Marine Polyphenols in Cardiovascular Health: Unraveling Structure–Activity Relationships, Mechanisms, and Therapeutic Implications
Abstract
:1. Introduction
2. Marine Polyphenolic Compounds: Diversity and Sources
2.1. Chemical Classification
2.1.1. Flavonoids
2.1.2. Non-Flavanoids
2.2. Extraction, Isolation, and Purification of Marine Polyphenolic Compounds
2.3. Structural Analysis of Marine Polyphenolic Compounds
3. Marine Polyphenolic Compounds against Cardiovascular Diseases
3.1. Marine Polyphenolic Compounds with Anti-Hypertensive Activities
3.1.1. Angiotensin-Converting Enzyme-Inhibitory Activity
3.1.2. Modulation of Lipid Metabolism
3.1.3. Vasorelaxant Properties
3.2. Marine Polyphenolic Compounds with Antioxidant Activities and Their Potential against Cardiovascular Diseases
3.3. Marine Polyphenolic Compounds with Anti-Inflammatory Activities and Their Potential against Cardiovascular Diseases
3.4. Metabolic Benefits of Marine Polyphenolic Compounds and Their Impact on Cardiovascular Diseases
4. Clinical and Pre-Clinical Evidence
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaziano, T.; Reddy, K.S.; Paccaud, F.; Horton, S.; Chaturvedi, V. Disease Control Priorities in Developing Countries, 2nd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M.; Milstein, A. Contributions of Health Care to Longevity: A Review of 4 Estimation Methods. Ann. Fam. Med. 2019, 17, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob. Food Secur. 2017, 12, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Xie, C.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. The Quest for Phenolic Compounds from Seaweed: Nutrition, Biological Activities and Applications. Food Rev. Int. 2022, 39, 5786–5813. [Google Scholar] [CrossRef]
- Liyanage, N.M.; Nagahawatta, D.P.; Jayawardena, T.U.; Jeon, Y.J. The Role of Seaweed Polysaccharides in Gastrointestinal Health: Protective Effect against Inflammatory Bowel Disease. Life 2023, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Nagahawatta, D.P.; Lu, Y.-A.; Yang, H.-W.; Je, J.-G.; Kim, S.-Y.; Jeon, Y.-J. Ishige okamurae and diphloroethohydoxycarmalol inhibit palmitic acid-impaired skeletal myogenesis and improve muscle regenerative potential. J. Funct. Foods 2021, 87, 104832. [Google Scholar] [CrossRef]
- Jayawardhana, H.H.A.C.K.; Lee, H.-G.; Liyanage, N.M.; Nagahawatta, D.P.; Ryu, B.; Jeon, Y.-J. Structural characterization and anti-inflammatory potential of sulfated polysaccharides from Scytosiphon lomentaria; attenuate inflammatory signaling pathways. J. Funct. Foods 2023, 102, 105446. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. An emerging trend in functional foods for the prevention of cardiovascular disease and diabetes: Marine algal polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1342–1358. [Google Scholar] [CrossRef]
- Yordi, E.G.; Pérez, E.M.; Matos, M.J.; Villares, E.U. Antioxidant and pro-oxidant effects of polyphenolic compounds and structure-activity relationship evidence. In Nutritional Science and Health, 1st ed.; IntechOpen: London, UK, 2012; pp. 23–48. [Google Scholar]
- Cai, Y.Z.; Mei, S.; Jie, X.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Nagahawatta, D.P.; Kim, H.S.; Jee, Y.H.; Jayawardena, T.U.; Ahn, G.; Namgung, J.; Yeo, I.K.; Sanjeewa, K.K.A.; Jeon, Y.J. Sargachromenol Isolated from Sargassum horneri Inhibits Particulate Matter-Induced Inflammation in Macrophages through Toll-like Receptor-Mediated Cell Signaling Pathways. Mar. Drugs 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.; Jayawardena, T.U.; Lee, H.G.; Heo, M.S.; Jeon, Y.J. Eckmaxol Isolated from Ecklonia maxima Attenuates Particulate-Matter-Induced Inflammation in MH-S Lung Macrophage. Mar. Drugs 2022, 20, 766. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Kim, H.-S.; Lee, H.-G.; Jayawardena, T.U.; Nagahawatta, D.P.; Yang, H.-W.; Udayanga, D.; Kim, J.-I.; Jeon, Y.-J. 3-Hydroxy-5,6-epoxy-β-ionone Isolated from Invasive Harmful Brown Seaweed Sargassum Horneri Protects MH-S Mouse Lung Cells from Urban Particulate Matter-Induced Inflammation. Appl. Sci. 2021, 11, 10929. [Google Scholar] [CrossRef]
- Rodríguez, P.F.; Murillo-González, L.; Rodríguez, E.; Pérez, A.M. Marine phenolic compounds: Sources, commercial value, and biological activities. In Marine Phenolic Compounds, 1st ed.; Springer: Cham, Switzerland, 2023; pp. 47–86. [Google Scholar]
- Nagahawatta, D.P.; Liyanage, N.M.; Je, J.G.; Jayawardhana, H.; Jayawardena, T.U.; Jeong, S.H.; Kwon, H.J.; Choi, C.S.; Jeon, Y.J. Polyphenolic Compounds Isolated from Marine Algae Attenuate the Replication of SARS-CoV-2 in the Host Cell through a Multi-Target Approach of 3CL(pro) and PL(pro). Mar. Drugs 2022, 20, 786. [Google Scholar] [CrossRef] [PubMed]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardena, T.U.; Jayawardhana, H.; Jeong, S.H.; Kwon, H.J.; Jeon, Y.J. Role of marine natural products in the development of antiviral agents against SARS-CoV-2: Potential and prospects. Mar. Life Sci. Technol. 2024, 6, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Gager, L.; Lalegerie, F.; Connan, S.; Stiger-Pouvreau, V. Marine algal derived phenolic compounds and their biological activities for medicinal and cosmetic applications. In Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives, 1st ed.; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 278–334. [Google Scholar]
- Jayawardhana, H.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Liyanage, N.M.; Nagahawatta, D.P.; Lee, H.G.; Kim, J.I.; Jeon, Y.J. Marine Algal Polyphenols as Skin Protective Agents: Current Status and Future Prospectives. Mar. Drugs 2023, 21, 285. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Perez-Correa, J.R.; Dominguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Felix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.; Kim, M.; Son, K.T.; Jeong, Y.; Jeon, Y.J. Antioxidant Activity of Marine Algal Polyphenolic Compounds: A Mechanistic Approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef]
- Martins, B.T.; Correia da Silva, M.; Pinto, M.; Cidade, H.; Kijjoa, A. Marine natural flavonoids: Chemistry and biological activities. Nat. Prod. Res. 2019, 33, 3260–3272. [Google Scholar] [CrossRef]
- Arora, V.; Sharma, N.; Tarique, M.; Vyas, G.; Sharma, R.B. An Overview of Flavonoids: A Diverse Group of Bioactive Phytoconstituents. Curr. Tradit. Med. 2023, 9, 1–12. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Rajabiyan, A.; Nabizade, N.; Meygoli Nezhad, N.; Zarei-Ahmady, A. Seaweed-derived phenolic compounds as diverse bioactive molecules: A review on identification, application, extraction and purification strategies. Int. J. Biol. Macromol. 2024, 266, 131147. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenco-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef] [PubMed]
- De Luca, D.; Lauritano, C. In Silico Identification of Type III PKS Chalcone and Stilbene Synthase Homologs in Marine Photosynthetic Organisms. Biology 2020, 9, 110. [Google Scholar] [CrossRef]
- Jayatilake, G.S.; Baker, B.J.; McClintock, J.B. Isolation and Identification of a Stilbene Derivative from the Antarctic Sponge Kirkpatrickia variolosa. J. Nat. Prod. 2004, 58, 1958–1960. [Google Scholar] [CrossRef]
- Lu, P.; Wang, W.; Zhang, G.; Li, W.; Jiang, A.; Cao, M.; Zhang, X.; Xing, K.; Peng, X.; Yuan, B.; et al. Isolation and characterization marine bacteria capable of degrading lignin-derived compounds. PLoS ONE 2020, 15, e0240187. [Google Scholar] [CrossRef]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed]
- Matulja, D.; Vranjesevic, F.; Kolympadi Markovic, M.; Pavelic, S.K.; Markovic, D. Anticancer Activities of Marine-Derived Phenolic Compounds and Their Derivatives. Molecules 2022, 27, 1449. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S. Preface. In Natural Products From Marine Algae; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2015; Volume 1308, pp. v–vii. [Google Scholar]
- Michalak, I. Experimental processing of seaweeds for biofuels. WIREs Energy Environ. 2018, 7, e288. [Google Scholar] [CrossRef]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging Technologies for the Extraction of Marine Phenolics: Opportunities and Challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on alpha-amylase, alpha-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, S.; Wang, Z.; Wang, C.; Wang, E.; Zhang, Y.; Liu, J. Supercritical fluid extraction of flavonoids from Maydis stigma and its nitrite-scavenging ability. Food Bioprod. Process. 2011, 89, 333–339. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Rajauria, G. Optimization and validation of reverse phase HPLC method for qualitative and quantitative assessment of polyphenols in seaweed. J. Pharm. Biomed. Anal. 2018, 148, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Lojková, L.; Vlcek, J. Hyphenated Solid Phase Extraction/Supercritical Fluid Extraction Methods for Extraction of Phenolic Compounds from Algae. Curr. Anal. Chem. 2013, 10, 86–98. [Google Scholar] [CrossRef]
- Gager, L.; Connan, S.; Molla, M.; Couteau, C.; Arbona, J.-F.; Coiffard, L.; Cérantola, S.; Stiger-Pouvreau, V. Active phlorotannins from seven brown seaweeds commercially harvested in Brittany (France) detected by 1H NMR and in vitro assays: Temporal variation and potential valorization in cosmetic applications. J. Appl. Phycol. 2020, 32, 2375–2386. [Google Scholar] [CrossRef]
- Sang, V.T.; Hung, N.D.; Se-kwon, K. Pharmaceutical properties of marine polyphenols: An overview. ACTA Pharm. Sci. 2019, 57, 217. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.K. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-C.; Kang, M.C.; Kang, N.; Kim, H.-S.; Lee, S.-H.; Ahn, G.; Jung, W.-K.; Jeon, Y.-J. Effect of angiotensin I-converting enzyme (ACE) inhibition and nitric oxide (NO) production of 6,6′-bieckol, a marine algal polyphenol and its anti-hypertensive effect in spontaneously hypertensive rats. Process Biochem. 2017, 58, 326–332. [Google Scholar] [CrossRef]
- Muradian, K.; Vaiserman, A.; Min, K.J.; Fraifeld, V.E. Fucoxanthin and lipid metabolism: A minireview. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ko, J.Y.; Samarakoon, K.; Oh, J.Y.; Heo, S.J.; Kim, C.Y.; Nah, J.W.; Jang, M.K.; Lee, J.S.; Jeon, Y.J. Preparative isolation of sargachromanol E from Sargassum siliquastrum by centrifugal partition chromatography and its anti-inflammatory activity. Food Chem. Toxicol. 2013, 62, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Tuorkey, M.J. Solar ultraviolet radiation from cancer induction to cancer prevention: Solar ultraviolet radiation and cell biology. Eur. J. Cancer Prev. 2015, 24, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, R.; Santhanam, R.; Suleria, H. Biology and Ecology of Pharmaceutical Marine Plants; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Birringer, M.; Siems, K.; Maxones, A.; Frank, J.; Lorkowski, S. Natural 6-hydroxy-chromanols and -chromenols: Structural diversity, biosynthetic pathways and health implications. RSC Adv. 2018, 8, 4803–4841. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure-function and mechanisms of action and opportunities for drug development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef]
- Hakim, M.M.; Patel, I.C. A review on phytoconstituents of marine brown algae. Future J. Pharm. Sci. 2020, 6, 129. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity—A review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.R.; Jung, H.A.; Choi, J.S. Phlorotannins with Potential Anti-tyrosinase and Antioxidant Activity Isolated from the Marine Seaweed Ecklonia stolonifera. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Lee, Y.; Kim, S.Y.; Kim, H.S.; Joo, H.G.; Park, J.W.; et al. Eckol isolated from Ecklonia cava attenuates oxidative stress induced cell damage in lung fibroblast cells. FEBS Lett. 2005, 579, 6295–6304. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Karadeniz, F.; Kim, M.M.; Kim, S.K. α-Glucosidase and α-amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. J. Sci. Food Agric. 2009, 89, 1552–1558. [Google Scholar] [CrossRef]
- Yuan, Y.; Zheng, Y.; Zhou, J.; Geng, Y.; Zou, P.; Li, Y.; Zhang, C. Polyphenol-Rich Extracts from Brown Macroalgae Lessonia trabeculate Attenuate Hyperglycemia and Modulate Gut Microbiota in High-Fat Diet and Streptozotocin-Induced Diabetic Rats. J. Agric. Food Chem. 2019, 67, 12472–12480. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Luo, J.; Wu, N.; Zhang, R.; Shi, D. BPN, a marine-derived PTP1B inhibitor, activates insulin signaling and improves insulin resistance in C2C12 myotubes. Int. J. Biol. Macromol. 2018, 106, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hou, Y.; Xie, M.; Ma, W.; Shi, D.; Jiang, B. CYC31, A Natural Bromophenol PTP1B Inhibitor, Activates Insulin Signaling and Improves Long Chain-Fatty Acid Oxidation in C2C12 Myotubes. Mar. Drugs 2020, 18, 267. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.H.; Paudel, P.; Jung, H.A.; Choi, J.S. Identifying Phlorofucofuroeckol-A as a Dual Inhibitor of Amyloid-beta(25-35) Self-Aggregation and Insulin Glycation: Elucidation of the Molecular Mechanism of Action. Mar. Drugs 2019, 17, 600. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, S.M.; Ko, S.C.; Kang, M.C.; Jeon, Y.J. Octaphlorethol A, a novel phenolic compound isolated from Ishige foliacea, protects against streptozotocin-induced pancreatic beta cell damage by reducing oxidative stress and apoptosis. Food Chem. Toxicol. 2013, 59, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-A.; Kang, M.-C.; Lee, J.-H.; Kang, N.; Lee, W.; Oh, J.-Y.; Yang, H.-W.; Lee, J.-S.; Jeon, Y.-J. Protective effect of marine brown algal polyphenols against oxidative stressed zebrafish with high glucose. RSC Adv. 2015, 5, 25738–25746. [Google Scholar] [CrossRef]
- Yan, X.; Yang, C.; Lin, G.; Chen, Y.; Miao, S.; Liu, B.; Zhao, C. Antidiabetic Potential of Green Seaweed Enteromorpha prolifera Flavonoids Regulating Insulin Signaling Pathway and Gut Microbiota in Type 2 Diabetic Mice. J. Food Sci. 2019, 84, 165–173. [Google Scholar] [CrossRef]
- Shibata, T.; Yamaguchi, K.; Nagayama, K.; Kawaguchi, S.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against glycosidases from the viscera of the turban shellTurbo cornutus. Eur. J. Phycol. 2002, 37, 493–500. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, H.S.; Lee, W.; Han, E.J.; Kim, S.Y.; Fernando, I.P.S.; Ahn, G.; Kim, K.N. Eckol from Ecklonia cava ameliorates TNF-alpha/IFN-gamma-induced inflammatory responses via regulating MAPKs and NF-kappaB signaling pathway in HaCaT cells. Int. Immunopharmacol. 2020, 82, 106146. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Son, M.; Lee, H.S.; Kim, H.S.; Jeon, Y.J.; Byun, K. Protective Effect of Pyrogallol-Phloroglucinol-6,6-Bieckol from Ecklonia cava on Monocyte-Associated Vascular Dysfunction. Mar. Drugs 2018, 16, 441. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Bang, M.H.; Jeon, H.J.; Hwang, T.; Yang, S.A. Anti-inflammatory and Anti-allergic Effects of Phlorofucofuroeckol A and Dieckol Isolated from Ecklonia cava. J. Life Sci. 2018, 28, 1170–1178. [Google Scholar]
- Jun, Y.J.; Lee, M.; Shin, T.; Yoon, N.; Kim, J.H.; Kim, H.R. Eckol enhances heme oxygenase-1 expression through activation of Nrf2/JNK pathway in HepG2 cells. Molecules 2014, 19, 15638–15652. [Google Scholar] [CrossRef]
- Lee, J.W.; Seok, J.K.; Boo, Y.C. Ecklonia cava Extract and Dieckol Attenuate Cellular Lipid Peroxidation in Keratinocytes Exposed to PM10. Evid. Based Complement. Altern. Med. 2018, 2018, 8248323. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. In Nineteenth International Seaweed Symposium: Proceedings of the 19th International Seaweed Symposium, Kobe, Japan, 26–31 March 2007; Borowitzka, M.A., Critchley, A.T., Kraan, S., Peters, A., Sjøtun, K., Notoya, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 243–252. [Google Scholar]
- Zou, Y.; Qian, Z.J.; Li, Y.; Kim, M.M.; Lee, S.H.; Kim, S.K. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Lee, M.S.; Lee, E.W.; Kim, Y.M.; Kim, T.H. Pancreatic lipase inhibitory activity of phlorotannins isolated from Eisenia bicyclis. Phytother. Res. 2013, 27, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Wu, Y.X.; Kim, J.S.; Woo, J.H.; Park, K.T.; Kwon, O.J.; Seo, H.J.; Kim, T.; Park, N.H. 6,6’-Bieckol inhibits adipocyte differentiation through downregulation of adipogenesis and lipogenesis in 3T3-L1 cells. J. Sci. Food Agric. 2015, 95, 1830–1837. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPalpha and PPARgamma. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef]
- Oh, S.; Son, M.; Choi, J.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. Phlorotannins from Ecklonia cava Attenuates Palmitate-Induced Endoplasmic Reticulum Stress and Leptin Resistance in Hypothalamic Neurons. Mar. Drugs 2019, 17, 570. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Jeon, H.J.; Lee, O.H.; Lee, B.Y. Dieckol, a major phlorotannin in Ecklonia cava, suppresses lipid accumulation in the adipocytes of high-fat diet-fed zebrafish and mice: Inhibition of early adipogenesis via cell-cycle arrest and AMPKalpha activation. Mol. Nutr. Food Res. 2015, 59, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-C.; Jung, W.-K.; Kang, S.-M.; Lee, S.-H.; Kang, M.C.; Heo, S.-J.; Kang, K.-H.; Kim, Y.-T.; Park, S.-J.; Jeong, Y.; et al. Angiotensin I-converting enzyme (ACE) inhibition and nitric oxide (NO)-mediated antihypertensive effect of octaphlorethol A isolated from Ishige sinicola: In vitro molecular mechanism and in vivo SHR model. J. Funct. Foods 2015, 18, 289–299. [Google Scholar] [CrossRef]
- Lu, Y.A.; Jiang, Y.; Yang, H.W.; Hwang, J.; Jeon, Y.J.; Ryu, B. Diphlorethohydroxycarmalol Isolated from Ishige okamurae Exerts Vasodilatory Effects via Calcium Signaling and PI3K/Akt/eNOS Pathway. Int. J. Mol. Sci. 2021, 22, 1610. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Hyun, S.K.; Kim, H.R.; Choi, J.S. Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fish. Sci. 2006, 72, 1292–1299. [Google Scholar] [CrossRef]
- Son, M.; Oh, S.; Lee, H.S.; Ryu, B.; Jiang, Y.; Jang, J.T.; Jeon, Y.J.; Byun, K. Pyrogallol-Phloroglucinol-6,6’-Bieckol from Ecklonia cava Improved Blood Circulation in Diet-Induced Obese and Diet-Induced Hypertension Mouse Models. Mar. Drugs 2019, 17, 272. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.; Ko, S.C.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on angiotensin I-converting enzyme (ACE) inhibitory activity. Nutr. Res. Pract. 2011, 5, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mei-Mei, Z.; Ke-Wu, Z.J.T.M.R. Marine natural products with anti-inflammation effects. Tradit. Med. Res. 2020, 5, 252. [Google Scholar]
- Degl’Innocenti, D.; Vasarri, M. Marine Anti-Inflammatory Agents 2020, 1st ed.; MDPI: Basel, Switzerland, 2021. [Google Scholar]
- Sobhani, M.; Farzaei, M.H.; Kiani, S.; Khodarahmi, R. Immunomodulatory; Anti-inflammatory/antioxidant Effects of Polyphenols: A Comparative Review on the Parental Compounds and Their Metabolites. Food Rev. Int. 2020, 37, 759–811. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Bonham, M.P.; Ryan, L. Do marine algal polyphenols have antidiabetic, antihyperlipidemic or anti-inflammatory effects in humans? A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2039–2054. [Google Scholar] [CrossRef]
- Poss, J.; Custodis, F.; Werner, C.; Weingartner, O.; Bohm, M.; Laufs, U. Cardiovascular disease and dyslipidemia: Beyond LDL. Curr. Pharm. Des. 2011, 17, 861–870. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuniga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Vita, J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005, 81, 292S–297S. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Guzman, M.; Rodriguez-Nogales, A.; Algieri, F.; Galvez, J. Potential Role of Seaweed Polyphenols in Cardiovascular-Associated Disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Jeon, Y.-J. Screening for Angiotensin 1-Converting Enzyme Inhibitory Activity of Ecklonia cava. Prev. Nutr. Food Sci. 2005, 10, 134–139. [Google Scholar] [CrossRef]
- Cha, S.-H.; Lee, K.-W.; Jeon, Y.-J. Screening of extracts from red algae in Jeju for potentials MarineAngiotensin-I converting enzyme (ACE) inhibitory activity. Algae 2006, 21, 343–348. [Google Scholar] [CrossRef]
- Liu, J.C.; Hsu, F.L.; Tsai, J.C.; Chan, P.; Liu, J.Y.; Thomas, G.N.; Tomlinson, B.; Lo, M.Y.; Lin, J.Y. Antihypertensive effects of tannins isolated from traditional Chinese herbs as non-specific inhibitors of angiontensin converting enzyme. Life Sci. 2003, 73, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Park, B.G.; Shin, W.S.; Oh, S.; Park, G.M.; Kim, N.I.; Lee, S. A novel antihypertension agent, sargachromenol D from marine brown algae, Sargassum siliquastrum, exerts dual action as an L-type Ca(2+) channel blocker and endothelin A/B(2) receptor antagonist. Bioorg. Med. Chem. 2017, 25, 4649–4655. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Kim, H.-S.; Sanjeewa, K.K.A.; Kim, S.-Y.; Rho, J.-R.; Jee, Y.; Ahn, G.; Jeon, Y.-J. Sargassum horneri and isolated 6-hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one (HTT); LPS-induced inflammation attenuation via suppressing NF-κB, MAPK and oxidative stress through Nrf2/HO-1 pathways in RAW 264.7 macrophages. Algal Res. 2019, 40, 101513. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Asanka Sanjeewa, K.K.; Shanura Fernando, I.P.; Ryu, B.M.; Kang, M.C.; Jee, Y.; Lee, W.W.; Jeon, Y.J. Sargassum horneri (Turner) C. Agardh ethanol extract inhibits the fine dust inflammation response via activating Nrf2/HO-1 signaling in RAW 264.7 cells. BMC Complement. Altern. Med. 2018, 18, 249. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Ahn, G.; Kim, H.-J.; Fu, X.; Jee, Y.; Jeon, Y.-J. Ethanol extract separated from Sargassum horneri (Turner) abate LPS-induced inflammation in RAW 264.7 macrophages. Fish. Aquat. Sci. 2019, 22, 6. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Li, J.; Yuan, F.; Li, M.; Zhang, Q.; Huang, Y.Y.; Pang, J.Y.; Zhang, B.; Sun, F.Y.; Sun, H.S.; et al. Xyloketal B attenuates atherosclerotic plaque formation and endothelial dysfunction in apolipoprotein e deficient mice. Mar. Drugs 2015, 13, 2306–2326. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Yang, Z.; Yoon, B.; He, Y.; Uhm, S.; Shin, H.C.; Lee, B.H.; Yoo, Y.C.; Lee, K.B.; Han, S.Y.; et al. Blood-brain barrier-permeable fluorone-labeled dieckols acting as neuronal ER stress signaling inhibitors. Biomaterials 2015, 61, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kwon, O.; Kim, J.Y. Supplementation of a polyphenol extract from Ecklonia cava reduces body fat, oxidative and inflammatory stress in overweight healthy subjects with abdominal obesity: A randomized, placebo-controlled, double-blind trial. J. Funct. Foods 2018, 46, 356–364. [Google Scholar] [CrossRef]
- Almutairi, M.G.; Aldubayan, K.; Molla, H. Effect of seaweed (Ecklonia cava extract) on blood glucose and insulin level on prediabetic patients: A double-blind randomized controlled trial. Food Sci. Nutr. 2023, 11, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef]
- Hata, Y.; Nakajima, K.; Uchida, J.-i.; Hidaka, H.; Nakano, T. Clinical Effects of Brown Seaweed, Undaria pinnatifida (wakame), on Blood Pressure in Hypertensive Subjects. J. Clin. Biochem. Nutr. 2001, 30, 43–53. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. The Impact of a Single Dose of a Polyphenol-Rich Seaweed Extract on Postprandial Glycaemic Control in Healthy Adults: A Randomised Cross-Over Trial. Nutrients 2018, 10, 270. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Zhao, W.; Subbiah, V.; Xie, C.; Yang, Z.; Shi, L.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Bioaccessibility and Bioavailability of Phenolic Compounds in Seaweed. Food Rev. Int. 2022, 39, 5729–5760. [Google Scholar] [CrossRef]
- Hanuka Katz, I.; Eran Nagar, E.; Okun, Z.; Shpigelman, A. The Link between Polyphenol Structure, Antioxidant Capacity and Shelf-Life Stability in the Presence of Fructose and Ascorbic Acid. Molecules 2020, 25, 225. [Google Scholar] [CrossRef] [PubMed]
- van het Hof, K.H.; Kivits, G.A.; Weststrate, J.A.; Tijburg, L.B. Bioavailability of catechins from tea: The effect of milk. Eur. J. Clin. Nutr. 1998, 52, 356–359. [Google Scholar] [CrossRef]
- Haminiuk, C.W.; Plata-Oviedo, M.S.; de Mattos, G.; Carpes, S.T.; Branco, I.G. Extraction and quantification of phenolic acids and flavonols from Eugenia pyriformis using different solvents. J. Food Sci. Technol. 2014, 51, 2862–2866. [Google Scholar] [CrossRef] [PubMed]
- Trobo-Maseda, L.; Romero-Fernandez, M.; Guisan, J.M.; Rocha-Martin, J. Glycosylation of polyphenolic compounds: Design of a self-sufficient biocatalyst by co-immobilization of a glycosyltransferase, a sucrose synthase and the cofactor UDP. Int. J. Biol. Macromol. 2023, 250, 126009. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Pichler, A.; Simunovic, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Sakane, T.; Harada, M.; Sugiura, N.; Koda, H.; Kiso, Y.; Sezaki, H. Absorption and metabolism of antioxidative polyphenolic compounds in red wine. Ann. N. Y. Acad. Sci. 2002, 957, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Cáceres-Jiménez, S.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Pereira-Caro, G. Bioaccessibility and bioavailability of marine polyphenols. In Marine Phenolic Compounds, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 265–298. [Google Scholar]
- Sanna, V.; Pala, N.; Sechi, M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar]
- Chen, L.; Gnanaraj, C.; Arulselvan, P.; El-Seedi, H.; Teng, H. A review on advanced microencapsulation technology to enhance bioavailability of phenolic compounds: Based on its activity in the treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Oladimeji, F.A.; Adegbola, A.J.; Onyeji, C.O. Appraisal of Bioenhancers in Improving Oral Bioavailability: Applications to Herbal Medicinal Products. J. Pharm. Res. Int. 2018, 24, 1–23. [Google Scholar] [CrossRef]
- Wen, H.; Jung, H.; Li, X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Kyselova, Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip. Toxicol. 2011, 4, 173–183. [Google Scholar] [CrossRef]
- Zeisel, S.H. Precision (Personalized) Nutrition: Understanding Metabolic Heterogeneity. Annu. Rev. Food Sci. Technol. 2020, 11, 71–92. [Google Scholar] [CrossRef] [PubMed]
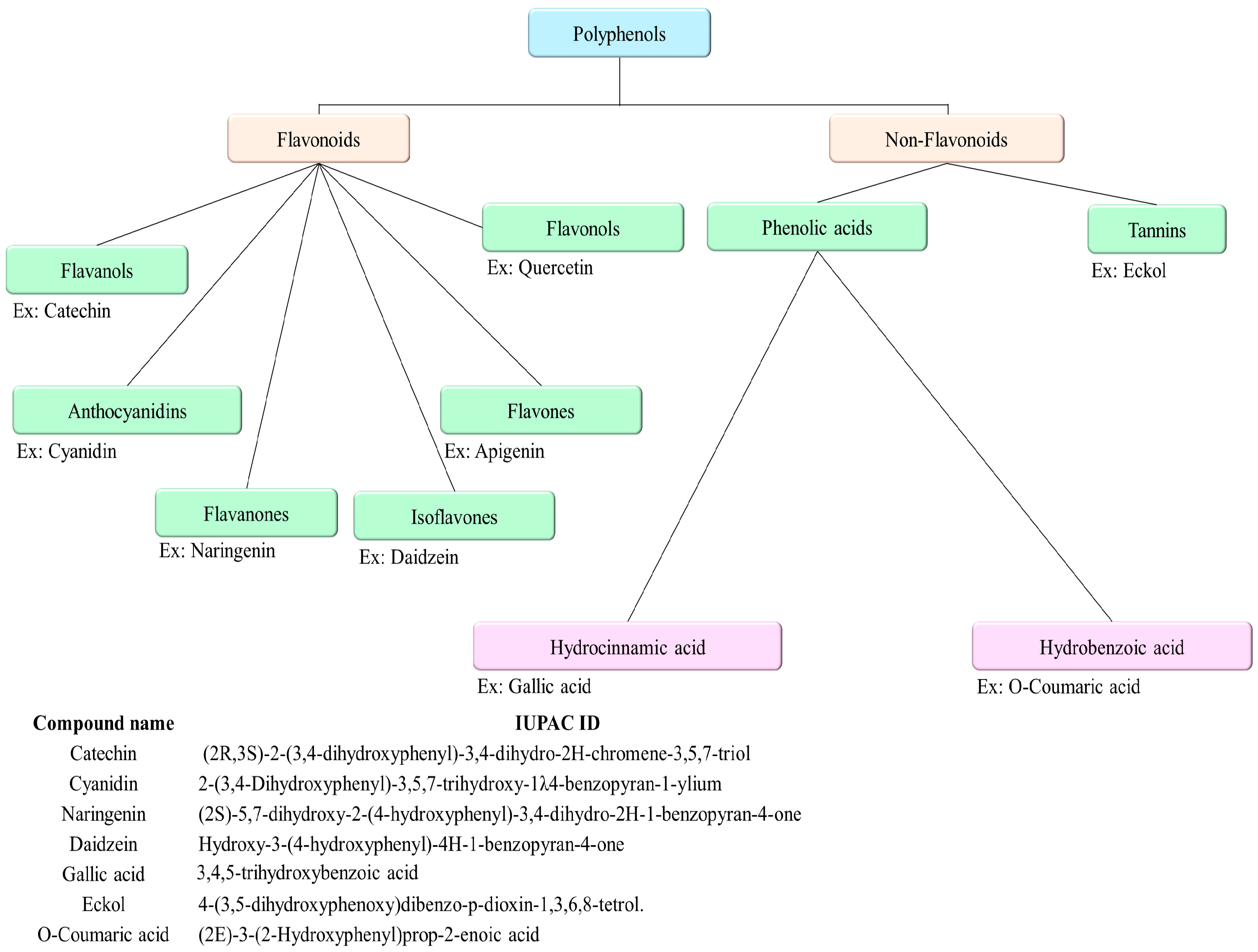


| Group | Structure | Structural Characterization | Common Source | Primary Bioactivity |
|---|---|---|---|---|
| Flavones | 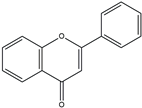 | Double bond between C2 and C3, no hydroxyl group at C3 | Marine plants | Antioxidant |
| Flavonols |  | Hydroxyl group at C3 | Seaweeds | Anti-inflammatory Cardioprotective |
| Flavanones |  | No double bond between C2 and C3 (saturated C chain) | Various marine algae | Antioxidant Anti-inflammatory |
| Isoflavones | 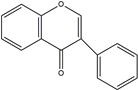 | B ring attached at C3 position | Marine fungi and bacteria | Estrogenic Anticancer |
| Anthocyanidins |  | Glycosylated forms of anthocyanins | Some marine plants | Antioxidant |
| Flavanols | 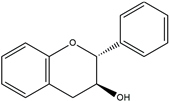 | Catechins, often found as part of larger tannin molecules | Green algae, seaweeds | Antioxidant, anti-carcinogenic |
| Group | Structural Characterization | Common Source | Primary Bioactivity |
|---|---|---|---|
| Phenolic Acids | C6-C1 structure, presence of carboxylic acid group and hydroxyl groups on an aromatic ring | Seaweeds, marine fungi | Antioxidant, radical scavenging |
| Tannins | High molecular weight, ability to bind and precipitate proteins | Marine plants, algae | Antioxidant |
| Stilbenes | C6-C2-C6 structure, 1,2-diphenylethylene nucleus | Mangroves, marine sponges | Anti-inflammatory, cardioprotective |
| Lignans | C6-C3-C6 structure, dimerization of two phenylpropanoid units | Marine algae, seagrasses | Antioxidant, anti-cancer |
| Phlorotannins | Polymers of phloroglucinol units, varying degrees of polymerization | Brown algae | Antioxidant, anti-inflammatory |
| Technique | Purpose | Description | Advantages | Disadvantages |
|---|---|---|---|---|
| High-Performance Liquid Chromatography (HPLC) | Separation, Identification, Quantification | Utilized for its precision in handling complex samples, essential for polyphenols. | High resolution and sensitivity; suitable for a wide range of polyphenols. | Can be time-consuming; requires derivatization for some compounds. |
| Gas Chromatography (GC) | Separation | Powerful for analyzing volatile and semi-volatile polyphenolic compounds when coupled with MS (GC-MS). | Good for volatile compounds; high resolution. | Not suitable for high molecular weight or non-volatile compounds. |
| Nuclear Magnetic Resonance (NMR) Spectroscopy | Structural elucidation | Provides detailed information about the molecular framework and spatial arrangement of atoms. | Non-destructive; provides detailed structural information. | Requires large sample amounts; less sensitive compared to MS. |
| Mass Spectrometry (MS) | Molecular weight determination | Determines molecular weight and structural features, crucial for structural elucidation. | Highly sensitive and accurate; provides molecular fingerprints. | Complex data analysis; high cost of equipment. |
| Ultraviolet-visible (UV-Vis) Spectroscopy | Absorbance study | Used to study the absorbance of polyphenols, correlating with structure and concentration. | Simple and quick; good for concentration determination. | Limited structural information; interference from other absorbing species. |
| Fourier-Transform Infrared (FTIR) Spectroscopy | Functional group analysis | Provides information on the functional groups within the polyphenolic compounds based on their infrared absorption spectra. | Non-destructive; no need for sample preparation. | Lower sensitivity; can be challenging to interpret overlapping peaks. |
| Capillary Electrophoresis (CE) | Separation, Identification | Separates compounds based on their charge and size under an electric field. | High efficiency and resolution; minimal sample requirement. | Limited to charged or chargeable analytes; lower sensitivity for non-UV absorbing compounds. |
| Liquid Chromatography-Mass Spectrometry (LC-MS) | Structural elucidation, Quantification | Combines the separation power of LC with the mass analysis capabilities of MS. | Provides detailed structural information; high sensitivity and selectivity. | Requires skilled operation; high cost of maintenance. |
| High-Resolution Mass Spectrometry (HRMS) | Structural elucidation | Offers accurate mass measurements for precise molecular formula determination. | High accuracy and resolution; useful for complex mixtures. | Expensive; requires expertise for data interpretation. |
| Primary Bioactivity | Description | Potential Impact on Cardiovascular Diseases | Example | Source |
|---|---|---|---|---|
| Antioxidant properties | Scavenge free radicals and protect cells from oxidative damage. | Prevents cardiovascular diseases by reducing oxidative stress. | Phlorotannins | Ecklonia cava (brown algae) |
| Anti-inflammatory effects | Modulate inflammatory responses. | Reduces the risk of atherosclerosis and endothelial dysfunction. | Eckol | Ecklonia stolonifera (brown algae) |
| Anti-hypertensive effects | Contribute to blood pressure regulation. | Manages hypertension, a major risk factor for cardiovascular diseases. | Dieckol | Ecklonia cava (brown algae) |
| Anti-hyperlipidemia effects | Regulate lipid metabolism and maintain healthy cholesterol levels. | Reduces the risk of hyperlipidemia-related heart diseases. | Fucoxanthin | Undaria pinnatifida (brown algae) |
| Anti-obesity effects | Aid in weight management and prevent obesity-related complications. | Lowers cardiovascular risks linked to obesity. | Fucosterol | Various brown algae species |
| Cardioprotective effects | Protect against ischemic heart disease and myocardial infarction. | Provides protection against various cardiac conditions. | Xyloketal B | Marine fungi species |
| Metabolic benefits | Improve insulin sensitivity and glucose metabolism. | Reduces the risk of type 2 diabetes and metabolic syndrome. | Sargachromanol G | Sargassum siliquastrum (brown algae) |
| Prevention of cardiac fibrosis | Help prevent cardiac fibrosis associated with heart failure. | Aids in preventing conditions that can lead to heart failure. | Saringosterol | Sargassum muticum (brown algae) |
| Disease Type Related to CVDs | Source | MPCs | Assay Type | Activity | Ref. |
|---|---|---|---|---|---|
| Diabetes mellitus | E. cava | Fucodiphloroethol G Dieckol 6,6′-bieckol 7-phloroeckol Phlorofucofuroeckol-A | In vitro | Inhibition of α-glucosidase IC50 values Dieckol: 10.79 μM 7-phloroeckol: 49.49 μM PC—NA Inhibition of α-α amylase Dieckol: 125 μM 7-phloroeckol: 250 μM Other compounds: <500 μM PC—NA | [58] |
| Lessonia trabeculate | Polyphenol-rich extracts | In vitro | Inhibition of α-glucosidase and lipase activities IC50 value <0.25 mg/mL PC—Acarbose—<0.25 mg/mL PC—Orlistat < 0.25 mg/mL | [59] | |
| F. vesiculosus | Crude extract and semi-purified phlorotannins composed by fucols, fucophlorethols, fuhalols, other phlorotannin derivatives | In vitro | Inhibition of α-amylase IC50 value 28.8–2.8 μg/mL PC—Acarbose—0.7 μg/mL Inhibition of α-glucosidase IC50 value 4.5–0.82 μg/mL PC—Acarbose—206.6 μg/mL Inhibition of pancreatic lipase IC50 value 45.9–19.0 μg/mL PC—Orlistat—1.8 μg/mL | [60] | |
| Rhodomela confervoides | 3,4-dibromo-5-(2-bromo-3,4-dihydroxy-6-(ethoxymethyl)benzyl)benzene-1,2-diol) | In vitro | Inhibition of PTP1B activity IC50 value 0.84 μM Improve insulin sensitivity by activating insulin signaling pathways IC50 value 0.1–0.5 μM PC—NA | [61] | |
| Rhodomela confervoides | 3-Bromo-4,5-bis(2,3-dibromo-4,5-dihydroxybenzyl)-1,2-benzenediol | In vitro | Inhibition of PTP1B activity IC50 value 2 μM PC—NAActivation of insulin signaling and prevent palmitate-induced insulin resistance. IC50 value 0.5–2 μM PC—NA | [62] | |
| E. stolonifera | Phlorofucofuroeckol-A | In vitro | Inhibition of AGEs formation IC50 value D-ribose-induced insulin glycation: 29.5 μM PC—vanillin—>500 μM D-glucose-induced insulin glycation: 43.55 μM PC—rutin—5.19 μM | [63] | |
| Ishige foliacea | Octaphlorethol A | In vitro | Decreased the death of STZ-treated pancreatic β-cellsDecreased the TBARS and ROSIncreased the activity of antioxidant enzymes IC50 value 12.5–50.0 μg/mL PC—NA | [64] | |
| E. cava | 6,6-Bieckol, Phloroeckol Dieckol Phlorofucofuroeckol | In vivo | Inhibition of high glucose-induced ROS and cell deathDieckol reduced heart rates, ROS, NO, and lipid peroxidationDieckol reduced the overexpression of iNOS and COX-2 IC50 value 10–20 μM PC—NA AM—zebrafish larvae (n = 15) | [65] | |
| Ulva prolifera | Extract rich in flavonoids | In vivo | Diminished the fasting blood glucose and improved oral glucose toleranceHypoglycemic effect by increasing IRS1/PI3K/Akt and suppressing JNK1/2 in liver IC50 value 150 mg/kg/day for 4 weeks by gavage PC—NA AM—Kunming male Icr mice (n = 6) | [66] | |
| Eisenia bicyclis | Phloroglucinol, Phloroglucinol tetramer, Eckol Phlorofucofuroeckol A Dieckol 8,8′-bieckol | In vitro | Inhibit β-galactosidase, and β-mannosidase activity PC—NA | [67] | |
| Anti-inflammation/anti-oxidant | Ecklonia cava | Eckol | In vitro | Inhibit the expression of TNF-α/IFN-γ-induced inflammatory responses via regulating MAPKs and NF-κB signaling pathway in HaCaT cells PC—NA | [68] |
| Dieckol | In vitro and in vivo | Regulate HO-1/Nrf-2 expression in LPS-stimulated macrophages and a periodontitis rat model PC—NA | [45] | ||
| 6,6′-Bieckol | Inhibit monocyte-associated vascular dysfunction PC—NA | [69] | |||
| Phlorofucofuroeckol- A | In vitro | Inhibit pro-inflammatory responses in LPS-induced RAW 264.7 macrophages PC—Wortmannin—500 nM | [70] | ||
| Ecklonia maxima | Eckmaxol | In vitro | Inhibit pro-inflammatory responses in particulate matter-induced lung macrophages PC—NA | [14] | |
| Sargassum horneri | Sargachromenol | In vitro | Inhibit particulate matter-induced TLR expressions | [13] | |
| Ecklonia stolonifera | Eckol | In vitro | Regulate HO-1/Nrf-2 and inhibit intercellular reactive oxygen species PC—NA | [71] | |
| Ecklonia cava | Dieckol | In vitro | Attenuate cellular lipid peroxidation in keratinocytes exposed to PM10 PC—NA | [72] | |
| Japanese Laminariaceae | 1,3,5-trihydroxybenzene Eckol Phlorofucofuroeckol A Dieckol 8,8′-bieckol | In vitro | Inhibition of phospholipid peroxidation in the liposome system PC—NA | [73] | |
| Ishige okamurae | Diphlorethohydroxycarmalol 6,6′-bieckol | In vitro | Inhibit reactive oxygen species in free radical mediate oxidative system PC-α-tocopherol—45.3 μM | [74] | |
| Obesity | Eisenia bicyclis | Eckol, Fucofuroeckol A 7-phloroeckol Dioxindehydroeckol Phlorofucofuroeckol A Dieckol | In vitro | Inhibit pancreatic lipase activity IC50 value Eckol: 76.6 μM Fucofuroeckol A: 37.2 μM 7-phloroeckol1: 2.7 μM Dioxindehydroeckol: >200 μM Phlorofucofuroeckol A: >200 μM Dieckol: 99.3 μM PC—Orlistat—0.7 μM | [75] |
| 6,6′-bieckol 6,8′-bieckol 8,8′-bieckol Dieckol Phlorofucofuroeckol-A | In vitro | Inhibit lipid accumulation and adipogenesis by downregulating differentiation of 3T3-L1 adipocytes PC—NA | [76] | ||
| Ecklonia stolonifera | Phloroglucinol, Eckol Dieckol Dioxinodehydroeckol Plorofucofuroeckol A | In vitro | Inhibition of lipid accumulation by suppression adipocyte differentiation through inhibiting C/EBPα and PPARγ expression PC—NA | [77] | |
| Ecklonia cava | Dieckol 2,7-phloroglucinol-6 6-bieckol Progallol-Ploroglucinol-6,6-bieckol Phlorofucofuroeckol A | In vitro | Inhibit the leptin resistance PC—NA | [78] | |
| Dieckol | In vitro and in vivo | Inhibit lipid accumulation and adipogenesis by regulating AMPKα, ERK, and AKT signaling in high-fat diet-fed zebrafish, mice, and 3T3-L1 models PC-Curcumin 2.5 μM AM—mouse (n = 10) and zebrafish larvae (n = 20) | [79] | ||
| Hypertension | Ishige sinicola | Octaphlorethol A | In vitro | Inhibition of hypertension by inhibiting ACE activity and regulating AMPK and Akt activation IC50 value 59 μM | [80] |
| Ishige okamurae | Diphlorethohydroxycarmalol | In vitro and in vivo | Inhibit hypertension through vasodilation PC—Captopril 10 mg/kg body weight AM: Spontaneously hypertensive rats | [81] | |
| Ecklonia stolonifera | Phloroglucinol Eckstolonol Eckol Phlorofucofuroeckol A Dieckol Triphlorethol-A Fucosterol | In vitro | Inhibit hypertension by inhibiting ACE activity IC50 values Phloroglucinol: N.A. Eckstolonol: 410.12 μM Eckol: 70.82 μM Phlorofucofuroeckol A: 12.74 μM Dieckol: 34.25 μM Triphlorethol-A: 700.9 μM Fucosterol: N.A. PC—captopril 1.63–3.26 ng/mL | [82] | |
| Ecklonia cava | Pyrogallol-phloroglucinol-6,6′-bieckol | In vitro and in vivo | Inhibit hypertension by improving blood circulation PC—Ginkgo biloba extract | [83] | |
| 6,6′-Bieckol | In vitro | Inhibit hypertension by regulating ACE activity IC50 value 0.42 mM PC—captopril 20 mg/kg body weight AM—Spontaneously hypertensive rats | [47] | ||
| Phloroglucinol Triphlorethol-A Eckol Dieckol Eckstolonol | In vitro | Inhibit hypertension by regulating ACE activity IC50 values Phloroglucinol: 2.57 mM Triphlorethol-A: 2.01 mM Eckol: 2.27 mM Dieckol: 1.47 mM Eckstolonol: 2.95 mM PC—NA | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagahawatta, D.P.; Liyanage, N.M.; Jayawardena, T.U.; Jeon, Y.-J. Marine Polyphenols in Cardiovascular Health: Unraveling Structure–Activity Relationships, Mechanisms, and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 8419. https://doi.org/10.3390/ijms25158419
Nagahawatta DP, Liyanage NM, Jayawardena TU, Jeon Y-J. Marine Polyphenols in Cardiovascular Health: Unraveling Structure–Activity Relationships, Mechanisms, and Therapeutic Implications. International Journal of Molecular Sciences. 2024; 25(15):8419. https://doi.org/10.3390/ijms25158419
Chicago/Turabian StyleNagahawatta, D. P., N. M. Liyanage, Thilina U. Jayawardena, and You-Jin Jeon. 2024. "Marine Polyphenols in Cardiovascular Health: Unraveling Structure–Activity Relationships, Mechanisms, and Therapeutic Implications" International Journal of Molecular Sciences 25, no. 15: 8419. https://doi.org/10.3390/ijms25158419





