Identification and Functional Studies on the Role of PlSPL14 in Herbaceous Peony Stem Development
Abstract
:1. Introduction
2. Results
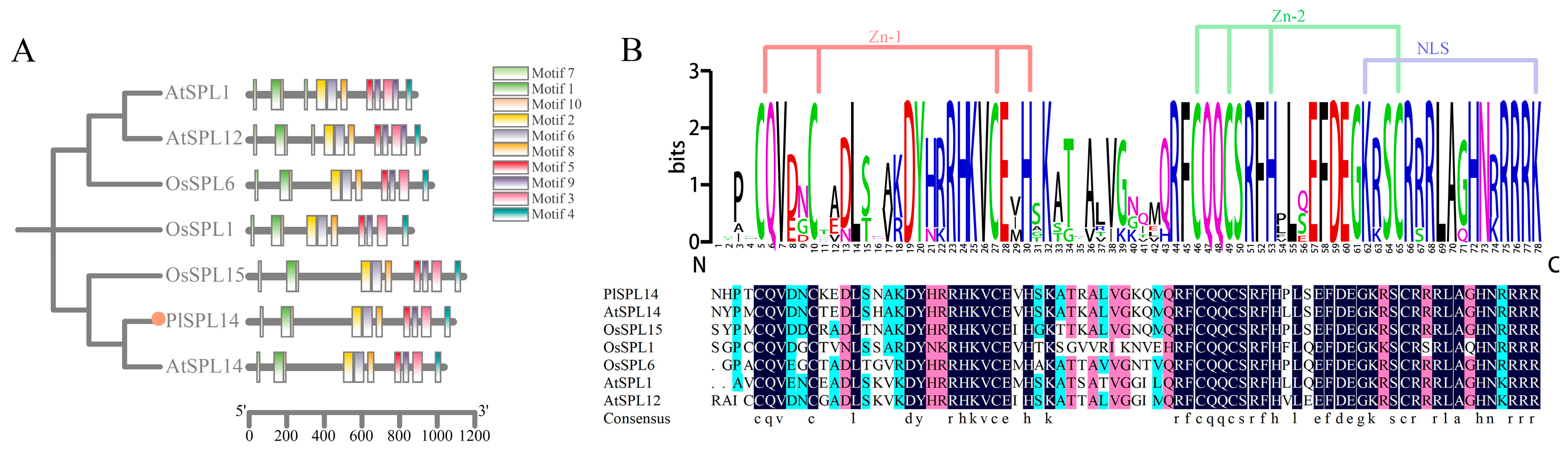
2.1. Identification and Phylogenetic Analysis of PlSPL14
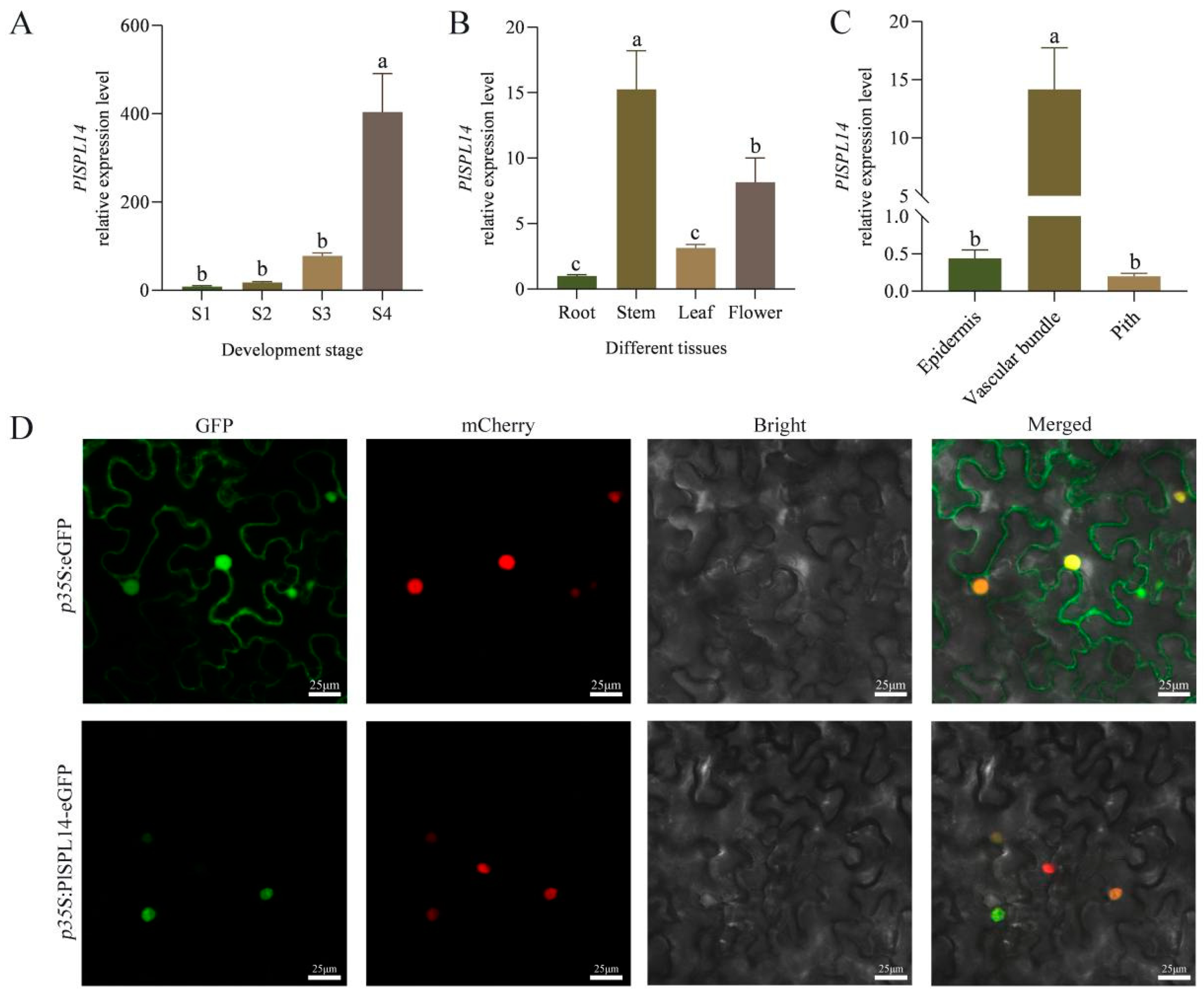
2.2. Expression and Characteristic Analysis of PlSPL14
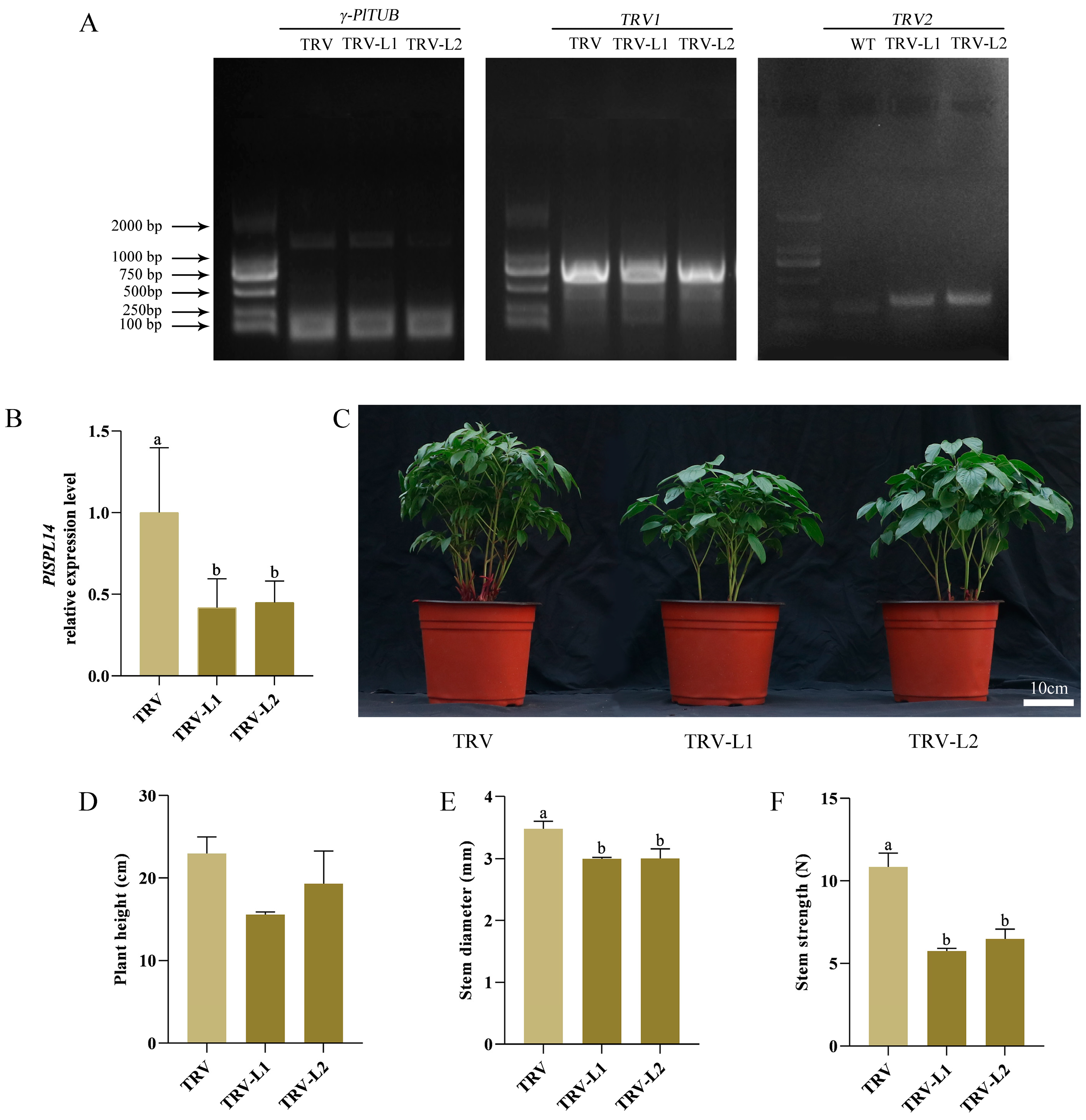
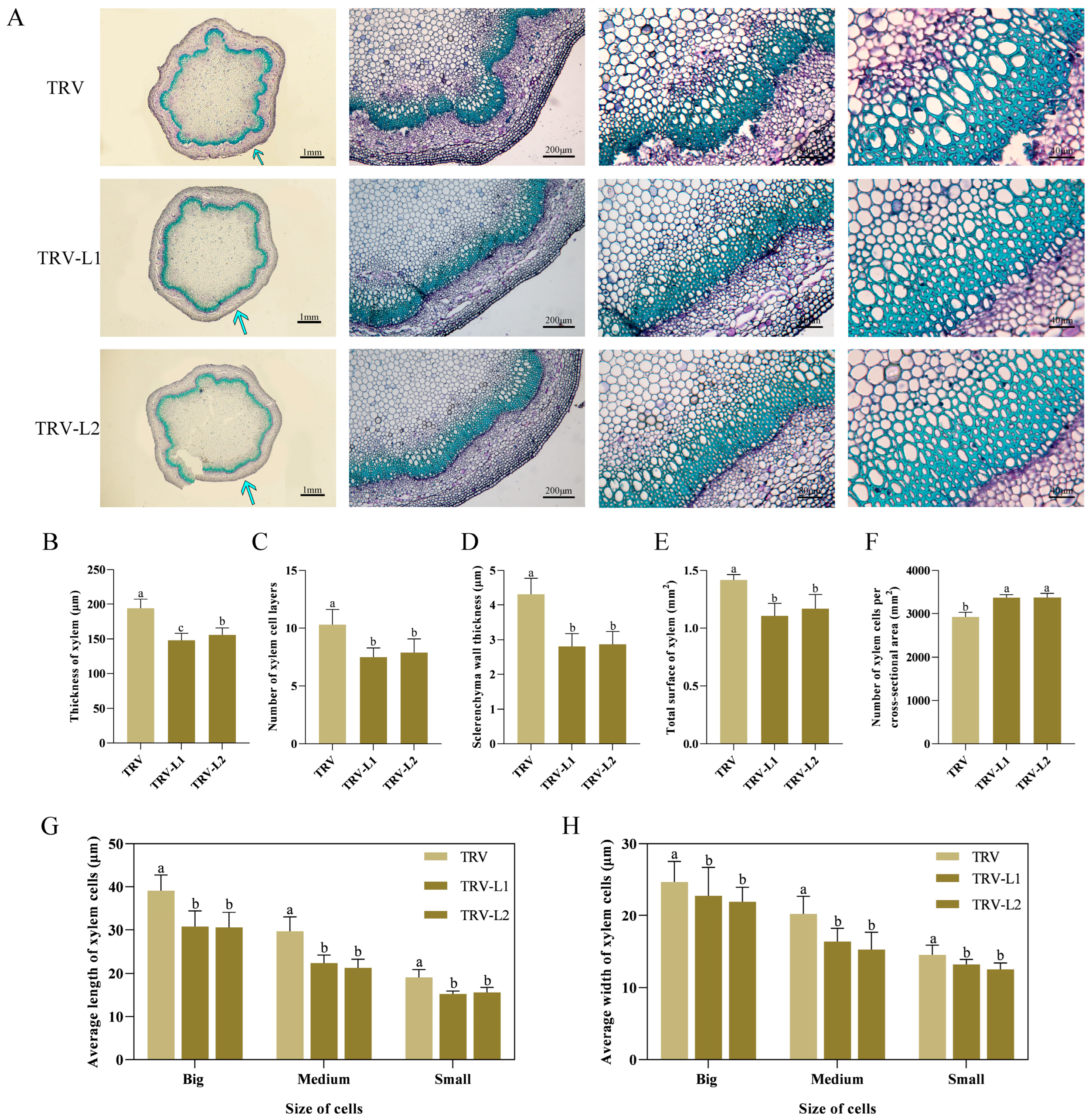
2.3. Silencing PlSPL14 in P. lactiflora Negatively Regulates Stem Development by Attenuating Stem Strength
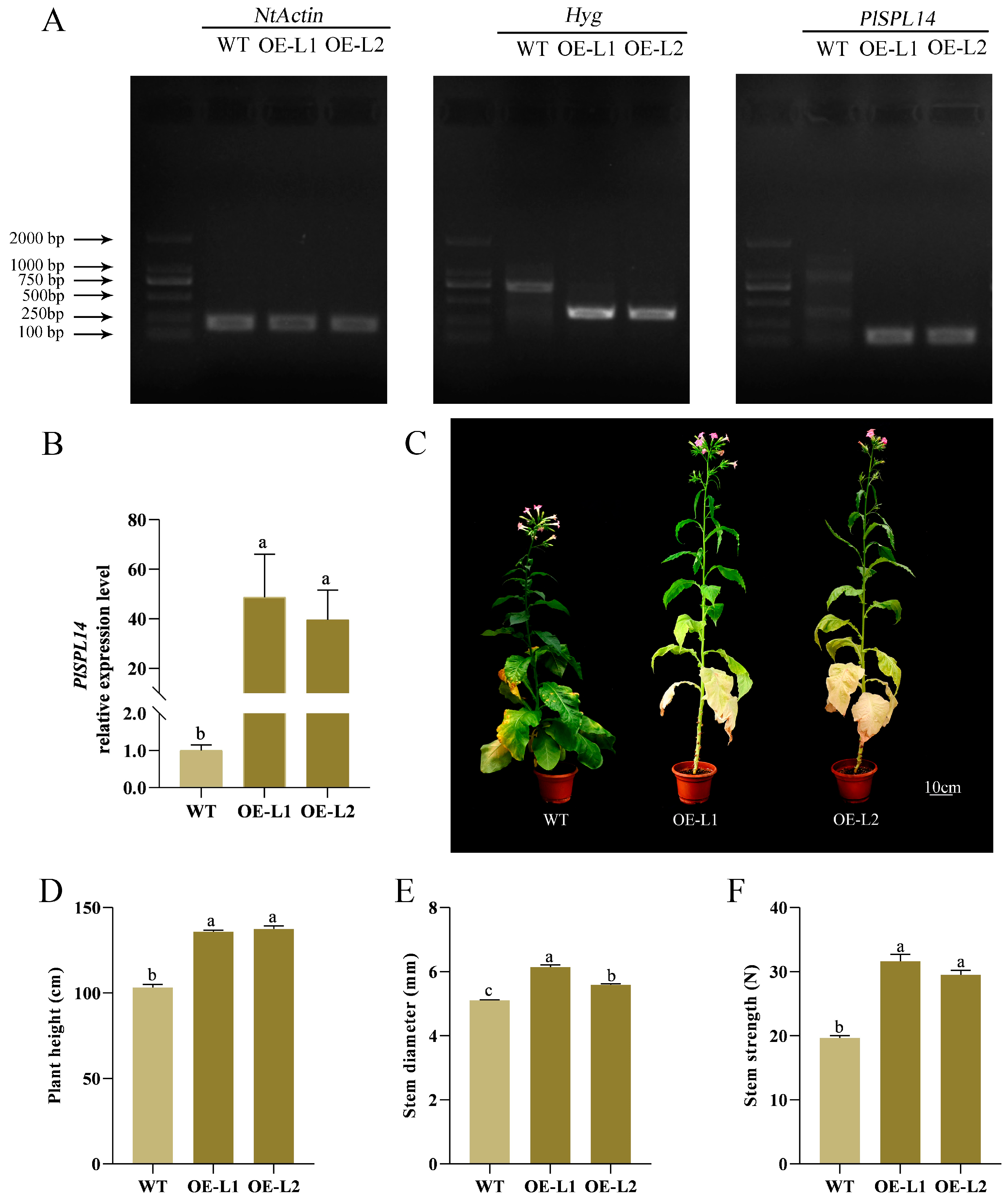
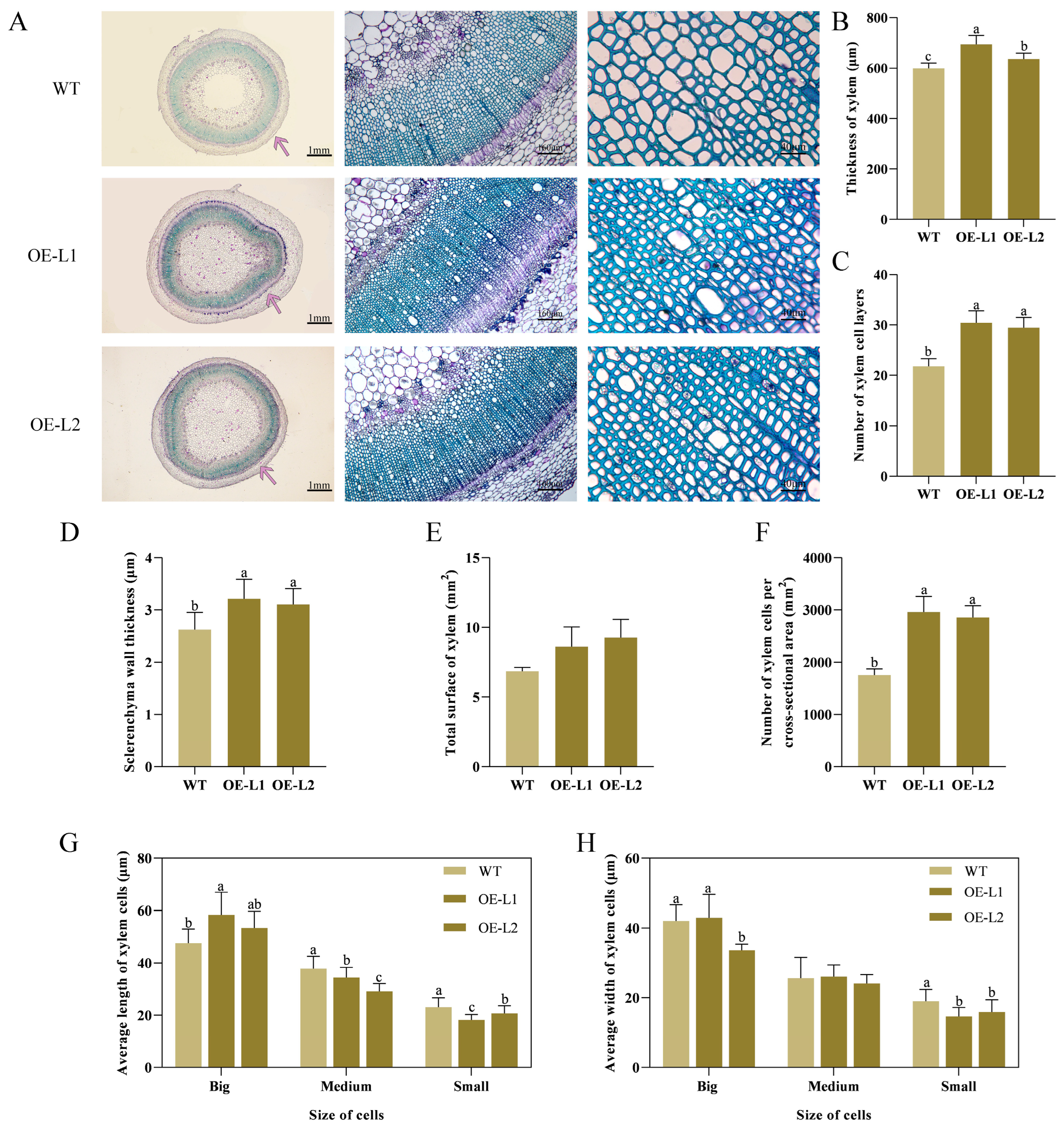
2.4. PlSPL14-Overexpressing Plant Exhibits Increased Stem Strength
2.5. Analysis of the Protein Interaction of PlSPL14
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. RNA Extraction and Quantitative RT-PCR (qRT-PCR)
4.3. Cloning and Bioinformatics Analysis of PlSPL14
4.4. Subcellular Localization
4.5. Virus-Induced Gene Silencing (VIGS) of PlSPL14 in P. lactiflora
4.6. Overexpressing PlSPL14 in Tobacco
4.7. Yeast Two-Hybrid Screening
4.8. Microscopy Observation and Cell Counting
4.9. Statistic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, Y.L.; Zhang, M.; Hong, A.Y.; Lan, X.Y.; Yang, H.Y.; Liu, Y. Transcriptome and weighted correlation network analyses provide insights into inflorescence stem straightness in Paeonia lactiflora. Plant Mol. Biol. 2020, 102, 239–252. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zheng, T.; Zhuo, X.; Li, P.; Qiu, L.; Liu, W.; Wang, J.; Cheng, T.; Zhang, Q. Comparative gene expression analysis reveals that multiple mechanisms regulate the weeping trait in Prunus mume. Sci. Rep. 2021, 11, 2675. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Rehman, A.u.; Anjum, S.A.; Iqbal, J.; Ahmad, R. Lodging stress in cereal—Effects and management: An overview. Environ. Sci. Pollut. Res. 2017, 24, 5222–5237. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, L.; Huang, X.; Zhao, D.; Tao, J. The herbaceous peony transcription factor WRKY41a promotes secondary cell wall thickening to enhance stem strength. Plant Physiol. 2023, 191, 428–445. [Google Scholar] [CrossRef]
- Zhang, J.; Nieminen, K.; Serra, J.A.A.; Helariutta, Y. The formation of wood and its control. Curr. Opin. Plant Biol. 2014, 17, 56–63. [Google Scholar] [CrossRef]
- Fukuda, H.; Ohashi-Ito, K. Chapter Six—Vascular tissue development in plants. Curr. Top. Dev. Biol. 2019, 131, 141–160. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.R.; Ren, A.Q.; Wan, Y.L.; Liu, Y. Xylem development and phloem conductivity in relation to the stem mechanical strength of Paeonia lactiflora. J. Plant Physiol. 2023, 283, 153963. [Google Scholar] [CrossRef]
- Xu, C.; Shen, Y.; He, F.; Fu, X.; Yu, H.; Lu, W.; Li, Y.; Li, C.; Fan, D.; Wang, H.C.; et al. Auxin-mediated Aux/IAA-ARF-HB signaling cascade regulates secondary xylem development in Populus. New Phytol. 2019, 222, 752–767. [Google Scholar] [CrossRef]
- Miyashima, S.; Sebastian, J.; Lee, J.-Y.; Helariutta, Y. Stem cell function during plant vascular development. EMBO J. 2013, 32, 178–193. [Google Scholar] [CrossRef]
- Fischer, U.; Kucukoglu, M.; Helariutta, Y.; Bhalerao, R.-P. The dynamics of cambial stem cell activity. Annu. Rev. Plant Biol. 2019, 70, 293–319. [Google Scholar] [CrossRef]
- Ruonala, R.; Ko, D.; Helariutta, Y. Genetic Networks in Plant Vascular Development. Annu. Rev. Genet. 2017, 51, 335–359. [Google Scholar] [CrossRef] [PubMed]
- De Rybel, B.; Mähönen, A.P.; Helariutta, Y.; Weijers, D. Plant vascular development: From early specification to differentiation. Nat. Rev. Mol. Cell Biol. 2016, 17, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Zhou, Z.; Qin, H.; Tan, J.; Ding, G. Exogenous Brassinosteroid Facilitates Xylem Development in Pinus massoniana Seedlings. Int. J. Mol. Sci. 2021, 22, 7615. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Carlsbecker, A.; Etchells, J.P. Class III HD-ZIPs govern vascular cell fate: An HD view on patterning and differentiation. J. Exp. Bot. 2017, 68, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Kucukoglu, M.; Chaabouni, S.; Zheng, B.; Mähönen, A.P.; Helariutta, Y.; Nilsson, O. Peptide encoding Populus CLV3/ESR-RELATED 47 (PttCLE47) promotes cambial development and secondary xylem formation in hybrid aspen. New Phytol. 2020, 226, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, M.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Recent Advances in the Transcriptional Regulation of Secondary Cell Wall Biosynthesis in the Woody Plants. Front. Plant Sci. 2018, 9, 1535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Song, D.; Xu, P.; Sun, J.; Li, L. A HD-ZIP III gene, PtrHB4, is required for interfascicular cambium development in Populus. Plant Biotechnol. J. 2018, 16, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lu, L.; Sheng, Z.; Zhao, D.; Tao, J. An R2R3-MYB network modulates stem strength by regulating lignin biosynthesis and secondary cell wall thickening in herbaceous peony. Plant J. 2023, 113, 1237–1258. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yoo, C.G.; Rottmann, W.; Winkeler, K.A.; Collins, C.M.; Gunter, L.E.; Jawdy, S.S.; Yang, X.; Pu, Y.; Ragauskas, A.J.; et al. PdWND3A, a wood-associated NAC domain-containing protein, affects lignin biosynthesis and composition in Populus. BMC Plant Biol. 2019, 19, 486. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, M.; Yu, J.; Guo, C. SPL9 mediates freezing tolerance by directly regulating the expression of CBF2 in Arabidopsis thaliana. BMC Plant Biol. 2022, 22, 59. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Huang, Y.-Y.; Zheng, Y.-P.; Liu, X.-X.; Zhou, S.-X.; Yang, X.-M.; Liu, S.-L.; Li, Y.; Li, J.-L.; Zhao, S.-L.; et al. Osa-miR535 targets SQUAMOSA promoter binding protein-like 4 to regulate blast disease resistance in rice. Plant J. 2022, 110, 166–178. [Google Scholar] [CrossRef]
- Cheng, H.; Hao, M.; Wang, W.; Mei, D.; Tong, C.; Wang, H.; Liu, J.; Fu, L.; Hu, Q. Genomic identification, characterization and differential expression analysis of SBP-box gene family in Brassica napus. BMC Plant Biol. 2016, 16, 196. [Google Scholar] [CrossRef]
- Hou, H.; Li, J.; Gao, M.; Singer, S.D.; Wang, H.; Mao, L.; Fei, Z.; Wang, X. Genomic Organization, Phylogenetic Comparison and Differential Expression of the SBP-Box Family Genes in Grape. PLoS ONE 2013, 8, e59358. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Wu, M.-F.; Yang, L.; Wu, G.; Poethig, R.S.; Wagner, D. The MicroRNA-Regulated SBP-Box Transcription Factor SPL3 Is a Direct Upstream Activator of LEAFY, FRUITFULL, and APETALA1. Dev. Cell 2009, 17, 268–278. [Google Scholar] [CrossRef]
- Yu, N.; Cai, W.-J.; Wang, S.; Shan, C.-M.; Wang, L.-J.; Chen, X.-Y. Temporal Control of Trichome Distribution by MicroRNA156-Targeted SPL Genes in Arabidopsis thaliana. Plant Cell 2010, 22, 2322–2335. [Google Scholar] [CrossRef]
- Li, Y.; Han, S.; Sun, X.; Khan, N.U.; Zhong, Q.; Zhang, Z.; Zhang, H.; Ming, F.; Li, Z.; Li, J. Variations in OsSPL10 confer drought tolerance by directly regulating OsNAC2 expression and ROS production in rice. J. Integr. Plant Biol. 2023, 65, 918–933. [Google Scholar] [CrossRef]
- Gupta, A.; Hua, L.; Zhang, Z.; Yang, B.; Li, W. CRISPR-induced miRNA156-recognition element mutations in TaSPL13 improve multiple agronomic traits in wheat. Plant Biotechnol. J. 2023, 21, 536–548. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, Y.; Xue, D.; Wang, J.; Yan, M.; Liu, G.; Dong, G.; Zeng, D.; Lu, Z.; Zhu, X.; et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 2010, 42, 541–544. [Google Scholar] [CrossRef]
- Miura, K.; Ikeda, M.; Matsubara, A.; Song, X.-J.; Ito, M.; Asano, K.; Matsuoka, M.; Kitano, H.; Ashikari, M. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 2010, 42, 545–549. [Google Scholar] [CrossRef]
- Duan, E.; Wang, Y.; Li, X.; Lin, Q.; Zhang, T.; Wang, Y.; Zhou, C.; Zhang, H.; Jiang, L.; Wang, J.; et al. OsSHI1 Regulates Plant Architecture Through Modulating the Transcriptional Activity of IPA1 in Rice. Plant Cell 2019, 31, 1026–1042. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Q.; Dong, Z.-J.; Yin, Y.-J.; da Silva, J.A.T.; Yu, X.-N. Advances in molecular biology of Paeonia L. Planta 2020, 251, 23. [Google Scholar] [CrossRef]
- Xu, H.; Lu, L.; Tang, Y.; Zhao, D.; Meng, J.; Tao, J. Cloning and Expression Characteristic Analysis of PISPL1 Gene in Paeonia lactiflora Pall. Acta Agric. Boreali-Sin. 2024, 39, 55–61. [Google Scholar]
- Cao, J.; Liu, K.; Song, W.; Zhang, J.; Yao, Y.; Xin, M.; Hu, Z.; Peng, H.; Ni, Z.; Sun, Q.; et al. Pleiotropic function of the SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE gene TaSPL14 in wheat plant architecture. Planta 2021, 253, 44. [Google Scholar] [CrossRef]
- Cardon, G.; Höhmann, S.; Klein, J.; Nettesheim, K.; Saedler, H.; Huijser, P. Molecular characterisation of the Arabidopsis SBP-box genes. Gene 1999, 237, 91–104. [Google Scholar] [CrossRef]
- Zhao, D.; Luan, Y.; Xia, X.; Shi, W.; Tang, Y.; Tao, J. Lignin provides mechanical support to herbaceous peony (Paeonia lactiflora Pall.) stems. Hortic. Res. 2020, 7, 213. [Google Scholar] [CrossRef]
- Liu, W.-g.; Hussain, S.; Liu, T.; Zou, J.-l.; Ren, M.-l.; Zhou, T.; Liu, J.; Yang, F.; Yang, W.-y. Shade stress decreases stem strength of soybean through restraining lignin biosynthesis. J. Integr. Agr. 2019, 18, 43–53. [Google Scholar] [CrossRef]
- Peng, J. RegulationFunctional Characterization of EjSPLs on Loquat Flowering Time. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2019. [Google Scholar]
- Liang, X.; Nazarenus, T.J.; Stone, J.M. Identification of a Consensus DNA-Binding Site for the Arabidopsis thaliana SBP Domain Transcription Factor, AtSPL14, and Binding Kinetics by Surface Plasmon Resonance. Biochemistry 2008, 47, 3645–3653. [Google Scholar] [CrossRef]
- Olas, J.J.; Van Dingenen, J.; Abel, C.; Działo, M.A.; Feil, R.; Krapp, A.; Schlereth, A.; Wahl, V. Nitrate acts at the Arabidopsis thaliana shoot apical meristem to regulate flowering time. New Phytol. 2019, 223, 814–827. [Google Scholar] [CrossRef]
- Gao, J.; Peng, H.; Chen, F.; Liu, Y.; Chen, B.; Li, W. Genome-wide identification and characterization, phylogenetic comparison and expression profiles of SPL transcription factor family in B. juncea (Cruciferae). PLoS ONE 2019, 14, e0224704. [Google Scholar] [CrossRef]
- Hanly, A.; Karagiannis, J.; Lu, Q.S.; Tian, L.; Hannoufa, A. Characterization of the Role of SPL9 in Drought Stress Tolerance in Medicago sativa. Int. J. Mol. Sci. 2020, 21, 6003. [Google Scholar] [CrossRef]
- He, Y.; Zhu, M.; Li, Z.; Jiang, S.; He, Z.; Xu, S.; Chen, X.; Hu, Z.; Zhang, Z. IPA1 Negatively Regulates Early Rice Seedling Development by Interfering with Starch Metabolism via the GA and WRKY Pathways. Int. J. Mol. Sci. 2021, 22, 6605. [Google Scholar] [CrossRef]
- Zhang, L.; He, G.; Li, Y.; Yang, Z.; Liu, T.; Xie, X.; Kong, X.; Sun, J. PIL transcription factors directly interact with SPLs and repress tillering/branching in plants. New Phytol. 2022, 233, 1414–1425. [Google Scholar] [CrossRef]
- Wang, L.; Yu, P.; Lyu, J.; Hu, Y.; Han, C.; Bai, M.Y.; Fan, M. BZR1 Physically Interacts with SPL9 to Regulate the Vegetative Phase Change and Cell Elongation in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 10415. [Google Scholar] [CrossRef]
- Liao, Z.; Yu, H.; Duan, J.; Yuan, K.; Yu, C.; Meng, X.; Kou, L.; Chen, M.; Jing, Y.; Liu, G.; et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 2019, 10, 2738. [Google Scholar] [CrossRef]
- Lu, L.; Tang, Y.; Xu, H.; Qian, Y.; Tao, J.; Zhao, D. Selection and verification of reliable internal reference genes in stem development of herbaceous peony (Paeonia lactiflora Pall.). Physiol. Mol. Biol. Plants 2023, 29, 773–782. [Google Scholar] [CrossRef]
- Liu, Y.; Schiff, M.; Marathe, R.; Dinesh-Kumar, S.P. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002, 30, 415–429. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Yu, R.; Tang, Y.; Meng, J.; Tao, J. Identification and Functional Studies on the Role of PlSPL14 in Herbaceous Peony Stem Development. Int. J. Mol. Sci. 2024, 25, 8443. https://doi.org/10.3390/ijms25158443
Xu H, Yu R, Tang Y, Meng J, Tao J. Identification and Functional Studies on the Role of PlSPL14 in Herbaceous Peony Stem Development. International Journal of Molecular Sciences. 2024; 25(15):8443. https://doi.org/10.3390/ijms25158443
Chicago/Turabian StyleXu, Huajie, Renkui Yu, Yuhan Tang, Jiasong Meng, and Jun Tao. 2024. "Identification and Functional Studies on the Role of PlSPL14 in Herbaceous Peony Stem Development" International Journal of Molecular Sciences 25, no. 15: 8443. https://doi.org/10.3390/ijms25158443




