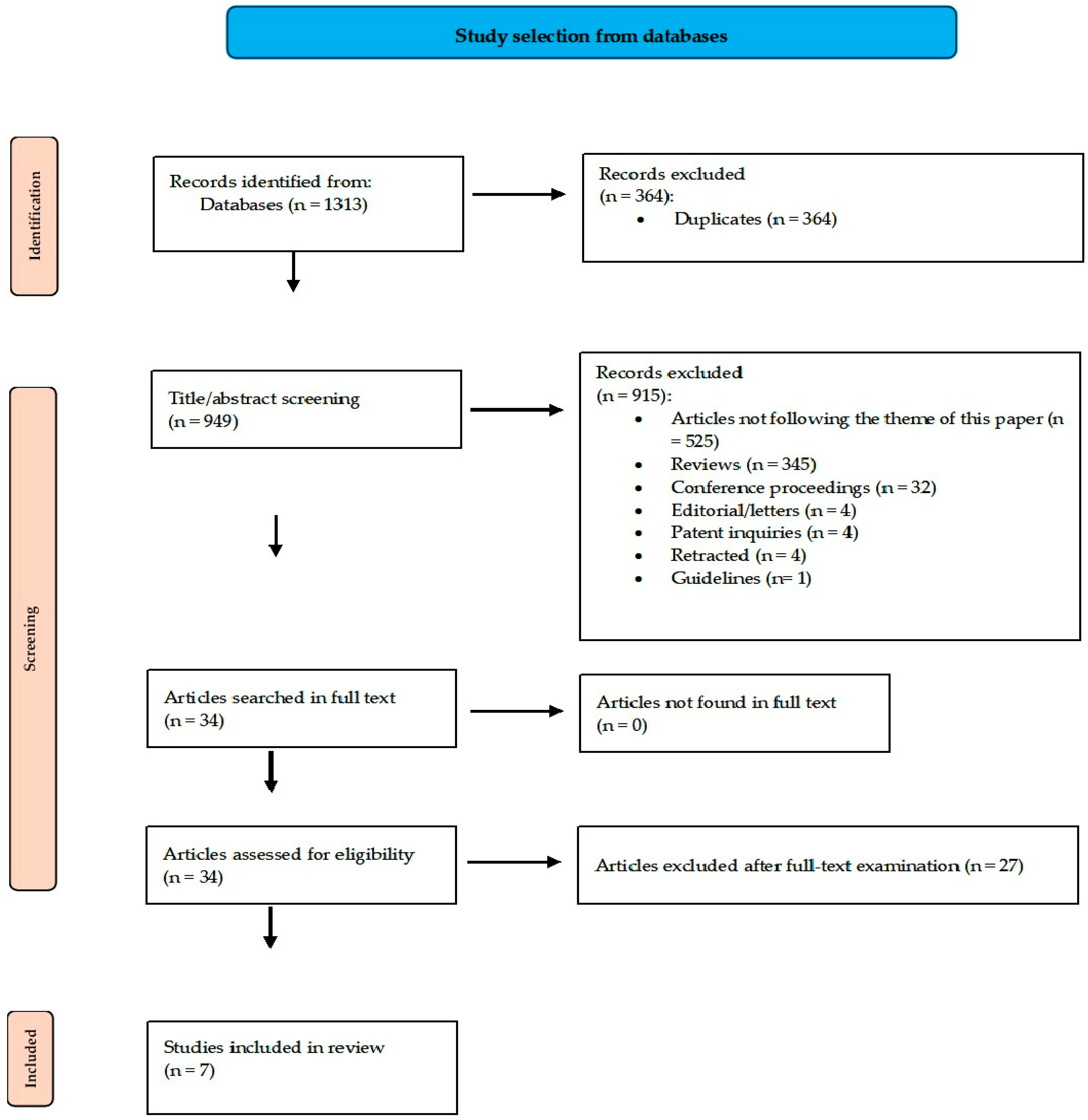
3.1. The Article Selection Scheme
3.2. Main Article Data
3.3. Meta-Correlation of Spearman Results
3.4. Meta-Correlation of Pearson Results
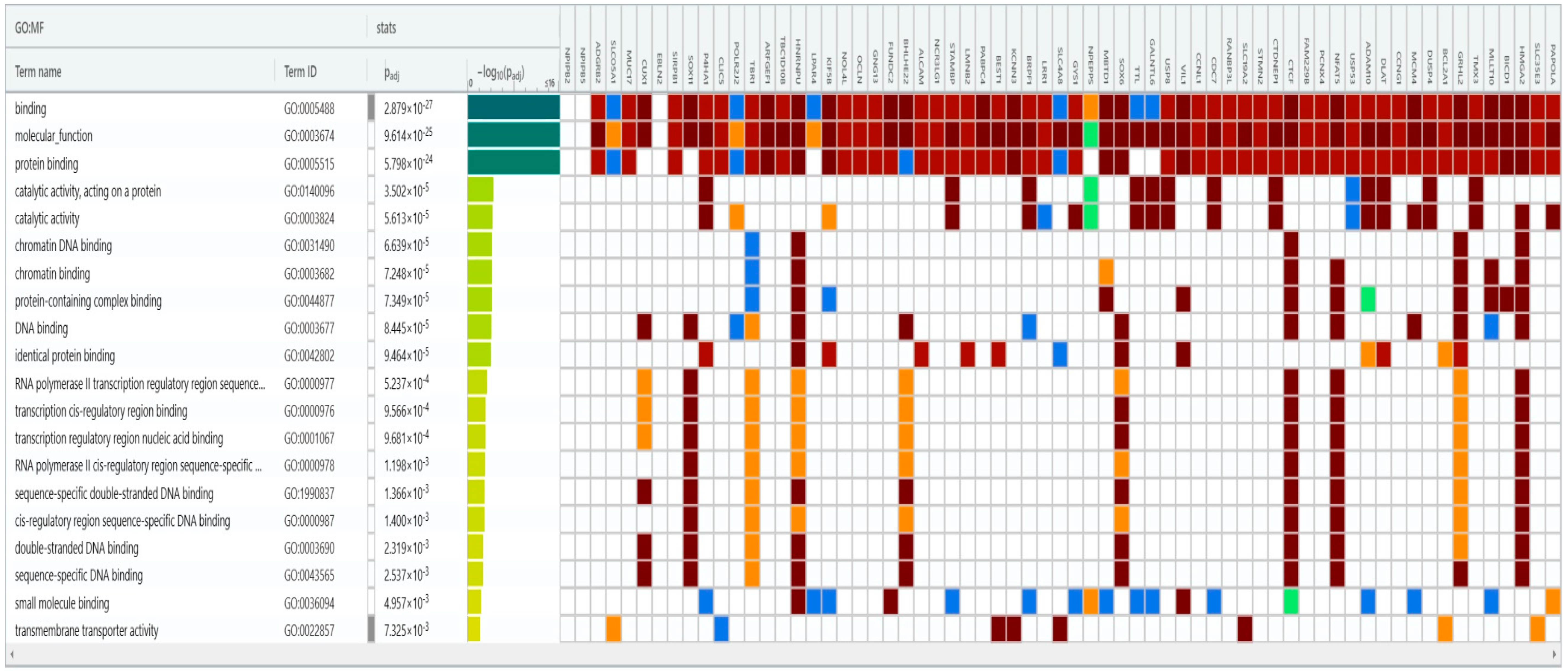
3.5. Bioinformatics Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Prevention of Hepatitis B and C in the EU/EEA; Publications Office: Luxembourg, 2024. Available online: https://data.europa.eu/doi/10.2900/703244 (accessed on 31 May 2024).
- Hepatitis, B. Surveillance Guidance. Available online: https://www.cdc.gov/hepatitis/statistics/SurveillanceGuidance/HepatitisB.htm (accessed on 30 May 2024).
- Hepatitis, B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 30 May 2024).
- Torimiro, J.N.E.; Duri, K.; Goumkwa, N.M.; Atah, S.M.; Ondigui, J.-L.N.; Lobe, C.; Bouyou, M.; Ndeboko, B.; Moussa, A.M.; Police, C.; et al. Toward the elimination of hepatitis B: Networking to promote the prevention of vertical transmission of hepatitis B virus through population-based interventions and multidisciplinary groups in Africa. Front. Public Health 2024, 12, 1283350. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, G.K.; Chemaitelly, H.; Nizamuddin, P.B.; Al-Sadeq, D.W.; Shurrab, F.M.; Amanullah, F.H.; Al-Hamad, T.H.; Mohammad, K.N.; Alabdulmalek, M.A.; Al Kahlout, R.A.; et al. Prevalence of hepatitis B and C viruses among migrant workers in Qatar. Sci. Rep. 2024, 14, 11275. [Google Scholar] [CrossRef] [PubMed]
- Zovich, B.; Freeland, C.; Moore, H.; Sapp, K.; Qureshi, A.; Holbert, R.; Zambrano, J.; Bhangoo, D.; Cohen, C.; Hass, R.W.; et al. Dismantling Barriers to Hepatitis B and Delta Screening, Prevention, and Linkage to Care among the PWUD Community in Philadelphia. Viruses 2024, 16, 628. [Google Scholar] [CrossRef] [PubMed]
- Demirchyan, A.; Dudareva, S.; Sahakyan, S.; Aslanyan, L.; Muradyan, D.; Musheghyan, L.; Mozalevskis, A.; Sargsyants, N.; Ghukasyan, G.; Petrosyan, V. Prevalence of hepatitis B virus infection among general population of Armenia in 2021 and factors associated with it: A cross-sectional study. BMJ Open 2024, 14, e080281. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Y.; Wang, M.; Wu, Y.; Dong, J.; Liu, J.; Hu, W. Establishing a screening strategy for non-discriminatory reactive blood donors by constructing a predictive model of hepatitis B virus infection status from a single blood center in China. Front. Public Health 2024, 12, 1366431. [Google Scholar] [CrossRef] [PubMed]
- Gherlan, G.S. Occult hepatitis B-the result of the host immune response interaction with different genomic expressions of the virus. World J. Clin. Cases 2022, 10, 5518–5530. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Moriyama, M.; Omata, M. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections: A Review. Int. J. Mol. Sci. 2019, 20, 1358. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Z.; Liu, J.; Li, K.; Zhang, Y. The Effect of Low HBV-DNA Viral Load on Recurrence in Hepatocellular Carcinoma Patients Who Underwent Primary Locoregional Treatment and the Development of a Nomogram Prediction Model. Microorganisms 2024, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Guo, S.; Schrodi, S.J. Mechanisms of DNA Methylation in Virus-Host Interaction in Hepatitis B Infection: Pathogenesis and Oncogenetic Properties. Int. J. Mol. Sci. 2021, 22, 9858. [Google Scholar] [CrossRef]
- Cho, W.T.; Yoo, T.; Lee, J.M.; Lee, J.W.; Kim, H.; Lee, J.S.; Han, S.H. Hepatitis B Virus DNA-Level Change is Associated with Tumor Recurrence in Patients with Resected Hepatitis B Virus Hepatocellular Carcinoma. J. Surg. Res. 2024, 295, 231–239. [Google Scholar] [CrossRef]
- Sedohara, A.; Takahashi, K.; Arai, K.; Arizono, K.; Tuvshinjargal, K.; Saito, M.; Nakahara, F.; Tsutsumi, T.; Ikeuchi, K.; Adachi, E.; et al. Characterization of mutations in hepatitis B virus DNA isolated from Japanese HBsAg-positive blood donors in 2021 and 2022. Arch. Virol. 2024, 169, 103. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Lee, S.K.; Cho, E.-J.; Kim, H.-S.; Lee, Y.K.; Kim, J.-S.; Song, W.; Kim, H.S. Performance Evaluation of the Roche Cobas 5800 HBV and HCV Tests: Comparison of the 200 and 500 μL Protocols. Ann. Lab. Med. 2023, 44, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-C.; Hosaka, T.; Liu, C.-J.; Suzuki, F.; Chiang, C.; Hong, C.-M.; Kumada, H.; Yang, W.-T.; Su, T.-H.; Yang, H.-C.; et al. HBcrAg-based risk score performs better than the HBV DNA-based scores for HCC prediction in grey zone patients who are HBeAg-negative. JHEP Rep. 2024, 6, 100956. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, V.; Prezioso, C.; Moens, U. Role of Virus-Induced Host Cell Epigenetic Changes in Cancer. Int. J. Mol. Sci. 2021, 22, 8346. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; Guandique, D.M.A.; Caroleo, M.C.; Luciani, F.; Colosimo, M.; Cannataro, R. Liver Damage and microRNAs: An Update. Curr. Issues Mol. Biol. 2022, 45, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach-Rivière, L.; Bergez, M.; Mönch, S.; Qu, B.; Riess, M.; Vondran, F.W.R.; Liese, J.; Hornung, V.; Urban, S.; König, R. Hepatitis B Virus DNA is a Substrate for the cGAS/STING Pathway but is not Sensed in Infected Hepatocytes. Viruses 2020, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Kouwaki, T.; Okamoto, M.; Tsukamoto, H.; Fukushima, Y.; Oshiumi, H. Extracellular Vesicles Deliver Host and Virus RNA and Regulate Innate Immune Response. Int. J. Mol. Sci. 2017, 18, 666. [Google Scholar] [CrossRef] [PubMed]
- Thirion, M.; Ochiya, T. Roles of microRNAs in the Hepatitis B Virus Infection and Related Diseases. Viruses 2013, 5, 2690–2703. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-R.; Liu, A.-R.; Jiang, L.-Y.; Wang, B.-G. Non-coding RNA and hepatitis B virus-related hepatocellular carcinoma: A bibliometric analysis and systematic review. Front. Med. 2022, 9, 995943. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, L.; Yan, X.; Jin, W.; Wang, Y.; Chen, L.; Wu, E.; Ye, X.; Gao, G.F.; Wang, F.; et al. Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G1-modulated P53 activity. Hepatology 2012, 55, 730–741. [Google Scholar] [CrossRef]
- Maepa, M.B.; Ely, A.; Grayson, W.; Arbuthnot, P. Sustained Inhibition of HBV Replication In Vivo after Systemic Injection of AAVs Encoding Artificial Antiviral Primary MicroRNAs. Mol. Ther. Nucleic Acids 2017, 7, 190–199. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Glasziou, P.; Del Mar, C.; Bannach-Brown, A.; Stehlik, P.; Scott, A.M. A full systematic review was completed in 2 weeks using automation tools: A case study. J. Clin. Epidemiol. 2020, 121, 81–90. [Google Scholar] [CrossRef]
- ZOTERO. Available online: http://www.zotero.org (accessed on 1 February 2023).
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Miami, FL, USA, 2020; Available online: https://synthesismanual.jbi.global (accessed on 1 February 2024).
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis. 2024. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 29 June 2024).
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G. Settings EWG on CR in LR. Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, 0147601. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews Systematic reviews and Meta-Analyses. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tastsoglou, S.; Alexiou, A.; Karagkouni, D.; Skoufos, G.; Zacharopoulou, E.; Hatzigeorgiou, A.G. DIANA-microT 2023: Including predicted targets of virally encoded miRNAs. Nucleic Acids Res. 2023, 51, W148–W153. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. g: Profiler—interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 March 2023).
- Cooper, H.; Hedges, L.V.; Valentine, J.C. The Handbook of Research Synthesis and Metaanalysis, 2nd ed.; Russell Sage Foundation: New York, NY, USA, 2009; pp. 264–265, 273–274. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Guo, H.; Liu, H.; Mitchelson, K.; Rao, H.; Luo, M.; Xie, L.; Sun, Y.; Zhang, L.; Lu, Y.; Liu, R.; et al. MicroRNAs-372/373 promote the expression of hepatitis B virus through the targeting of nuclear factor I/B. Hepatology 2011, 54, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Waidmann, O.; Bihrer, V.; Pleli, T.; Farnik, H.; Berger, A.; Zeuzem, S.; Kronenberger, B.; Piiper, A. Serum microRNA-122 levels in different groups of patients with chronic hepatitis B virus infection. J. Viral Hepat. 2011, 19, 58–65. [Google Scholar] [CrossRef]
- Arataki, K.; Hayes, C.N.; Akamatsu, S.; Akiyama, R.; Abe, H.; Tsuge, M.; Miki, D.; Ochi, H.; Hiraga, N.; Imamura, M.; et al. Circulating microRNA-22 correlates with microRNA-122 and represents viral replication and liver injury in patients with chronic hepatitis B. J. Med. Virol. 2013, 85, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Coppola, N.; Potenza, N.; Pisaturo, M.; Mosca, N.; Tonziello, G.; Signoriello, G.; Messina, V.; Sagnelli, C.; Russo, A.; Sagnelli, E. Liver microRNA hsa-miR-125a-5p in HBV Chronic Infection: Correlation with HBV Replication and Disease Progression. PLoS ONE 2013, 8, 65336. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wei, X.; Tang, C.; Li, J.; Liu, R.; Shen, A.; Wu, Z. Circulating microRNA-101 as a potential biomarker for hepatitis B virus-related hepatocellular carcinoma. Oncol. Lett. 2013, 6, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Winther, T.N.; Bang-Berthelsen, C.H.; Heiberg, I.L.; Pociot, F.; Hogh, B. Differential Plasma MicroRNA Profiles in HBeAg Positive and HBeAg Negative Children with Chronic Hepatitis B. PLoS ONE 2013, 8, 58236. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, M.R.; Cavallone, D.; Oliveri, F.; Moriconi, F.; Colombatto, P.; Coco, B.; Ciccorossi, P.; Rastelli, C.; Romagnoli, V.; Cherubini, B.; et al. A Serum MicroRNA Signature Is Associated with the Immune Control of Chronic Hepatitis B Virus Infection. PLoS ONE 2014, 9, 110782. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yao, Q.; Butt, A.M.; Guo, J.; Tian, Z.; Bao, X.; Li, H.; Meng, Q.; Lu, J. Expression profiling of serum microRNA-101 in HBV-associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Cancer Biol. Ther. 2014, 15, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Jiang, D.; Huang, J.; Xu, Z. Expression and clinical significance of miR-122 and miR-29 in hepatitis B virus-related liver disease. Genet. Mol. Res. 2014, 13, 7912–7918. [Google Scholar] [CrossRef]
- Akamatsu, S.; Hayes, C.N.; Tsuge, M.; Miki, D.; Akiyama, R.; Abe, H.; Ochi, H.; Hiraga, N.; Imamura, M.; Takahashi, S.; et al. Differences in serum microRNA profiles in hepatitis B and C virus infection. J. Infect. 2014, 70, 273–287. [Google Scholar] [CrossRef]
- Jin, B.-X.; Zhang, Y.-H.; Jin, W.-J.; Sun, X.-Y.; Qiao, G.-F.; Wei, Y.-Y.; Sun, L.-B.; Zhang, W.-H.; Li, N. MicroRNA panels as disease biomarkers distinguishing hepatitis B virus infection caused hepatitis and liver cirrhosis. Sci. Rep. 2015, 5, 15026. [Google Scholar] [CrossRef] [PubMed]
- Mohamadkhani, A.; Bastani, F.; Khorrami, S.; Ghanbari, R.; Eghtesad, S.; Sharafkhah, M.; Montazeri, G.; Poustchi, H. Negative Association of Plasma Levels of Vitamin D and mir-378 With Viral Load in Patients with Chronic Hepatitis B Infection. Hepat. Mon. 2015, 15, 28315. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Xu, H.; Yu, W.; Wang, B.; Zhang, J. Expression profile and clinical significance of miRNAs at different stages of chronic hepatitis B virus infection. Int. J. Clin. Exp. Med. 2015, 8, 5611–5620. [Google Scholar]
- Li, F.; Zhou, P.; Deng, W.; Wang, J.; Mao, R.; Zhang, Y.; Li, J.; Yu, J.; Yang, F.; Huang, Y.; et al. Serum microRNA-125b correlates with hepatitis B viral replication and liver necroinflammation. Clin. Microbiol. Infect. 2016, 22, 384.e1–384.e10. [Google Scholar] [CrossRef]
- Yu, S.-L.; Deng, H.; Li, X.-H.; Huang, Y.-X.; Xie, D.-Y.; Gao, Z.-L. Expression of MicroRNA-155 is Downregulated in Peripheral Blood Mononuclear Cells of Chronic Hepatitis B Patients. Hepat. Mon. 2016, 16, 34483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Wu, J.; Wang, X.; Sun, Z.; Han, Q.; Zhao, L. Low-level expression of microRNA-375 predicts poor prognosis in hepatocellular carcinoma. Tumor Biol. 2016, 37, 2145–2152. [Google Scholar] [CrossRef]
- Qiao, D.-D.; Yang, J.; Lei, X.-F.; Mi, G.-L.; Li, S.-L.; Li, K.; Xu, C.-Q.; Yang, H.-L. Expression of microRNA-122 and microRNA-22 in HBV-related liver cancer and the correlation with clinical features. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 742–747. [Google Scholar]
- Yang, X.; Li, H.; Sun, H.; Fan, H.; Hu, Y.; Liu, M.; Li, X.; Tang, H. Hepatitis B Virus-Encoded MicroRNA Controls Viral Replication. J. Virol. 2017, 91, 01919. [Google Scholar] [CrossRef]
- Akuta, N.; Suzuki, F.; Hosaka, T.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; Kobayashi, M.; Saitoh, S.; Suzuki, Y.; Arase, Y.; et al. Circulating microRNA-122 levels are important predictor of hepatitis B virus surface antigen seroclearance. J. Med. Virol. 2018, 90, 1586–1592. [Google Scholar] [CrossRef]
- Shen, X.; Xue, Y.; Cong, H.; Wang, X.; Ju, S. Dysregulation of serum microRNA-574-3p and its clinical significance in hepatocellular carcinoma. Ann. Clin. Biochem. 2017, 55, 478–484. [Google Scholar] [CrossRef]
- Li, F.; Bian, H.; Wang, W.; Ning, L.; Xu, M.; Sun, S.; Ren, W.; Qin, C.; Qi, J. HBV infection suppresses the expression of inflammatory macrophage miR-210. Mol. Med. Rep. 2019, 19, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Yousefpouran, S.; Mostafaei, S.; Manesh, P.V.; Iranifar, E.; Bokharaei-Salim, F.; Nahand, J.S.; Mirzaei, H.; Taran, M.; Babaei, F.; Sayad, B.; et al. The assessment of selected MiRNAs profile in HIV, HBV, HCV, HIV/HCV, HIV/HBV Co-infection and elite controllers for determination of biomarker. Microb. Pathog. 2020, 147, 104355. [Google Scholar] [CrossRef] [PubMed]
- Laleh, R.T.A.; Sharifi, Z.; Pourfathollah, A.A. Correlation of serum microRNA-122 level with the levels of Alanine aminotransferase and HBV-DNA in Chronic HBV-infected patients. Med. J. Islam. Repub. Iran 2021, 35, 1028–1031. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, X.; Wang, J.; He, Q.; Li, J.; Zhang, Z.; Liu, H. MicroRNA-138 Regulates T-Cell Function by Targeting PD-1 in Patients with Hepatitis B Virus-Related Liver Diseases. Lab. Med. 2021, 52, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Chen, X.; Wu, Z.; Zhu, X.; Liu, J.; Wang, T.; Gao, Z. The relationship between serum exosome HBV-miR-3 and current virological markers and its dynamics in chronic hepatitis B patients on antiviral treatment. Ann. Transl. Med. 2022, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Loukachov, V.; van Dort, K.A.; Jansen, L.; Reesink, H.W.; Kootstra, N.A. Identification of a Novel HBV Encoded miRNA Using Next Generation Sequencing. Viruses 2022, 14, 1223. [Google Scholar] [CrossRef] [PubMed]
- Loukachov, V.; van Dort, K.; Maurer, I.; Takkenberg, R.; de Niet, A.; Reesink, H.; Willemse, S.; Kootstra, N. Identification of Liver and Plasma microRNAs in Chronic Hepatitis B Virus infection. Front. Cell. Infect. Microbiol. 2022, 12, 790964. [Google Scholar] [CrossRef] [PubMed]
- Loukachov, V.V.; van Dort, K.A.; Maurer, I.; Takkenberg, R.B.; de Niet, A.; Reesink, H.W.; Willemse, S.B.; Kootstra, N.A. Serum microRNA-210 levels in different groups of chronic hepatitis B patients. Clin. Chim. Acta 2015, 450, 203–209. [Google Scholar] [CrossRef]
- van der Ree, M.H.; Jansen, L.; Kruize, Z.; van Nuenen, A.C.; van Dort, K.A.; Takkenberg, R.B.; Reesink, H.W.; Kootstra, N.A. Plasma MicroRNA Levels Are Associated with Hepatitis B e Antigen Status and Treatment Response in Chronic Hepatitis B Patients. J. Infect. Dis. 2017, 215, 1421–1429. [Google Scholar] [CrossRef]
- Zhou, P.; Dong, M.; Wang, J.; Li, F.; Zhang, J.; Gu, J. Baseline serum miR-125b levels predict virologic response to nucleos(t)ide analogue treatment in patients with HBeAg-positive chronic hepatitis B. Exp. Ther. Med. 2018, 16, 3805–3812. [Google Scholar] [CrossRef]
- Liu, W.; He, X.; Huang, F. Analysis of Serum MicroRNA-122 Expression at Different Stages of Chronic Hepatitis B Virus Infection. BioMed Res. Int. 2021, 2021, 9957440. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Liu, M.; Wang, L.; Wang, J.; Xiong, F.; Bao, X.; Gao, Y.; Yu, L.; Lu, J. Serum microRNAs predict response of patients with chronic hepatitis B to antiviral therapy. Int. J. Infect. Dis. 2021, 108, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, Y.; Zhang, S.; Zhu, B.; Wang, J.; Wang, J.; Wang, X.; Zhao, Z.; Deng, W.; Mao, R.; et al. Human hepatocyte-enriched miRNA-192-3p promotes HBV replication through inhibiting Akt/mTOR signalling by targeting ZNF143 in hepatic cell lines. Emerg. Microbes Infect. 2022, 11, 616–628. [Google Scholar] [CrossRef]
- Nagura, Y.; Matsuura, K.; Iio, E.; Fujita, K.; Inoue, T.; Matsumoto, A.; Tanaka, E.; Nishiguchi, S.; Kang, J.-H.; Matsui, T.; et al. Serum miR-192-5p levels predict the efficacy of pegylated interferon therapy for chronic hepatitis B. PLoS ONE 2022, 17, 0263844. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, E.; Potenza, N.; Onorato, L.; Sagnelli, C.; Coppola, N.; Russo, A. Micro-RNAs in hepatitis B virus-related chronic liver diseases and hepatocellular carcinoma. World J. Hepatol. 2018, 10, 558–570. [Google Scholar] [CrossRef]
- Cao, M.; Yuan, D.; Jiang, H.; Zhou, G.; Chen, C.; Han, G. Long non-coding RNA WAC antisense RNA 1 mediates hepatitis B virus replication in vitro by reinforcing miR-192-5p/ATG7-induced autophagy. Eur. J. Histochem. 2022, 66, 3438. [Google Scholar] [CrossRef]
- Duan, X.; Li, S.; Holmes, J.A.; Tu, Z.; Li, Y.; Cai, D.; Liu, X.; Li, W.; Yang, C.; Jiao, B.; et al. MicroRNA 130a Regulates both Hepatitis C Virus and Hepatitis B Virus Replication through a Central Metabolic Pathway. J. Virol. 2018, 92, 02009-17. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, L.; Zhang, Y.; Li, H. Circ-ATP5H Induces Hepatitis B Virus Replication and Expression by Regulating miR-138-5p/TNFAIP3 Axis. Cancer Manag. Res. 2020, 12, 11031–11040. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Chen, H.-L.; Shih, C. MicroRNA miR-204 and miR-1236 inhibit hepatitis B virus replication via two different mechanisms. Sci. Rep. 2016, 6, 34740. [Google Scholar] [CrossRef]
- Yang, H.; Rui, F.; Li, R.; Yin, S.; Xue, Q.; Hu, X.; Xu, Y.; Wu, C.; Shi, J.; Li, J. ADAR1 Inhibits HBV DNA Replication via Regulating miR-122-5p in Palmitic Acid Treated HepG2.2.15 Cells. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 4035–4047. [Google Scholar] [CrossRef]
- Xu, J.; An, P.; Winkler, C.A.; Yu, Y. Dysregulated microRNAs in Hepatitis B Virus-Related Hepatocellular Carcinoma: Potential as Biomarkers and Therapeutic Targets. Front. Oncol. 2020, 10, 1271. [Google Scholar] [CrossRef] [PubMed]
- Karamichali, E.; Foka, P.; Papadopoulou, G.; Loukaki-Gkountara, D.; Andresaki, K.; Koskinas, I.; Georgopoulou, U. Hepatitis Viruses Control Host Immune Responses by Modifying the Exosomal Biogenesis Pathway and Cargo. Int. J. Mol. Sci. 2022, 23, 10862. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Su, H.; Liu, M.; Qian, Y.; Fan, H. miRNA-mRNA network contributes to HBV-related hepatocellular carcinoma via immune infiltration induced by GRB2. Biomed. Rep. 2024, 20, 90. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Marumo, M.; Ekawa, K.; Daimon, T. Differences in serum and plasma levels of microRNAs and their time-course changes after blood collection. Pract. Lab. Med. 2024, 39, 00376. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yuan, Y.; Cho, J.-H.; McClarty, S.; Baxter, D.; Galas, D.J. Comparing the MicroRNA Spectrum between Serum and Plasma. PLoS ONE 2012, 7, e41561. [Google Scholar] [CrossRef] [PubMed]
- Git, A.; Dvinge, H.; Salmon-Divon, M.; Osborne, M.; Kutter, C.; Hadfield, J.; Bertone, P.; Caldas, C. Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA 2010, 16, 991–1006. [Google Scholar] [CrossRef]
- Farr, R.J.; Januszewski, A.S.; Joglekar, M.V.; Liang, H.; McAulley, A.K.; Hewitt, A.W.; Thomas, H.E.; Loudovaris, T.; Kay, T.W.H.; Jenkins, A.; et al. A comparative analysis of high-throughput platforms for validation of a circulating microRNA signature in diabetic retinopathy. Sci. Rep. 2015, 5, 10375. [Google Scholar] [CrossRef]
- Yen, Y.-H.; Huang, C.-M.; Wei, K.-L.; Wang, J.-H.; Lu, S.-N.; Lee, C.-M.; Hung, C.-H.; Chen, C.-H.; Tseng, P.-L.; Chang, K.-C.; et al. MicroRNA-122 as a predictor of HBsAg seroclearance in hepatitis B and C dual infected patients treated with interferon and ribavirin. Sci. Rep. 2016, 6, 33816. [Google Scholar] [CrossRef]
- Bandopadhyay, M.; Sarkar, N.; Datta, S.; Das, D.; Pal, A.; Panigrahi, R.; Banerjee, A.; Panda, C.K.; Das, C.; Chakrabarti, S.; et al. Hepatitis B virus X protein mediated suppression of miRNA-122 expression enhances hepatoblastoma cell proliferation through cyclin G1-p53 axis. Infect. Agents Cancer 2016, 11, 40. [Google Scholar] [CrossRef][Green Version]
- Spaniel, C.; Honda, M.; Selitsky, S.R.; Yamane, D.; Shimakami, T.; Kaneko, S.; Lanford, R.E.; Lemon, S.M. microRNA-122 Abundance in hepatocellular carcinoma and non-tumor liver tissue from Japanese patients with persistent HCV versus HBV infection. PLoS ONE 2013, 8, 76867. [Google Scholar] [CrossRef]
- Koduru, S.V.; Leberfinger, A.N.; Kawasawa, Y.I.; Mahajan, M.; Gusani, N.J.; Sanyal, A.J.; Ravnic, D.J. Non-coding RNAs in Various Stages of Liver Disease Leading to Hepatocellular Carcinoma: Differential Expression of miRNAs, piRNAs, lncRNAs, circRNAs, and sno/mt-RNAs. Sci. Rep. 2018, 8, 7967. [Google Scholar] [CrossRef]
- Ma, L.; Song, H.; Zhang, C.-Y.; Hou, D. MiR-192-5p Ameliorates Hepatic Lipid Metabolism in Non-Alcoholic Fatty Liver Disease by Targeting Yy1. Biomolecules 2024, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, A.; Zollinger, L.; Saely, C.H.; Muendlein, A.; Evangelakos, I.; Nasias, D.; Charizopoulou, N.; Schofield, J.D.; Othman, A.; Soran, H.; et al. Circulating microRNAs-192 and-194 are associated with the presence and incidence of diabetes mellitus. Sci. Rep. 2018, 8, 14274. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.; Hu, T.; Lu, S.; Kuo, F.; Chen, C.; Wang, J.; Huang, C.; Lee, C.; Lin, C.; Yen, Y.; et al. Circulating microRNAs as biomarkers for diagnosis of early hepatocellular carcinoma associated with hepatitis B virus. Int. J. Cancer 2016, 138, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Zulian, V.; Fiscon, G.; Paci, P.; Garbuglia, A.R. Hepatitis B Virus and microRNAs: A Bioinformatics Approach. Int. J. Mol. Sci. 2023, 24, 17224. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wu, Z.; Yang, M.; Zuo, X.; Yin, G.; Chen, W. LMNB2 is a prognostic biomarker and correlated with immune infiltrates in hepatocellular carcinoma. IUBMB Life 2020, 72, 2672–2685. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Chen, D.; Yin, J.; Mo, Z.; Sun, B.; Yang, T.; Zhang, X.; Zhai, Z.; Li, Y.; et al. Comprehensive analysis of a TPX2-related TRHDE-AS1/PKIA ceRNA network involving prognostic signatures in Hepatitis B virus-infected hepatocellular carcinoma. Front. Cell. Infect. Microbiol. 2022, 12, 1025900. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, H.; Geng, Y.; Ji, Q. How Kinesin-1 Utilize the Energy of Nucleotide: The Conformational Changes and Mechanochemical Coupling in the Unidirectional Motion of Kinesin-1. Int. J. Mol. Sci. 2020, 21, 6977. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, Y.; Li, Y.; Shi, T.; Luan, Y.; Yin, C. Exosome and virus infection. Front. Immunol. 2023, 14, 1154217. [Google Scholar] [CrossRef]
- Chen, P.; Edelman, J.D.; Gharib, S.A. Comparative Evaluation of miRNA Expression between in Vitro and in Vivo Airway Epithelium Demonstrates Widespread Differences. Am. J. Pathol. 2013, 183, 1405–1410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sneller, L.; Lin, C.; Price, A.; Kottilil, S.; Chua, J.V. RNA Interference Therapeutics for Chronic Hepatitis B: Progress, Challenges, and Future Prospects. Microorganisms 2024, 12, 599. [Google Scholar] [CrossRef] [PubMed]



| Article Reference | Study Site | Article Type | Patient Counts | Patient Average Age | Identified microRNA | Detection Method | Correlation Assessment (between microRNAs and Viral Loads) | NOS Score * |
|---|---|---|---|---|---|---|---|---|
| Yu et al., 2015 [66] | China | Cross-sectional | CHB group: 115 HC: 20 | CHB group: 52.84 ± 3.82 HC: 49.99 ± 5.16 | miR-210 (serum samples) | RT-PCR | Spearman: r = 0.525 p < 0.001 | 7 |
| van der Ree et al., 2017 [67] | Nether-lands | Cohort | CHB group: 92 | Cohort 1: HBeAg positives- 35 ± 10 HBeAg negatives- 42 ± 12 Cohort 2: HBeAg positives- 35 ± 9 HBeAg negatives- 44 ± 10 | miR-122-5p miR-125b-5p miR-192-5p miR-193b-3p miR-194-5p miR-200a-3p miR-204-5p miR-29a-5p (plasma samples) | RT-PCR | Pearson: miR-122-5p- r = 0.468 p = 0.01 miR-125b-5p- r = 0.599 p < 0.01 miR-192-5p- r = 0.483 p < 0.01 miR-193b-3p- r = 0.422 p < 0.01 miR-194-5p- r = 0.585 p < 0.01 miR-200a-3p- r = 0.130 p > 0.05 miR-204-5p- r = −0.003 p > 0.05 miR-29a-5p- r = 0.228 p < 0.05 | 8 |
| Zhou et al., 2018 [68] | China | Cohort | CHB group HBeAg negative treated with NA: 66 | CHB group: 31.55 ± 10 | miR-125b (serum samples) | RT-PCR | Spearman: r = 0.353 p = 0.004 (baseline measurement) | 9 |
| Liu. et al., 2021 [69] | China | Cross-sectional | HBV group: 62 Controls: 11 | HBV carrier group: 30.29 ± 11.19 CHB group: 38 ± 10.81 Cirrhosis group: 43.29 ± 6.28 Control group: 27.45 ± 6.2 | miR-122 (serum samples) | RT-PCR | Spearman: r = 0.354 p = 0.005 | 7 |
| Tan et al., 2021 [70] | China | Cohort | CHB group with HBeAg seroconversion after treatment: 36 | CHB responsive group to treatment: 33.72 ± 10.47 CHB non-responsive group to treatment: 33.78 ± 8.72 | miR-122-5p miR-320a-3p (serum samples) | Sequencing technique | Spearman: miR-122-5p- r = 0.438 p = 0.008 Pearson: miR-320a-3p- r = −0.366 p = 0.028 | 8 |
| Li et al., 2022 [71] | China | Cross-sectional+ in vitro analysis | CHB group: 109 HC group: 20 | - | miR-192-3p (serum samples) | RT-PCR | Spearman: r = 0.37 p = 0.0002 | 7 |
| Nagura et al., 2022 [72] | Japan | Cohort | CHB group: 61 | CHB group: 36.06 ± 8.35 | miR-192-5p (serum samples) | RT-PCR | Pearson: baseline- r = 0.484 p < 0.001 Pearson: In week 24- r = 0.655 p < 0.001 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manea, M.; Mărunțelu, I.; Constantinescu, I. An In-Depth Approach to the Associations between MicroRNAs and Viral Load in Patients with Chronic Hepatitis B—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 8410. https://doi.org/10.3390/ijms25158410
Manea M, Mărunțelu I, Constantinescu I. An In-Depth Approach to the Associations between MicroRNAs and Viral Load in Patients with Chronic Hepatitis B—A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2024; 25(15):8410. https://doi.org/10.3390/ijms25158410
Chicago/Turabian StyleManea, Marina, Ion Mărunțelu, and Ileana Constantinescu. 2024. "An In-Depth Approach to the Associations between MicroRNAs and Viral Load in Patients with Chronic Hepatitis B—A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 25, no. 15: 8410. https://doi.org/10.3390/ijms25158410
APA StyleManea, M., Mărunțelu, I., & Constantinescu, I. (2024). An In-Depth Approach to the Associations between MicroRNAs and Viral Load in Patients with Chronic Hepatitis B—A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 25(15), 8410. https://doi.org/10.3390/ijms25158410





