Environmentally Friendly UV Absorbers: Synthetic Characterization and Biosecurity Studies of the Host–Guest Supramolecular Complex
Abstract
:1. Introduction
2. Results and Discussions
2.1. Standard Curves and the Inclusion Rate of IMC-SBE-β-CD
2.2. Quantitative Calculation of IMC-SBE-β-CD Optimized Conformation
2.3. FT-IR and XRD Analysis of Materials
2.4. Cell Viability and Morphology
2.5. Effect of IMC-SBE-β-CD on Apoptosis
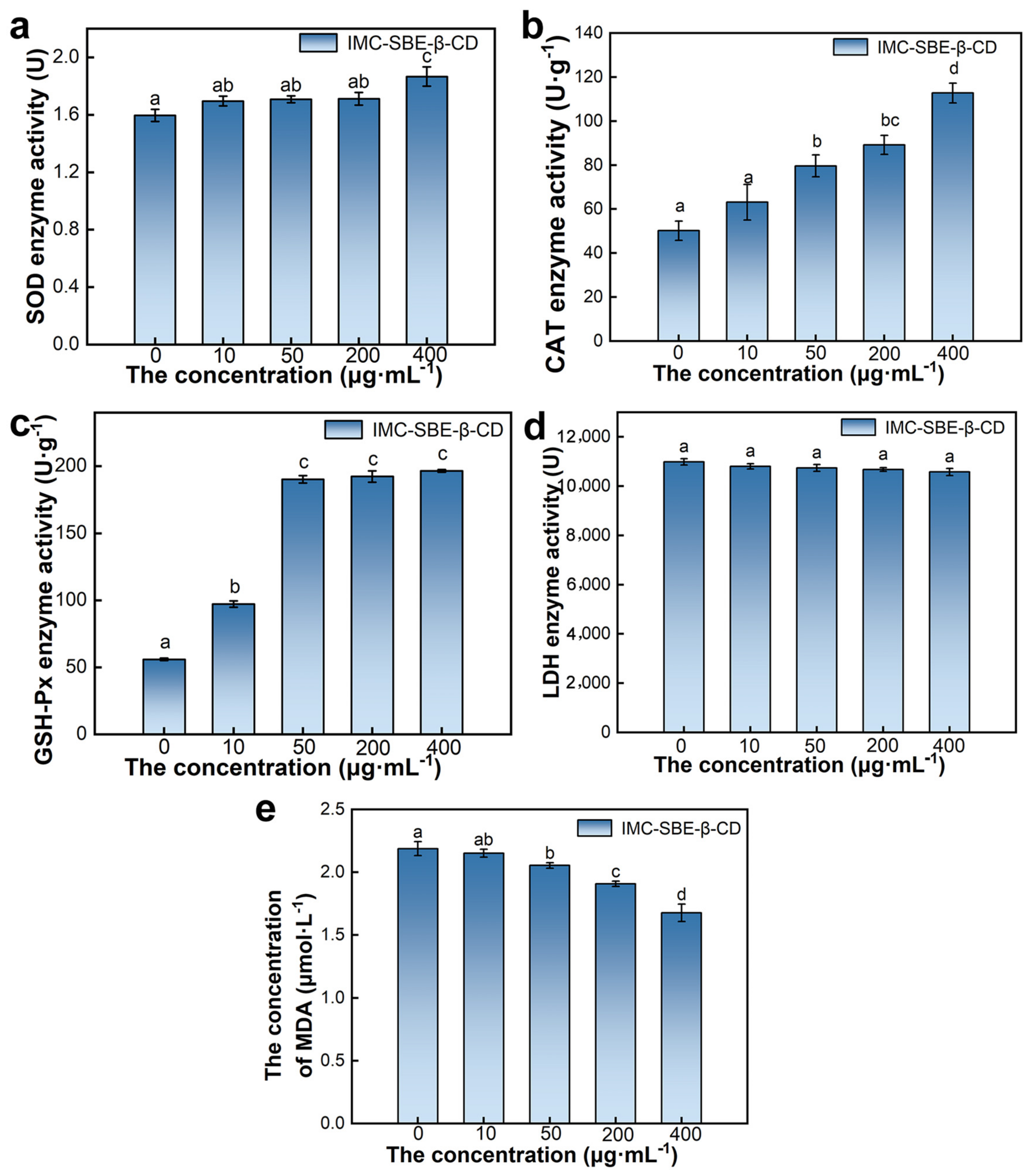
2.6. Effect of IMC-SBE-β-CD on Oxidative Stress System
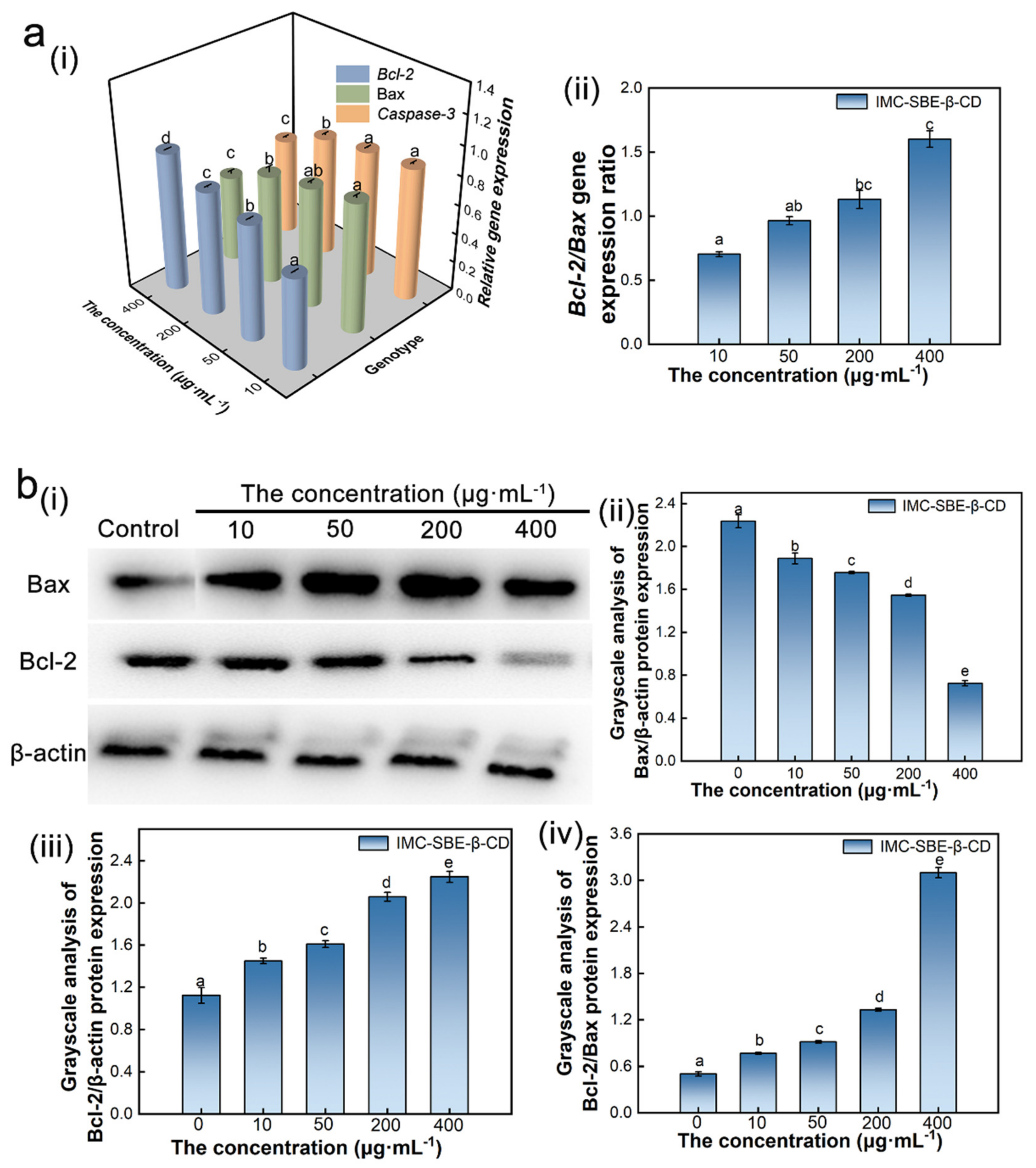
2.7. Effect of IMC-SBE-β-CD on Apoptotic Gene Expression
3. Materials and Methods
3.1. Materials and Equipment
3.2. Saturated Solution Method
3.3. Solid-Phase Synthesis Method
3.4. Characterization of Supramolecular Inclusion Complex
3.5. CCK-8 Assay
3.6. Cytomorphology Analysis
3.7. Apoptosis Assay
3.8. ROS Assay
3.9. Mitochondrial Membrane Potential (MMP) Assay
3.10. Measuring the Activity of Antioxidant Enzymes and the Content of Active Substance
3.11. Evaluation of Gene Expression
3.12. Evaluation of Protein Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apel, C.; Tang, J.; Ebinghaus, R. Environmental occurrence and distribution of organic UV stabilizers and UV filters in the sediment of Chinese Bohai and Yellow Seas. Environ. Pollut. 2018, 235, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yuan, T.; Zhou, C.; Cheng, P.; Bai, Q.; Ao, J.; Wang, W.; Zhang, H. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere 2013, 93, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yoo, J. History of sunscreen: An updated view. J. Cosmet. Dermatol. 2021, 20, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.; Freeman, S. Report of 19 cases of photoallergic contact dermatitis to sunscreens seen at the Skin and Cancer Foundation. Aust. J. Dermatol. 2001, 42, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sahu, S.; Gond, S.P.; Singh, B.; Rajendiran, A.; Singh, A. Pharmacological review of chemical agents used in sunscreen preparations. J. Pharm. Negat. Results 2022, 13, 2692–2702. [Google Scholar]
- Qu, X.; Feng, H.; Ma, C.; Yang, Y.; Yu, X. Synthesis, crystal structure and anti-tumor activity of a novel 3D supramolecular compound constructed from Strandberg-type polyoxometalate and benzimidazole. Inorg. Chem. Commun. 2017, 81, 22–26. [Google Scholar] [CrossRef]
- Hu, Q.D.; Tang, G.P.; Chu, P.K. Cyclodextrin-based host–guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Fourmentin, S.; Fenyvesi É Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Kothawade, R.V.; Markad, A.R.; Pardeshi, S.R.; Kulkarni, A.D.; Chaudhari, P.J.; Longhi, M.R.; Dhas, N.; Naik, J.B.; Surana, S.J.; et al. Sulfobutylether-β-cyclodextrin: A functional biopolymer for drug delivery applications. Carbohydr. Polym. 2022, 301, 120347. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ghate, V.M.; Lewis, S.A. Supramolecular cyclodextrin complex: Diversity, safety, and applications in ocular therapeutics. Exp. Eye Res. 2019, 189, 107829. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Wang, X.F.; Huang, T.; Yang, Y.; Tu, J.S.; Zou, J.; Yang, H.; Yang, R. Application of sodium sulfobutylether-β-cyclodextrin based on encapsulation. Carbohydr. Polym. 2024, 333, 121985. [Google Scholar] [CrossRef]
- Nanda, A.; Sahoo, R.N.; Pramanik, A.; Mohapatra, R.; Pradhan, S.K.; Thirumurugan, A.; Das, D.; Mallick, S. Drug-in-mucoadhesive type film for ocular anti-inflammatory potential of amlodipine: Effect of sulphobutyl-ether-beta-cyclodextrin on permeation and molecular docking characterization. Colloids Surf. B 2018, 172, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kuwahara, H.; Yang, P.; Song, L.; Gao, X. PGCN: Disease gene prioritization by disease and gene embedding through graph convolutional neural networks. bioRxiv 2019. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Mohandoss, S.; Atchudan, R.; Edison TN, J.I.; Mandal, T.K.; Palanisamy, S.; You, S.G.; Napoleon, A.A.; Shim, J.J.; Lee, Y.R. Enhanced solubility of guanosine by inclusion complexes with cyclodextrin derivatives: Preparation, characterization, and evaluation. Carbohydr. Polym. 2019, 224, 115166. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhu, W.J.; Wu, J.L.; Guo, X.B.; Sun, X.Y.; Li, Q.X.; Wang, J.H.; Chen, L. Development and physicochemical characterization of chitosan hydrochloride/sulfobutyl ether-β-cyclodextrin nanoparticles for cinnamaldehyde entrapment. J. Food Biochem. 2020, 44, e13197. [Google Scholar] [CrossRef]
- Arjunan, V.; Anitha, R.; Thenmozhi, S.; Marchewk, M.K.; Mohan, S. Potential energy profile, structural, vibrational and reactivity descriptors of trans–2–methoxycinnamic acid by FTIR, FT-Raman and quantum chemical studies. J. Mol. Struct. 2016, 1113, 42–54. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.Y.; Jiang, J.Y.; Ji, Q.Y.; Fu, Y.; Zhao, L.X.; Li, C.Y.; Ye, F. Physicochemical properties and fungicidal activity of inclusion complexes of fungicide chlorothalonil with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J. Mol. Liq. 2019, 293, 111513. [Google Scholar] [CrossRef]
- Yi, C.R.; Li, Y.; Zhang, S.X.; Fan, H.; Cheng, Z.Q. CSH/SBE-β-CD nanoparticles: Controlled synthesis and application for loading and pH-responsive drug release. New J. Chem. 2022, 46, 13498–13503. [Google Scholar] [CrossRef]
- Wang, X.; Han, L.; Hu, X.; Li, S.; Ma, W.; Song, W. Photostability of the inclusion complex of isoamyl4-(Dimethylamino) benzoate with sulfobutylether-β-cyclodextrin. J. Photochem. Photobiol. A 2022, 423, 113614. [Google Scholar] [CrossRef]
- Tian, L.W.; Guo, M.; Chen, H.L.; Wu, Y.N. Human health risk assessment of cinnamate UV absorbers: In vitro and in silico investigations. Environ. Int. 2023, 171, 107658. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Gu, Y.; Guo, M.; Hou, P.; Li, Y.; Wu, R.H. Perfluorooctanoic acid exposure induces apoptosis in SMMC-7721 hepatocellular cancer cells. Environ. Pollut. 2019, 247, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Ge, M.; Jin, J.; Xu, H.; Mao, L.; Geng, S.; Wu, J.; Zhu, J.; Li, X.; Zhong, C. Mechanism investigation on bisphenol S-induced oxidative stress and inflammation in murine RAW264. 7 cells: The role of NLRP3 inflammasome, TLR4, Nrf2 and MAPK. J. Hazard. Mater. 2020, 394, 122549. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.F.; Din, J.; Zhao, F.; Liu, X.Q. Effect of soybean peptides against hydrogen peroxide induced oxidative stress in HepG2 cells via Nrf2 signaling. Food Funct. 2020, 11, 2725–2737. [Google Scholar] [CrossRef] [PubMed]
- Delbridge AR, D.; Grabow, S.; Strasser, A.; Vaux, D.L. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer 2016, 16, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Black, K.A.; Stollar, E.J. Uncovering protein function: From classification to complexes. Essays Biochem. 2022, 66, 255–285. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]




| Materials | IMC Concentration in Inclusion Complex (μg·mL−1) | Inclusion Ratio (%) |
|---|---|---|
| IMC-SBE-β-CD | 10.48 | 84.45 |
| IMC/SBE-β-CD | 8.82 | 71.01 |
| Temperature (°C) | Phase Solubility Curve | R2 | K Values (L·mmol−1) |
|---|---|---|---|
| 25 | y = 0.0022x + 1.8569 | 0.9881 | 1.1874 |
| 30 | y = 0.0071x + 3.0122 | 0.9853 | 2.3739 |
| 35 | y = 0.0072x + 3.9635 | 0.9935 | 1.8298 |
| 40 | y = 0.0069x + 4.5450 | 0.9890 | 1.5287 |
| 45 | y = 0.0075x + 5.5185 | 0.9923 | 1.3693 |
| 50 | y = 0.0063x + 6.3348 | 0.9859 | 1.0008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Wu, Y.; Hou, Y.; Dong, Y.; Ni, K.; Guo, M. Environmentally Friendly UV Absorbers: Synthetic Characterization and Biosecurity Studies of the Host–Guest Supramolecular Complex. Int. J. Mol. Sci. 2024, 25, 8476. https://doi.org/10.3390/ijms25158476
Tian L, Wu Y, Hou Y, Dong Y, Ni K, Guo M. Environmentally Friendly UV Absorbers: Synthetic Characterization and Biosecurity Studies of the Host–Guest Supramolecular Complex. International Journal of Molecular Sciences. 2024; 25(15):8476. https://doi.org/10.3390/ijms25158476
Chicago/Turabian StyleTian, Luwei, Yanan Wu, Yetong Hou, Yaru Dong, Kaijie Ni, and Ming Guo. 2024. "Environmentally Friendly UV Absorbers: Synthetic Characterization and Biosecurity Studies of the Host–Guest Supramolecular Complex" International Journal of Molecular Sciences 25, no. 15: 8476. https://doi.org/10.3390/ijms25158476






