Redox Chemistry: Implications for Necrotizing Enterocolitis
Abstract
:1. Introduction
2. Necrotizing Enterocolitis
3. Redox Chemistry
3.1. Reactive Oxygen Species
3.2. Reactive Nitrogen Species
4. Physiologic Redox Chemistry
4.1. Reactive Species Sources and Functions
4.2. Enzymatic and Non-Enzymatic Regulation
5. Redox Chemistry and Necrotizing Enterocolitis
5.1. Implications of Redox Chemistry in Necrotizing Enterocolitis
5.2. Risk Factors
6. Reducing Oxidative Stress and Necrotizing Enterocolitis Risk
7. Future Treatments and Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trinci, M.; Piccolo, C.L.; Pallottino, A.A.; Esposito, F.; Zeccolini, M.; Miele, V. Necrotizing Enterocolitis. In Imaging Non-Traumatic Abdominal Emergencies in Pediatric Patients; Miele, V., Trinci, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 53–72. [Google Scholar]
- Henry, M.C.W.; Moss, R.L. Necrotizing Enterocolitis. Annu. Rev. Med. 2009, 60, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, S.C.; Ching, Y.; Yu, D.; Carpenter, J.; Kenny, M.; Weldon, C.; Lillehei, C.; Valim, C.; Horbar, J.D.; Jaksic, T. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 2009, 44, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Walker, W.A. Necrotizing Enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Ozsurekci, Y.; Aykac, K. Oxidative Stress Related Diseases in Newborns. Oxidative Med. Cell. Longev. 2016, 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, M.; Rizzo, M.; Scaturro, G.; Pitruzzella, A.; Gammazza, A.M.; Cappello, F.; Corsello, G.; Li Volti, G. Oxidative stress markers at birth: Analyses of a neonatal population. Acta Histochem. 2015, 117, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.J.; Upperman, J.S.; Ford, H.R.; Camerini, V. Understanding the Susceptibility of the Premature Infant to Necrotizing Enterocolitis (NEC). Pediatr. Res. 2008, 63, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.H.; Hall, N.J. Contemporary Outcomes for Infants with Necrotizing Enterocolitis-A Systematic Review. J. Pediatr. 2020, 220, 86–92.e83. [Google Scholar] [CrossRef] [PubMed]
- Maayan-Metzger, A.; Avivi, S.; Schushan-Eisen, I.; Kuint, J. Human Milk Versus Formula Feeding Among Preterm Infants: Short-Term Outcomes. Am. J. Perinatol. 2012, 29, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Snyder, K.B.; Hunter, C.J. The leaky gut: A narrative review on the role of epithelial cell permeability in necrotizing enterocolitis. Pediatr. Med. 2023, 7. [Google Scholar] [CrossRef]
- Markel, T.A.; Crisostomo, P.R.; Wairiuko, G.M.; Pitcher, J.; Tsai, B.M.; Meldrum, D.R. Cytokines in Necrotizing Enterocolitis. Shock 2006, 25, 329–337. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, Y.; Liu, Y. miR-34a increases inflammation and oxidative stress levels in patients with necrotizing enterocolitis by downregulating SIRT1 expression. Mol. Med. Rep. 2021, 24, 664. [Google Scholar] [CrossRef]
- Chan, K.L.; Wong, K.F.; Luk, J.M. Role of LPS/CD14/TLR4-mediated inflammation in necrotizing enterocolitis: Pathogenesis and therapeutic implications. World J. Gastroenterol. 2009, 15, 4745–4752. [Google Scholar] [CrossRef] [PubMed]
- Leaphart, C.L.; Cavallo, J.; Gribar, S.C.; Cetin, S.; Li, J.; Branca, M.F.; Dubowski, T.D.; Sodhi, C.P.; Hackam, D.J. A Critical Role for TLR4 in the Pathogenesis of Necrotizing Enterocolitis by Modulating Intestinal Injury and Repair1. J. Immunol. 2007, 179, 4808–4820. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.X.; Rudloff, I.; Lao, J.C.; Pang, M.A.; Goldberg, R.; Bui, C.B.; McLean, C.A.; Stock, M.; Klassert, T.E.; Slevogt, H.; et al. Characterization of the pathoimmunology of necrotizing enterocolitis reveals novel therapeutic opportunities. Nat. Commun. 2020, 11, 5794. [Google Scholar] [CrossRef]
- Cai, X.; Liebe, H.L.; Golubkova, A.; Leiva, T.; Hunter, C.J. A Review of the Diagnosis and Treatment of Necrotizing Enterocolitis. Curr. Pediatr. Rev. 2023, 19, 285–295. [Google Scholar] [CrossRef]
- Niño, D.F.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 590–600. [Google Scholar] [CrossRef]
- Redox Reactions. Available online: https://www.wiley.com/college/boyer/0470003790/reviews/redox/redox.htm (accessed on 10 May 2024).
- Bayr, H. Reactive oxygen species. Crit. Care Med. 2005, 33, S498–S501. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P.; McAndrew, J.; Sellak, H.; White, C.R.; Jo, H.; Freeman, B.A.; Darley-Usmar, V.M. Biological aspects of reactive nitrogen species. Biochim. Et Biophys. Acta (BBA)—Bioenerg. 1999, 1411, 385–400. [Google Scholar] [CrossRef]
- Michel, T.; Vanhoutte, P.M. Cellular signaling and NO production. Pflügers Arch.—Eur. J. Physiol. 2010, 459, 807–816. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Tretter, V.; Hochreiter, B.; Zach, M.L.; Krenn, K.; Klein, K.U. Understanding Cellular Redox Homeostasis: A Challenge for Precision Medicine. Int. J. Mol. Sci. 2022, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Corkey, B.E.; Deeney, J.T. The Redox Communication Network as a Regulator of Metabolism. Front. Physiol. 2020, 11, 567796. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Bai, Y.; Zhang, Q.; Wong, V.; Floering, L.M.; Daniel, K.; Reece, J.M.; Deeney, J.T.; Andersen, M.E.; Corkey, B.E.; et al. Reactive Oxygen Species as a Signal in Glucose-Stimulated Insulin Secretion. Diabetes 2007, 56, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Kohchi, C.; Inagawa, H.; Nishizawa, T.; Soma, G.-I. ROS and Innate Immunity. Anticancer Res. 2009, 29, 817. [Google Scholar]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.C.; Andriantsitohaina, R. Reactive Nitrogen Species: Molecular Mechanisms and Potential Significance in Health and Disease. Antioxid. Redox Signal. 2008, 11, 669–702. [Google Scholar] [CrossRef]
- Liu, V.W.; Huang, P.L. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008, 77, 19–29. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. Selenoproteins *. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Nordberg, J.; Arnér, E.S.J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system1 1This review is based on the licentiate thesis “Thioredoxin reductase—Interactions with the redox active compounds 1-chloro-2,4-dinitrobenzene and lipoic acid” by Jonas Nordberg, 2001, Karolinska Institute, Stockholm, ISBN 91-631-1064-4. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004, 58, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Caplan, M.S.; Sun, X.-M.; Hsueh, W.; Hageman, J.R. Role of platelet-activating factor and tumor necrosis factor-alpha in neonatal necrotizing enterocolitis. J. Pediatr. 1990, 116, 960–964. [Google Scholar] [CrossRef]
- Edelson, M.B.; Bagwell, C.E.; Rozycki, H.J. Circulating Pro- and Counterinflammatory Cytokine Levels and Severity in Necrotizing Enterocolitis. Pediatrics 1999, 103, 766–771. [Google Scholar] [CrossRef]
- Anand, R.J.; Leaphart, C.L.; Mollen, K.P.; Hackam, D.J. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock 2007, 27, 124–133. [Google Scholar] [CrossRef]
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive Oxygen Species in the Immune System. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, C.; Dilli, D.; Uras, N.; Ulu, H.O.; Oguz, S.S.; Erdeve, O.; Dilmen, U. Total oxidant status and oxidative stress are increased in infants with necrotizing enterocolitis. J. Pediatr. Surg. 2011, 46, 2096–2100. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Tataranno, M.L.; Negro, S.; Cornacchione, S.; Longini, M.; Proietti, F.; Soubasi, V.; Benders, M.J.; Van Bel, F.; Buonocore, G. May oxidative stress biomarkers in cord blood predict the occurrence of necrotizing enterocolitis in preterm infants? J. Matern.-Fetal Neonatal Med. 2012, 25, 128–131. [Google Scholar] [CrossRef]
- Zamora, R.; Bryan, N.S.; Boyle, P.; Wong, C.; Milsom, A.B.; Jaffe, R.; Feelisch, M.; Ford, H.R. Nitrosative stress in an animal model of necrotizing enterocolitis. Free Radic. Biol. Med. 2005, 39, 1428–1437. [Google Scholar] [CrossRef]
- Singer, I.I.; Kawka, D.W.; Schloemann, S.; Tessner, T.; Riehl, T.; Stenson, W.F. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 1998, 115, 297–306. [Google Scholar] [CrossRef]
- Chung, D.H.; Ethridge, R.T.; Kim, S.; Owens-Stovall, S.; Hernandez, A.; Kelly, D.R.; Evers, B.M. Molecular Mechanisms Contributing to Necrotizing Enterocolitis. Ann. Surg. 2001, 233, 835–842. [Google Scholar] [CrossRef]
- Upperman, J.S.; Potoka, D.; Grishin, A.; Hackam, D.; Zamora, R.; Ford, H.R. Mechanisms of nitric oxide-mediated intestinal barrier failure in necrotizing enterocolitis. Semin. Pediatr. Surg. 2005, 14, 159–166. [Google Scholar] [CrossRef]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol.-Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef]
- Gow, A.J.; Duran, D.; Malcolm, S.; Ischiropoulos, H. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett. 1996, 385, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Rubbo, H.; Trostchansky, A.; O’Donnell, V.B. Peroxynitrite-mediated lipid oxidation and nitration: Mechanisms and consequences. Arch. Biochem. Biophys. 2009, 484, 167–172. [Google Scholar] [CrossRef]
- Boughton-Smith, N.K.; Evans, S.M.; Whittle, B.J.R.; Moncada, S.; Hawkey, C.J.; Cole, A.T.; Balsitis, M. Nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Lancet 1993, 342, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Ford, H.; Watkins, S.; Reblock, K.; Rowe, M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J. Pediatr. Surg. 1997, 32, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Nadler, E.P.; Upperman, J.S.; Dickinson, E.C.; Ford, H.R. Nitric Oxide and Intestinal Barrier Failure. Semin. Pediatr. Surg. 1999, 8, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Anatoly Grishin, P.T.D.; Wang, J.; Mallicote, M.; Nguyen, M.; Philippe-Auguste, M.; Gayer, C.P.; Ford, H.R. Necrotizing Enterocolitis—Pathogenesis, Diagnosis and Treatment. In Necrotizing Enterocolitis, 1st ed.; Hackam, D.J., Ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Clark, D.A.; Fornabaio, D.M.; McNeill, H.; Mullane, K.M.; Caravella, S.J.; Miller, M.J. Contribution of oxygen-derived free radicals to experimental necrotizing enterocolitis. Am. J. Pathol. 1988, 130, 537–542. [Google Scholar] [PubMed]
- Nadler, E.P.; Dickinson, E.; Knisely, A.; Zhang, X.-R.; Boyle, P.; Beer-Stolz, D.; Watkins, S.C.; Ford, H.R. Expression of Inducible Nitric Oxide Synthase and Interleukin-12 in Experimental Necrotizing Enterocolitis. J. Surg. Res. 2000, 92, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Wedgwood, S.; Steinhorn, R.H. Role of Reactive Oxygen Species in Neonatal Pulmonary Vascular Disease. Antioxid. Redox Signal. 2013, 21, 1926–1942. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, D.H.; Coran, A.G. Perioperative Nutritional Support in Pediatrics. Nutrition 1998, 14, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Nguyen, M. Oxygen Therapy for Neonatal Resuscitation in the Delivery Room. NeoReviews 2019, 20, e500–e512. [Google Scholar] [CrossRef] [PubMed]
- Perez-de-Sa, V.; Cunha-Goncalves, D.; Nordh, A.; Hansson, S.; Larsson, A.; Ley, D.; Fellman, V.; Werner, O. High Brain Tissue Oxygen Tension During Ventilation With 100% Oxygen After Fetal Asphyxia in Newborn Sheep. Pediatr. Res. 2009, 65, 57–61. [Google Scholar] [CrossRef]
- Chen, Y.; Koike, Y.; Chi, L.; Ahmed, A.; Miyake, H.; Li, B.; Lee, C.; Delgado-Olguín, P.; Pierro, A. Formula feeding and immature gut microcirculation promote intestinal hypoxia, leading to necrotizing enterocolitis. Dis. Models Mech. 2019, 12, dmm040998. [Google Scholar] [CrossRef]
- Emerit, J.; Beaumont, C.; Trivin, F. Iron metabolism, free radicals, and oxidative injury. Biomed. Pharmacother. 2001, 55, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, A.J.; Mannion, T.; Lynch, S.M.; Frei, B. The effect of iron overload on rat plasma and liver oxidant status in vivo. Biochem. J. 1994, 300, 799–803. [Google Scholar] [CrossRef]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Laborie, S.; Lavoie, J.-C.; Chessex, P. Paradoxical Role of Ascorbic Acid and Riboflavin in Solutions of Total Parenteral Nutrition: Implication in Photoinduced Peroxide Generation. Pediatr. Res. 1998, 43, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Reiter, R.J.; Karbownik, M.; Tan, D.-X.; Gitto, P.; Barberi, S.; Barberi, I. Causes of Oxidative Stress in the Pre- and Perinatal Period. Biol. Neonate 2002, 81, 146–157. [Google Scholar] [CrossRef]
- Saugstad, O.D. Oxidative Stress in the Newborn—A 30-Year Perspective. Biol. Neonate 2005, 88, 228–236. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Davis, J.M.; Auten, R.L. Maturation of the antioxidant system and the effects on preterm birth. Semin. Fetal Neonatal Med. 2010, 15, 191–195. [Google Scholar] [CrossRef]
- Frank, L.; Ilene Sosenko, R.S. Development of lung antioxidant enzyme system in late gestation: Possible implications for the prematurely born infant. J. Pediatr. 1987, 110, 9–14. [Google Scholar] [CrossRef]
- Aceti, A.; Beghetti, I.; Martini, S.; Faldella, G.; Corvaglia, L. Oxidative Stress and Necrotizing Enterocolitis: Pathogenetic Mechanisms, Opportunities for Intervention, and Role of Human Milk. Oxidative Med. Cell. Longev. 2018, 2018, 7397659. [Google Scholar] [CrossRef]
- Ezaki, S.; Ito, T.; Suzuki, K.; Tamura, M. Association between Total Antioxidant Capacity in Breast Milk and Postnatal Age in Days in Premature Infants. J. Clin. Biochem. Nutr. 2008, 42, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Lyden, E.; Furtado, J.; Van Ormer, M.; Anderson-Berry, A. A Comparison of Nutritional Antioxidant Content in Breast Milk, Donor Milk, and Infant Formulas. Nutrients 2016, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Fulton, W.B.; Good, M.; Vurma, M.; Das, T.; Lai, C.-S.; Jia, H.; Yamaguchi, Y.; Lu, P.; Prindle, T.; et al. Fat composition in infant formula contributes to the severity of necrotizing enterocolitis. Br. J. Nutr. 2018, 120, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Lin, J. Too much short-chain fatty acids cause neonatal necrotizing enterocolitis. Med. Hypotheses 2004, 62, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Caplan, M.S.; Russell, T.; Xiao, Y.; Amer, M.; Kaup, S.; Jilling, T. Effect of Polyunsaturated Fatty Acid (PUFA) Supplementation on Intestinal Inflammation and Necrotizing Enterocolitis (NEC) in a Neonatal Rat Model. Pediatr. Res. 2001, 49, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Pozo, S.; Parra-Llorca, A.; Lara-Cantón, I.; Solaz, A.; García-Jiménez, J.L.; Pallardó, F.V.; Vento, M. Oxygen in the neonatal period: Oxidative stress, oxygen load and epigenetic changes. Semin. Fetal Neonatal Med. 2020, 25, 101090. [Google Scholar] [CrossRef] [PubMed]
- Vento, M. Oxygen Supplementation in the Neonatal Period: Changing the Paradigm. Neonatology 2014, 105, 323–331. [Google Scholar] [CrossRef]
- Reiter, R.J.; Paredes, S.D.; Manchester, L.C.; Tan, D.-X. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Aversa, S.; Reiter, R.J.; Antonuccio, P.; Centorrino, A.; Romeo, C.; Impellizzeri, P.; Gitto, E. Oxidative Stress-Mediated Damage in Newborns with Necrotizing Enterocolitis: A Possible Role of Melatonin. Am. J. Perinatol. 2015, 32, 905–909. [Google Scholar] [CrossRef]
- Costa, S.; Giannantonio, C.; Romagnoli, C.; Vento, G.; Gervasoni, J.; Persichilli, S.; Zuppi, C.; Cota, F. Effects of lutein supplementation on biological antioxidant status in preterm infants: A randomized clinical trial. J. Matern. Fetal Neonatal Med. 2013, 26, 1311–1315. [Google Scholar] [CrossRef]
- Guven, A.; Gundogdu, G.; Vurucu, S.; Uysal, B.; Oztas, E.; Ozturk, H.; Korkmaz, A. Medical ozone therapy reduces oxidative stress and intestinal damage in an experimental model of necrotizing enterocolitis in neonatal rats. J. Pediatr. Surg. 2009, 44, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, H.; Jiang, M.-Z.; Ohta, N.; Sato, S.; Tamura, S.; Hiraoka, M.; Maeda, M.; Mayumi, M. Oxidative stress in neonates: Evaluation using specific biomarkers. Life Sci. 2004, 75, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathol. Int. 1999, 49, 91–102. [Google Scholar] [CrossRef]
- Tsukahara, H. Oxidative Stress Biomarkers: Current Status and Future Perspective. In Studies on Pediatric Disorders; Tsukahara, H., Kaneko, K., Eds.; Springer: New York, NY, USA, 2014; pp. 87–113. [Google Scholar]
- Milne, G.L.; Yin, H.; Brooks, J.D.; Sanchez, S.; Jackson Roberts, L.; Morrow, J.D. Quantification of F2-Isoprostanes in Biological Fluids and Tissues as a Measure of Oxidant Stress. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2007; Volume 433, pp. 113–126. [Google Scholar]
- Bautista, G.M.; Cera, A.J.; Chaaban, H.; McElroy, S.J. State-of-the-art review and update of in vivo models of necrotizing enterocolitis. Front. Pediatr. 2023, 11, 1161342. [Google Scholar] [CrossRef] [PubMed]
- Mahe, M.M.; Sundaram, N.; Watson, C.L.; Shroyer, N.F.; Helmrath, M.A. Establishment of human epithelial enteroids and colonoids from whole tissue and biopsy. J. Vis. Exp. 2015, 97, e52483. [Google Scholar] [CrossRef]
- Ares, G.J.; Buonpane, C.; Yuan, C.; Wood, D.; Hunter, C.J.; Hunter, C.J. A Novel Human Epithelial Enteroid Model of Necrotizing Enterocolitis. JoVE (J. Vis. Exp.) 2019, 146, e59194. [Google Scholar] [CrossRef]
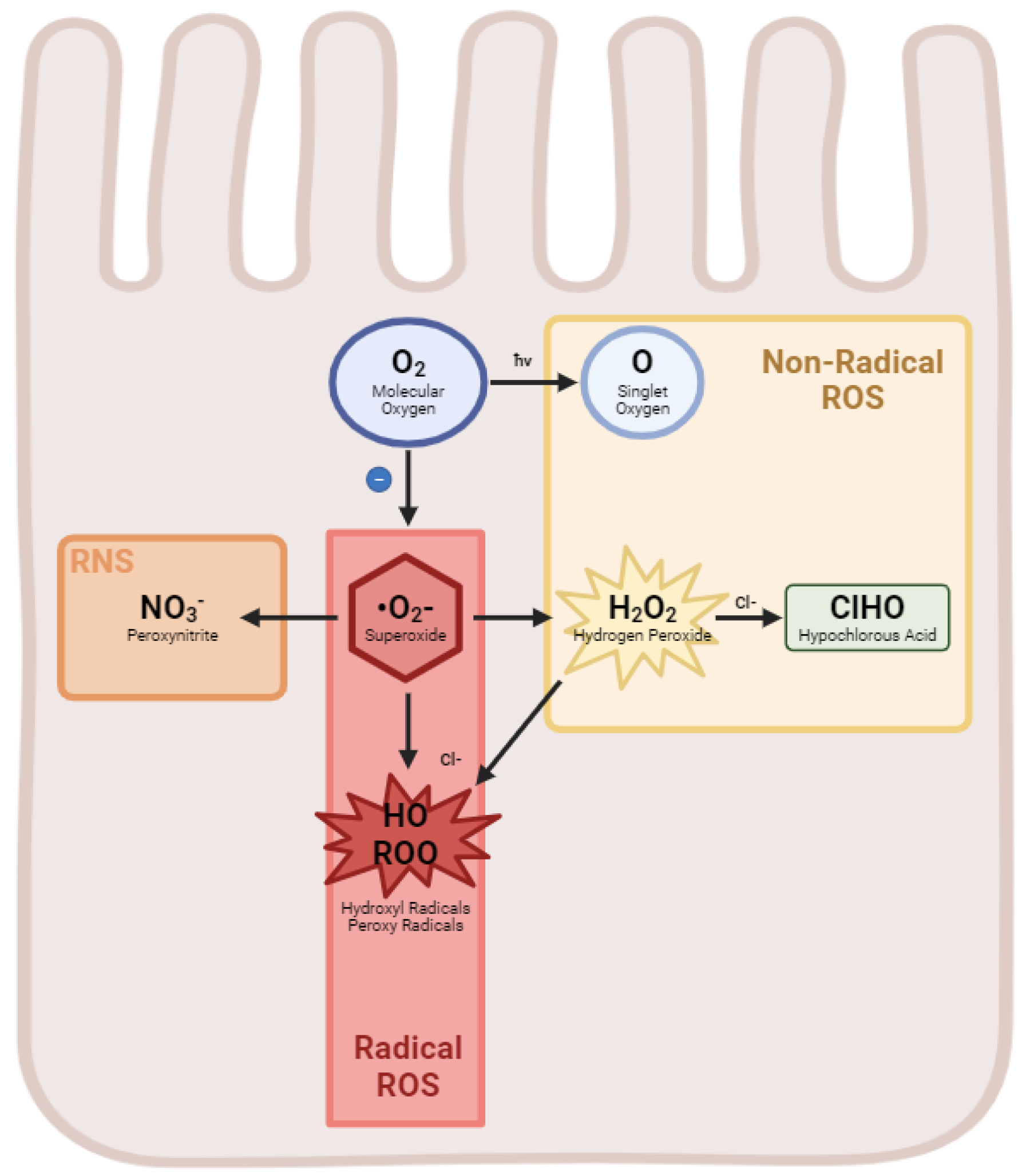

| Reactive Oxygen Species | Formula |
|---|---|
| Hydrogen Peroxide | H2O2 |
| Superoxide anion | °O2− |
| Hydroxyl Radical | HO |
| Singlet oxygen | O |
| Ozone | O3 |
| Peroxyl radical | ROO |
| Hypochlorous acid | ClHO |
| Reactive Nitrogen Species | |
| Nitric oxide | NO |
| Peroxynitrite anion | NO3− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gershner, G.H.; Hunter, C.J. Redox Chemistry: Implications for Necrotizing Enterocolitis. Int. J. Mol. Sci. 2024, 25, 8416. https://doi.org/10.3390/ijms25158416
Gershner GH, Hunter CJ. Redox Chemistry: Implications for Necrotizing Enterocolitis. International Journal of Molecular Sciences. 2024; 25(15):8416. https://doi.org/10.3390/ijms25158416
Chicago/Turabian StyleGershner, Grant H., and Catherine J. Hunter. 2024. "Redox Chemistry: Implications for Necrotizing Enterocolitis" International Journal of Molecular Sciences 25, no. 15: 8416. https://doi.org/10.3390/ijms25158416






