Acute Hyperglycemia-Induced Injury in Myocardial Infarction
Abstract
1. Introduction
2. Current Evidence: From the Bench to the Bedside
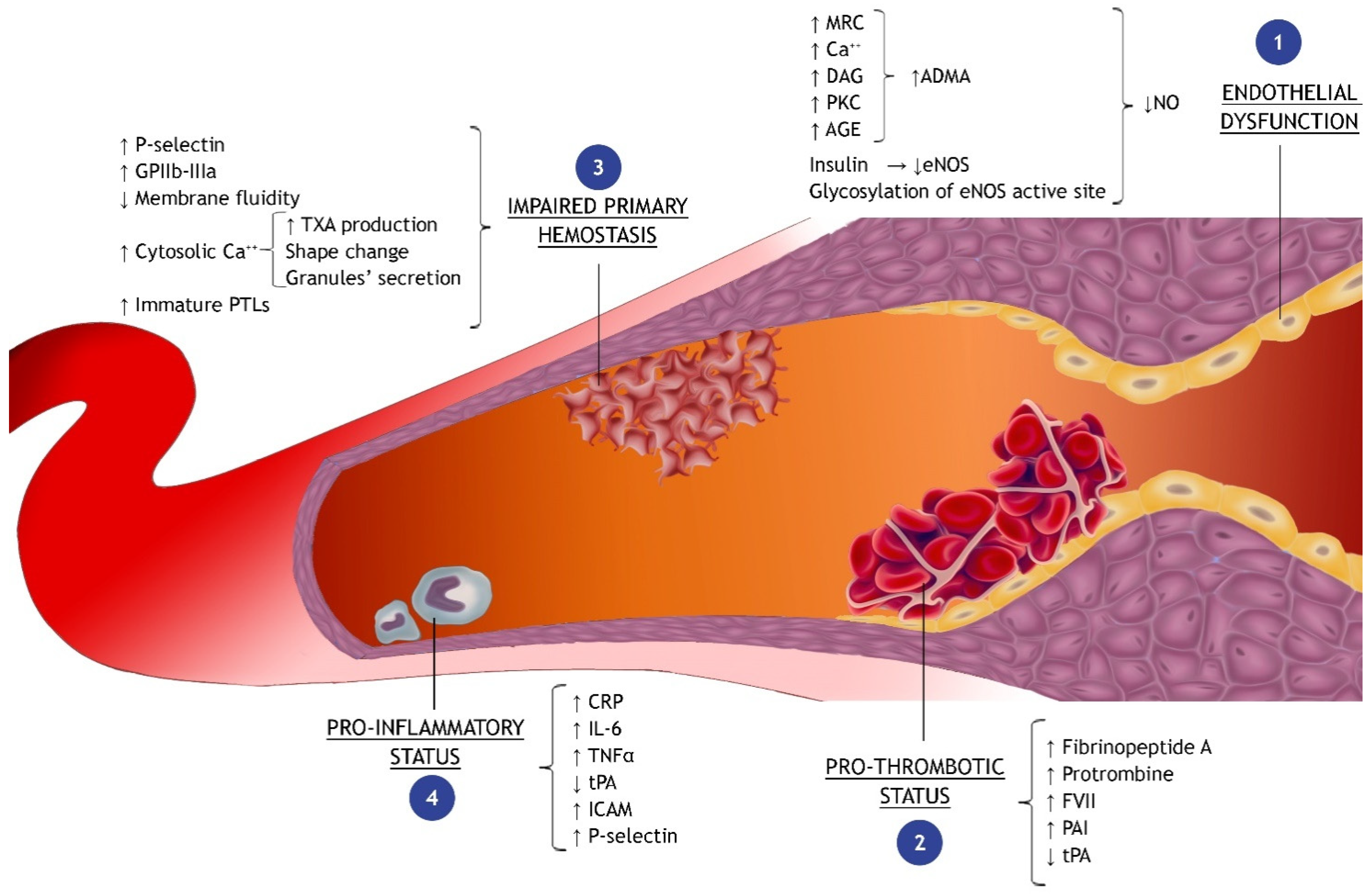
2.1. Pathophysiological Mechanisms Triggered by Hyperglycemic Status in the Setting of Acute Myocardial Infarction
2.2. Endothelial Dysfunction
2.2.1. Oxidative Stress
- The MRC is a protein complex within the internal mitochondrial membrane, which is involved in the redox reactions aimed to produce adenosine triphosphate (ATP) by using oxygen as the final electrons’ acceptor. Some studies identify ROS overproduction by MRC as the principal cause of hyperglycemia-induced tissue damage through the increase in glycolysis, tricarboxylic acid (TCA) cycle activity, ATP/ADP ratio, and the hyperpolarization of the mitochondrial membrane [38,39,40,41]. When the overproduction of electron donors by the TCA cycle occurs, the increased electrochemical potential difference (generated by the proton gradient across the inner mitochondrial membrane) increases the production of superoxide anions by endothelial cells [42]. Moreover, hyperglycemia seems also responsible for the impairment of the mitochondrial ATP-sensitive potassium channels (mKATP), which play a role in the protective preconditioning phenomenon [43,44];
- Several studies have also pointed out the PKC activation in vascular cells in response to high PGL [45,46,47]. PKC is triggered by intracellular signals, such as diacylglycerol (DAG) or calcium ions (Ca2+), whose concentration in endothelial cells increases during hyperglycemic states. PKC activation, in turn, via numerous different pathways (as described in Figure 1) contributes to the impairment of myocardial perfusion [42,48];
- In addition, a very suggestive discovery is that hyperglycemia stimulates the production of ROS in the heart through the activation of NADPH oxidase 2 (NOX2) via the sodiummyoinositol cotransporter-1 (SMIT1); the latter is an isoform of the sodium/glucose cotransporters (SGLT) and might explain some of the beneficial effects of the glifozines (SGLT-2 inhibitors) [49,50,51].
2.2.2. Nitric Oxide Metabolism
2.3. Impaired Primary Hemostasis and Pro-Thrombotic Status
2.4. Pro-Inflammatory Status
2.5. Autophagy
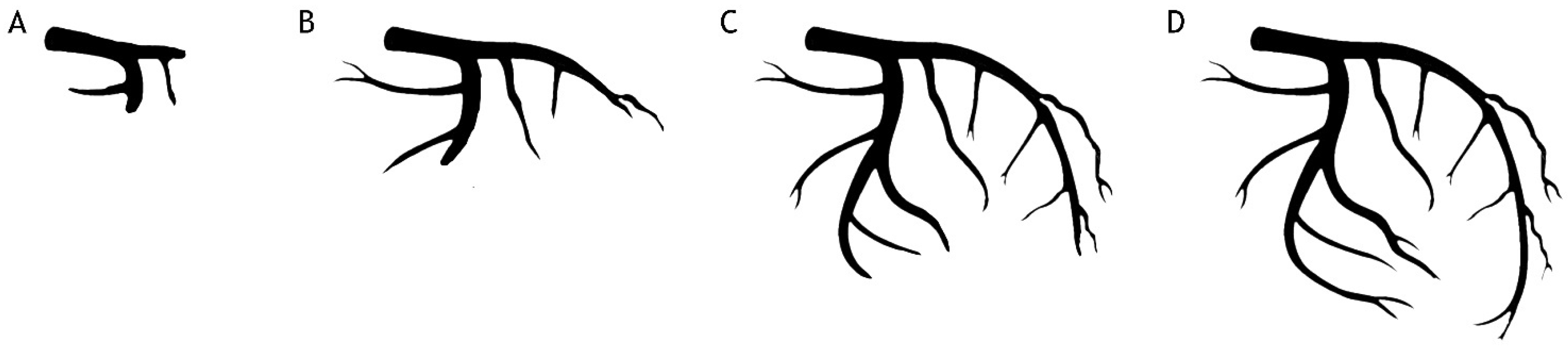
2.6. The Close Relationship between Acute Hyperglycemia, Coronary Flow and Myocardial Perfusion
2.7. Glucose Control Strategy in Patients with AMI
3. Conclusions
Funding
Conflicts of Interest
References
- Deedwania, P.; Kosiborod, M.; Barrett, E.; Ceriello, A.; Isley, W.; Mazzone, T.; Raskin, P. Hyperglycemia and acute coronary syndrome: A scientific statement from the american heart association diabetes committee of the council on nutrition, physical activity, and metabolism. Circulation 2008, 117, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.; Deedwania, P. An overview of glycemic control in the coronary care unit with recommendations for clinical management. J. Diabetes Sci. Technol. 2009, 3, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, M.; Kimura, K.; Kojima, S.; Sakamoto, T.; Matsui, K.; Ishihara, M.; Asada, Y.; Tei, C.; Miyazaki, S.; Sonoda, M.; et al. Effects of glucose abnormalities on in-hospital outcome after coronary intervention for acute myocardial infarction. Circ. J. 2005, 69, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.; Rathore, S.S.; Inzucchi, S.E.; Masoudi, F.A.; Wang, Y.; Havranek, E.P.; Krumholz, H.M. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: Implications for patients with and without recognized diabetes. Circulation 2005, 111, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Wahab, N.N.; Cowden, E.A.; Pearce, N.J.; Gardner, M.J.; Merry, H.; Cox, J.L. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J. Am. Coll. Cardiol. 2002, 40, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Alkatiri, A.H.; Qalby, N.; Mappangara, I.; Zainal, A.T.F.; Cramer, M.J.; Doevendans, P.A.; Qanitha, A. Stress hyperglycemia and poor outcomes in patients with st-elevation myocardial infarction: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1303685. [Google Scholar] [CrossRef] [PubMed]
- Capes, S.E.; Hunt, D.; Malmberg, K.; Gerstein, H.C. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systematic overview. Lancet 2000, 355, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M. Acute hyperglycemia in patients with acute myocardial infarction. Circ. J. 2012, 76, 563–571. [Google Scholar] [CrossRef]
- Finfer, S.; Chittock, D.R.; Su, S.Y.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; Henderson, W.R.; et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Kramer, D.G.; Lessard, D.; Yarzebski, J.; Gore, J.M. Serum glucose levels and hospital outcomes in patients with acute myocardial infarction without prior diabetes: A community-wide perspective. Coron. Artery Dis. 2007, 18, 125–131. [Google Scholar] [CrossRef]
- Hoebers, L.P.; Damman, P.; Claessen, B.E.; Vis, M.M.; Baan, J., Jr.; van Straalen, J.P.; Fischer, J.; Koch, K.T.; Tijssen, J.G.; de Winter, R.J.; et al. Predictive value of plasma glucose level on admission for short and long term mortality in patients with st-elevation myocardial infarction treated with primary percutaneous coronary intervention. Am. J. Cardiol. 2012, 109, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, K.; Ito, H.; Ikushima, M.; Kawano, S.; Okamura, A.; Asano, K.; Kuroda, T.; Tanaka, K.; Masuyama, T.; Hori, M.; et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2003, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Krinsley, J.S.; Egi, M.; Kiss, A.; Devendra, A.N.; Schuetz, P.; Maurer, P.M.; Schultz, M.J.; van Hooijdonk, R.T.; Kiyoshi, M.; Mackenzie, I.M.; et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: An international multicenter cohort study. Crit. Care 2013, 17, R37. [Google Scholar] [CrossRef] [PubMed]
- Mladenović, V.; Zdravković, V.; Jović, M.; Vucić, R.; Irić-Cupić, V.; Rosić, M. Influence of admission plasma glucose level on short- and long-term prognosis in patients with st-segment elevation myocardial infarction. Vojnosanit. Pregl. 2010, 67, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.J.; Hao, Z.X.; Liu, R.; Liu, Y. Admission glucose and risk of early death in non-diabetic patients with st-segment elevation myocardial infarction: A meta-analysis. Med. Sci. Monit. 2015, 21, 1387–1394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paolisso, P.; Foà, A.; Bergamaschi, L.; Angeli, F.; Fabrizio, M.; Donati, F.; Toniolo, S.; Chiti, C.; Rinaldi, A.; Stefanizzi, A.; et al. Impact of admission hyperglycemia on short and long-term prognosis in acute myocardial infarction: Minoca versus mioca. Cardiovasc. Diabetol. 2021, 20, 192. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Inoue, I.; Kawagoe, T.; Shimatani, Y.; Kurisu, S.; Nishioka, K.; Umemura, T.; Nakamura, S.; Yoshida, M. Impact of acute hyperglycemia on left ventricular function after reperfusion therapy in patients with a first anterior wall acute myocardial infarction. Am. Heart J. 2003, 146, 674–678. [Google Scholar] [CrossRef]
- Timmer, J.R.; van der Horst, I.C.; Ottervanger, J.P.; Henriques, J.P.; Hoorntje, J.C.; de Boer, M.J.; Suryapranata, H.; Zijlstra, F. Prognostic value of admission glucose in non-diabetic patients with myocardial infarction. Am. Heart J. 2004, 148, 399–404. [Google Scholar] [CrossRef]
- Singh, K.; Hibbert, B.; Singh, B.; Carson, K.; Premaratne, M.; Le May, M.; Chong, A.-Y.; Arstall, M.; So, D. Meta-analysis of admission hyperglycaemia in acute myocardial infarction patients treated with primary angioplasty: A cause or a marker of mortality? Eur. Heart J.-Cardiovasc. Pharmacother. 2015, 1, 220–228. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Guo, K.; Wu, Z.; Lao, Y.; Li, J.; Huang, X.; Feng, L.; Dong, J.; Yuan, Y. Association between fasting hyperglycemia and new-onset atrial fibrillation in patients with acute myocardial infarction and the impact on short- and long-term prognosis. Front. Cardiovasc. Med. 2021, 8, 667527. [Google Scholar] [CrossRef]
- Shechter, M.; Merz, C.N.; Paul-Labrador, M.J.; Kaul, S. Blood glucose and platelet-dependent thrombosis in patients with coronary artery disease. J. Am. Coll. Cardiol. 2000, 35, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular endothelial cell biology: An update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the european society of cardiology working groups on atherosclerosis and vascular biology, aorta and peripheral vascular diseases, coronary pathophysiology and microcirculation, and thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Lüscher, T.F. Endothelial function in coronary artery disease. Cardiologia 1997, 42, 1221–1227. [Google Scholar] [PubMed]
- He, Z.; King, G.L. Microvascular complications of diabetes. Endocrinol. Metab. Clin. N. Am. 2004, 33, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, T.F.; Barton, M. Biology of the endothelium. Clin. Cardiol. 1997, 20, II-3-10. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Esposito, K.; Giunta, R.; Coppola, G.; De Angelis, L.; Farzati, B.; Paolisso, G.; Giugliano, D. Circulating adhesion molecules in humans: Role of hyperglycemia and hyperinsulinemia. Circulation 2000, 101, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Siniscalchi, M.; Esposito, K.; Sellitto, A.; De Fanis, U.; Romano, C.; Portoghese, M.; Siciliano, S.; Nappo, F.; Sasso, F.C.; et al. Effects of stress hyperglycemia on acute myocardial infarction: Role of inflammatory immune process in functional cardiac outcome. Diabetes Care 2003, 26, 3129–3135. [Google Scholar] [CrossRef] [PubMed]
- European Diabetes Epidemiology Group. Glucose tolerance and mortality: Comparison of who and american diabetes association diagnostic criteria. The decode study group. Diabetes epidemiology: Collaborative analysis of diagnostic criteria in europe. Lancet 1999, 354, 617–621. [Google Scholar] [CrossRef]
- Lash, J.M.; Nase, G.P.; Bohlen, H.G. Acute hyperglycemia depresses arteriolar no formation in skeletal muscle. Am. J. Physiol. 1999, 277, H1513–H1520. [Google Scholar] [CrossRef]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.D.; Poston, L. The effect of hyperglycaemia on function of rat isolated mesenteric resistance artery. Br. J. Pharmacol. 1994, 113, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.B.; Goldfine, A.B.; Timimi, F.K.; Ting, H.H.; Roddy, M.A.; Simonson, D.C.; Creager, M.A. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation 1998, 97, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Luo, Z.; Zhou, J. Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovasc. Diabetol. 2021, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Addabbo, F.; Ratliff, B.; Park, H.C.; Kuo, M.C.; Ungvari, Z.; Csiszar, A.; Krasnikov, B.; Sodhi, K.; Zhang, F.; Nasjletti, A.; et al. The krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: Proteomic approach. Am. J. Pathol. 2009, 174, 34–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ciccone, M.M.; Scicchitano, P.; Zito, A.; Cortese, F.; Boninfante, B.; Falcone, V.A.; Quaranta, V.N.; Ventura, V.A.; Zucano, A.; Di Serio, F.; et al. Correlation between inflammatory markers of atherosclerosis and carotid intima-media thickness in obstructive sleep apnea. Molecules 2014, 19, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.; Napoli, G.; Carulli, E.; Moscarelli, M.; Forleo, C.; Nestola, P.L.; Biondi-Zoccai, G.; Giordano, A.; Favale, S. Autoimmune diseases in patients undergoing percutaneous coronary intervention: A risk factor for in-stent restenosis? Atherosclerosis 2021, 333, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase c—Dependent activation of nad(p)h oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Addabbo, F.; Montagnani, M. Vascular actions of insulin with implications for endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E568–E577. [Google Scholar] [CrossRef]
- Du, X.L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing sp1 glycosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12222–12226. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Asano, G.; Takashi, E.; Ishiwata, T.; Onda, M.; Yokoyama, M.; Naito, Z.; Ashraf, M.; Sugisaki, Y. Pathogenesis and protection of ischemia and reperfusion injury in myocardium. J. Nippon Med. Sch. 2003, 70, 384–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kersten, J.R.; Montgomery, M.W.; Ghassemi, T.; Gross, E.R.; Toller, W.G.; Pagel, P.S.; Warltier, D.C. Diabetes and hyperglycemia impair activation of mitochondrial k(atp) channels. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1744–H1750. [Google Scholar] [CrossRef]
- Inoguchi, T.; Xia, P.; Kunisaki, M.; Higashi, S.; Feener, E.P.; King, G.L. Insulin’s effect on protein kinase c and diacylglycerol induced by diabetes and glucose in vascular tissues. Am. J. Physiol. 1994, 267, E369–E379. [Google Scholar] [CrossRef]
- Tesfamariam, B. Free radicals in diabetic endothelial cell dysfunction. Free Radic. Biol. Med. 1994, 16, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.A.; Williamson, J.R.; Easom, R.A.; Chang, K.; Sherman, W.R.; Turk, J. Diacylglycerol accumulation and microvascular abnormalities induced by elevated glucose levels. J. Clin. Investig. 1991, 87, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Schrier, R.W. Characterization of glucose-induced in situ protein kinase c activity in cultured vascular smooth muscle cells. Diabetes 1992, 41, 1464–1472. [Google Scholar] [CrossRef]
- Baartscheer, A.; Schumacher, C.A.; Wüst, R.C.; Fiolet, J.W.; Stienen, G.J.; Coronel, R.; Zuurbier, C.J. Empagliflozin decreases myocardial cytoplasmic na(+) through inhibition of the cardiac na(+)/h(+) exchanger in rats and rabbits. Diabetologia 2017, 60, 568–573. [Google Scholar] [CrossRef]
- Van Steenbergen, A.; Balteau, M.; Ginion, A.; Ferté, L.; Battault, S.; Ravenstein, C.M.; Balligand, J.L.; Daskalopoulos, E.P.; Gilon, P.; Despa, F.; et al. Sodium-myoinositol cotransporter-1, smit1, mediates the production of reactive oxygen species induced by hyperglycemia in the heart. Sci. Rep. 2017, 7, 41166. [Google Scholar] [CrossRef]
- Balteau, M.; Tajeddine, N.; de Meester, C.; Ginion, A.; Rosiers, C.D.; Brady, N.R.; Sommereyns, C.; Horman, S.; Vanoverschelde, J.L.; Gailly, P.; et al. Nadph oxidase activation by hyperglycaemia in cardiomyocytes is independent of glucose metabolism but requires sglt1. Cardiovasc. Res. 2011, 92, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Wautier, M.P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.L. Activation of nadph oxidase by age links oxidant stress to altered gene expression via rage. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E685–E694. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Menini, T. The axis age-rage-soluble rage and oxidative stress in chronic kidney disease. Adv. Exp. Med. Biol. 2014, 824, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W.; Stuehr, D.J. Nitric oxide synthases: Properties and catalytic mechanism. Annu. Rev. Physiol. 1995, 57, 707–736. [Google Scholar] [CrossRef] [PubMed]
- Addabbo, F.; Nacci, C.; De Benedictis, L.; Leo, V.; Tarquinio, M.; Quon, M.J.; Montagnani, M. Globular adiponectin counteracts vcam-1-mediated monocyte adhesion via adipor1/nf-κb/cox-2 signaling in human aortic endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1143–E1154. [Google Scholar] [CrossRef] [PubMed]
- Govers, R.; Rabelink, T.J. Cellular regulation of endothelial nitric oxide synthase. Am. J. Physiol. Renal. Physiol. 2001, 280, F193–F206. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Edelstein, D.; Dimmeler, S.; Ju, Q.; Sui, C.; Brownlee, M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the akt site. J. Clin. Investig. 2001, 108, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Federici, M.; Menghini, R.; Mauriello, A.; Hribal, M.L.; Ferrelli, F.; Lauro, D.; Sbraccia, P.; Spagnoli, L.G.; Sesti, G.; Lauro, R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by o-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 2002, 106, 466–472. [Google Scholar] [CrossRef]
- Potashnik, R.; Bloch-Damti, A.; Bashan, N.; Rudich, A. Irs1 degradation and increased serine phosphorylation cannot predict the degree of metabolic insulin resistance induced by oxidative stress. Diabetologia 2003, 46, 639–648. [Google Scholar] [CrossRef]
- Bucala, R.; Tracey, K.J.; Cerami, A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J. Clin. Investig. 1991, 87, 432–438. [Google Scholar] [CrossRef]
- Vásquez-Vivar, J.; Kalyanaraman, B.; Martásek, P.; Hogg, N.; Masters, B.S.; Karoui, H.; Tordo, P.; Pritchard, K.A., Jr. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc. Natl. Acad. Sci. USA 1998, 95, 9220–9225. [Google Scholar] [CrossRef]
- Wever, R.M.; van Dam, T.; van Rijn, H.J.; de Groot, F.; Rabelink, T.J. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem. Biophys. Res. Commun. 1997, 237, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Milstien, S.; Katusic, Z. Oxidation of tetrahydrobiopterin by peroxynitrite: Implications for vascular endothelial function. Biochem. Biophys. Res. Commun. 1999, 263, 681–684. [Google Scholar] [CrossRef]
- Xia, Y.; Tsai, A.L.; Berka, V.; Zweier, J.L. Superoxide generation from endothelial nitric-oxide synthase. A ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J. Biol. Chem. 1998, 273, 25804–25808. [Google Scholar] [CrossRef]
- Heitzer, T.; Krohn, K.; Albers, S.; Meinertz, T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with type ii diabetes mellitus. Diabetologia 2000, 43, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.J. Diabetes mellitus as a prothrombotic condition. J. Intern. Med. 2007, 262, 157–172. [Google Scholar] [CrossRef]
- Ceriello, A.; Giugliano, D.; Quatraro, A.; Russo, P.D.; Marchi, E.; Torella, R. Hyperglycemia may determine fibrinopeptide a plasma level increase in humans. Metabolism 1989, 38, 1162–1163. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Giugliano, D.; Quatraro, A.; Russo, P.D.; Torella, R. Blood glucose may condition factor vii levels in diabetic and normal subjects. Diabetologia 1988, 31, 889–891. [Google Scholar] [CrossRef]
- Pandolfi, A.; Giaccari, A.; Cilli, C.; Alberta, M.M.; Morviducci, L.; De Filippis, E.A.; Buongiorno, A.; Pellegrini, G.; Capani, F.; Consoli, A. Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in the rat. Acta. Diabetol. 2001, 38, 71–76. [Google Scholar] [CrossRef]
- Enomoto, M.; Adachi, H.; Yamagishi, S.; Takeuchi, M.; Furuki, K.; Hino, A.; Hiratsuka, A.; Takajo, Y.; Imaizumi, T. Positive association of serum levels of advanced glycation end products with thrombogenic markers in humans. Metabolism 2006, 55, 912–917. [Google Scholar] [CrossRef]
- Ferreiro, J.L.; Angiolillo, D.J. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation 2011, 123, 798–813. [Google Scholar] [CrossRef]
- Gaiz, A.; Mosawy, S.; Colson, N.; Singh, I. Thrombotic and cardiovascular risks in type two diabetes; role of platelet hyperactivity. Biomed. Pharmacother. 2017, 94, 679–686. [Google Scholar] [CrossRef]
- Guthikonda, S.; Alviar, C.L.; Vaduganathan, M.; Arikan, M.; Tellez, A.; DeLao, T.; Granada, J.F.; Dong, J.F.; Kleiman, N.S.; Lev, E.I. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 2008, 52, 743–749. [Google Scholar] [CrossRef]
- Melchinger, H.; Jain, K.; Tyagi, T.; Hwa, J. Role of platelet mitochondria: Life in a nucleus-free zone. Front. Cardiovasc. Med. 2019, 6, 153. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef]
- Li, D.; Zhao, L.; Liu, M.; Du, X.; Ding, W.; Zhang, J.; Mehta, J.L. Kinetics of tumor necrosis factor alpha in plasma and the cardioprotective effect of a monoclonal antibody to tumor necrosis factor alpha in acute myocardial infarction. Am. Heart J. 1999, 137, 1145–1152. [Google Scholar] [CrossRef]
- Morohoshi, M.; Fujisawa, K.; Uchimura, I.; Numano, F. Glucose-dependent interleukin 6 and tumor necrosis factor production by human peripheral blood monocytes in vitro. Diabetes 1996, 45, 954–959. [Google Scholar] [CrossRef]
- Visser, L.; Zuurbier, C.J.; Hoek, F.J.; Opmeer, B.C.; de Jonge, E.; de Mol, B.A.; van Wezel, H.B. Glucose, insulin and potassium applied as perioperative hyperinsulinaemic normoglycaemic clamp: Effects on inflammatory response during coronary artery surgery. Br. J. Anaesth. 2005, 95, 448–457. [Google Scholar] [CrossRef]
- Booth, G.; Stalker, T.J.; Lefer, A.M.; Scalia, R. Elevated ambient glucose induces acute inflammatory events in the microvasculature: Effects of insulin. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E848–E856. [Google Scholar] [CrossRef]
- Hokama, J.Y.; Ritter, L.S.; Davis-Gorman, G.; Cimetta, A.D.; Copeland, J.G.; McDonagh, P.F. Diabetes enhances leukocyte accumulation in the coronary microcirculation early in reperfusion following ischemia. J. Diabetes Complicat. 2000, 14, 96–107. [Google Scholar] [CrossRef]
- Sekar, M.; Thirumurugan, K. Autophagy: A molecular switch to regulate adipogenesis and lipolysis. Mol. Cell Biochem. 2022, 477, 727–742. [Google Scholar] [CrossRef]
- Bharath, L.P.; Rockhold, J.D.; Conway, R. Selective autophagy in hyperglycemia-induced microvascular and macrovascular diseases. Cells 2021, 10, 2114. [Google Scholar] [CrossRef]
- García-Díez, E.; Pérez-Jiménez, J.; Martín, M.Á.; Ramos, S. (−)-epicatechin and colonic metabolite 2,3-dihydroxybenzoic acid, alone or in combination with metformin, protect cardiomyocytes from high glucose/high palmitic acid-induced damage by regulating redox status, apoptosis and autophagy. Food Funct. 2024, 15, 2536–2549. [Google Scholar] [CrossRef]
- Saad, R.; Tadmor, H.; Ertracht, O.; Nakhoul, N.; Nakhoul, F.; Evgeny, F.; Atar, S. The molecular effects of sglt2i empagliflozin on the autophagy pathway in diabetes mellitus type 2 and its complications. J. Diabetes Res. 2022, 2022, 8337823. [Google Scholar] [CrossRef]
- Hao, Y.; Lu, Q.; Li, T.; Yang, G.; Hu, P.; Ma, A. Admission hyperglycemia and adverse outcomes in diabetic and non-diabetic patients with non-st-elevation myocardial infarction undergoing percutaneous coronary intervention. BMC Cardiovasc. Disord. 2017, 17, 6. [Google Scholar] [CrossRef]
- Kosiborod, M.; Inzucchi, S.E.; Krumholz, H.M.; Xiao, L.; Jones, P.G.; Fiske, S.; Masoudi, F.A.; Marso, S.P.; Spertus, J.A. Glucometrics in patients hospitalized with acute myocardial infarction: Defining the optimal outcomes-based measure of risk. Circulation 2008, 117, 1018–1027. [Google Scholar] [CrossRef]
- Timmer, J.R.; Ottervanger, J.P.; de Boer, M.J.; Dambrink, J.H.; Hoorntje, J.C.; Gosselink, A.T.; Suryapranata, H.; Zijlstra, F.; Hof, A.W.V. Hyperglycemia is an important predictor of impaired coronary flow before reperfusion therapy in st-segment elevation myocardial infarction. J. Am. Coll Cardiol. 2005, 45, 999–1002. [Google Scholar] [CrossRef]
- Gibson, C.M.; Cannon, C.P.; Daley, W.L.; Dodge, J.T., Jr.; Alexander, B., Jr.; Marble, S.J.; McCabe, C.H.; Raymond, L.; Fortin, T.; Poole, W.K.; et al. Timi frame count: A quantitative method of assessing coronary artery flow. Circulation 1996, 93, 879–888. [Google Scholar] [CrossRef]
- Eshraghi, A.; Talasaz, A.H.; Salamzadeh, J.; Bahremand, M.; Salarifar, M.; Nozari, Y.; Jenab, Y.; Boroumand, M.A.; Vaseghi, G.; Eshraghi, N. Study of the possible medical and medication explanatory factors of angiographic outcomes in patients with acute st elevation myocardial infarction undergoing primary percutaneous intervention. Adv. Biomed. Res. 2014, 3, 186. [Google Scholar] [CrossRef]
- Engler, R.L.; Dahlgren, M.D.; Morris, D.D.; Peterson, M.A.; Schmid-Schönbein, G.W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am. J. Physiol. 1986, 251, H314–H323. [Google Scholar] [CrossRef]
- McDonagh, P.F.; Hokama, J.Y.; Copeland, J.G.; Reynolds, J.M. The blood contribution to early myocardial reperfusion injury is amplified in diabetes. Diabetes 1997, 46, 1859–1867. [Google Scholar] [CrossRef]
- Nakamura, T.; Ako, J.; Kadowaki, T.; Funayama, H.; Sugawara, Y.; Kubo, N.; Momomura, S. Impact of acute hyperglycemia during primary stent implantation in patients with st-elevation myocardial infarction. J. Cardiol. 2009, 53, 272–277. [Google Scholar] [CrossRef]
- Kersten, J.R.; Toller, W.G.; Tessmer, J.P.; Pagel, P.S.; Warltier, D.C. Hyperglycemia reduces coronary collateral blood flow through a nitric oxide-mediated mechanism. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H2097–H2104. [Google Scholar] [CrossRef]
- Montalescot, G.; Barragan, P.; Wittenberg, O.; Ecollan, P.; Elhadad, S.; Villain, P.; Boulenc, J.M.; Morice, M.C.; Maillard, L.; Pansiéri, M.; et al. Platelet glycoprotein iib/iiia inhibition with coronary stenting for acute myocardial infarction. N. Engl. J. Med. 2001, 344, 1895–1903. [Google Scholar] [CrossRef]
- Opie, L.H. Preconditioning and metabolic anti-ischaemic agents. Eur. Heart J. 2003, 24, 1854–1856. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.; Tian, Y.; Liu, Y.; Hennessy, S.; Kron, I.L.; French, B.A. Acute hyperglycemia abolishes ischemic preconditioning by inhibiting akt phosphorylation: Normalizing blood glucose before ischemia restores ischemic preconditioning. Oxid. Med. Cell Longev. 2013, 2013, 329183. [Google Scholar] [CrossRef]
- Zuurbier, C.J.; Demirci, C.; Koeman, A.; Vink, H.; Ince, C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J. Appl. Physiol. 2005, 99, 1471–1476. [Google Scholar] [CrossRef]
- Gibson, C.M.; Ryan, K.A.; Murphy, S.A.; Mesley, R.; Marble, S.J.; Giugliano, R.P.; Cannon, C.P.; Antman, E.M.; Braunwald, E. Impaired coronary blood flow in nonculprit arteries in the setting of acute myocardial infarction. The timi study group. Thrombolysis in myocardial infarction. J. Am. Coll Cardiol. 1999, 34, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.; Zanna, D.; Cafaro, A.; Marchese, A.; Addabbo, F.; Navarese, E.P.; Napodano, M.; Cecere, A.; Resta, F.; Paradies, V.; et al. Role of plasma glucose level on myocardial perfusion in st-segment elevation myocardial infarction patients. J. Diabetes Complicat. 2018, 32, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Baptista, R.M.; Monteiro, S.R.; Gonçalves, F.M.; Monteiro, P.F.; Gonçalves, L.M. Admission hyperglycemia and all-cause mortality in diabetic and non-diabetic patients with acute myocardial infarction: A tertiary center analysis. Intern. Emerg. Med. 2021, 16, 2109–2119. [Google Scholar] [CrossRef]
- Planer, D.; Witzenbichler, B.; Guagliumi, G.; Peruga, J.Z.; Brodie, B.R.; Xu, K.; Fahy, M.; Mehran, R.; Stone, G.W. Impact of hyperglycemia in patients with st-segment elevation myocardial infarction undergoing percutaneous coronary intervention: The horizons-ami trial. Int. J. Cardiol. 2013, 167, 2572–2579. [Google Scholar] [CrossRef]
- Stranders, I.; Diamant, M.; van Gelder, R.E.; Spruijt, H.J.; Twisk, J.W.; Heine, R.J.; Visser, F.C. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch. Intern. Med. 2004, 164, 982–988. [Google Scholar] [CrossRef]
- Demarchi, A.; Cornara, S.; Somaschini, A.; Fortuni, F.; Mandurino-Mirizzi, A.; Crimi, G.; Ferlini, M.; Gnecchi, M.; De Servi, S.; Visconti, L.O.; et al. Has hyperglycemia a different prognostic role in stemi patients with or without diabetes? Nutr. Metab. Cardiovasc. Dis. 2021, 31, 528–531. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Janicke, D.; Wilson, M.F.; Tripathy, D.; Garg, R.; Bandyopadhyay, A.; Calieri, J.; Hoffmeyer, D.; Syed, T.; Ghanim, H.; et al. Anti-inflammatory and profibrinolytic effect of insulin in acute st-segment-elevation myocardial infarction. Circulation 2004, 109, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Lautamäki, R.; Airaksinen, K.E.; Seppänen, M.; Toikka, J.; Härkönen, R.; Luotolahti, M.; Borra, R.; Sundell, J.; Knuuti, J.; Nuutila, P. Insulin improves myocardial blood flow in patients with type 2 diabetes and coronary artery disease. Diabetes 2006, 55, 511–516. [Google Scholar] [CrossRef] [PubMed]
- McNulty, P.; Pfau, S.; Deckelbaum, L. Effect of plasma insulin level on myocardial blood flow and its mechanism of action. Am. J. Cardiol. 2000, 85, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, U.; Randin, D.; Vollenweider, P.; Vollenweider, L.; Nicod, P. Nitric oxide release accounts for insulin’s vascular effects in humans. J. Clin. Investig. 1994, 94, 2511–2515. [Google Scholar] [CrossRef]
- Iliadis, F.; Kadoglou, N.; Didangelos, T. Insulin and the heart. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. S1), S86–S91. [Google Scholar] [CrossRef]
- Iguchi, T.; Hasegawa, T.; Otsuka, K.; Matsumoto, K.; Yamazaki, T.; Nishimura, S.; Nakata, S.; Ehara, S.; Kataoka, T.; Shimada, K.; et al. Insulin resistance is associated with coronary plaque vulnerability: Insight from optical coherence tomography analysis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 284–291. [Google Scholar] [CrossRef]
- Wang, C.C.; Goalstone, M.L.; Draznin, B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes 2004, 53, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.; Sardella, G.; Stefanini, G.G.; Corcione, N.; Nestola, P.L.; Morello, A.; Briguori, C.; Tamburino, C.; Fabbiocchi, F.; Rotolo, F.L.; et al. Impact of insulin-treated and noninsulin-treated diabetes mellitus in all-comer patients undergoing percutaneous coronary interventions with polymer-free biolimus-eluting stent (from the rudi-free registry). Am. J. Cardiol. 2019, 124, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, K.; Norhammar, A.; Wedel, H.; Rydén, L. Glycometabolic state at admission: Important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: Long-term results from the diabetes and insulin-glucose infusion in acute myocardial infarction (digami) study. Circulation 1999, 99, 2626–2632. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, K.; Rydén, L.; Wedel, H.; Birkeland, K.; Bootsma, A.; Dickstein, K.; Efendic, S.; Fisher, M.; Hamsten, A.; Herlitz, J.; et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (digami 2): Effects on mortality and morbidity. Eur. Heart J. 2005, 26, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 esc guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 esc guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 esc guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the easd. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 esc guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Ekmekci, A.; Cicek, G.; Uluganyan, M.; Gungor, B.; Osman, F.; Ozcan, K.S.; Bozbay, M.; Ertas, G.; Zencirci, A.; Sayar, N.; et al. Admission hyperglycemia predicts inhospital mortality and major adverse cardiac events after primary percutaneous coronary intervention in patients without diabetes mellitus. Angiology 2014, 65, 154–159. [Google Scholar] [CrossRef]
- Meier, J.J.; Deifuss, S.; Klamann, A.; Launhardt, V.; Schmiegel, W.H.; Nauck, M.A. Plasma glucose at hospital admission and previous metabolic control determine myocardial infarct size and survival in patients with and without type 2 diabetes: The langendreer myocardial infarction and blood glucose in diabetic patients assessment (lambda). Diabetes Care 2005, 28, 2551–2553. [Google Scholar] [CrossRef]

| Blood Glucose Level at Presentation | % of Population | 30-Days Mortality (%) | 1 Year Mortality (%) |
|---|---|---|---|
| ≤110 mg/dL | 15.07 | 11 | 22.8 |
| 111–140 mg/dL | 26.78 | 13.4 | 25.4 |
| 141–170 mg/dL | 17.81 | 17.7 | 30.4 |
| 171–240 mg/dL | 19.86 | 22.4 | 37.5 |
| ≥240 mg/dL | 20.48 | 27.8 | 44.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe, M.; Addabbo, F.; Cecere, A.; Tritto, R.; Napoli, G.; Nestola, P.L.; Cirillo, P.; Biondi-Zoccai, G.; Giordano, S.; Ciccone, M.M. Acute Hyperglycemia-Induced Injury in Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 8504. https://doi.org/10.3390/ijms25158504
Pepe M, Addabbo F, Cecere A, Tritto R, Napoli G, Nestola PL, Cirillo P, Biondi-Zoccai G, Giordano S, Ciccone MM. Acute Hyperglycemia-Induced Injury in Myocardial Infarction. International Journal of Molecular Sciences. 2024; 25(15):8504. https://doi.org/10.3390/ijms25158504
Chicago/Turabian StylePepe, Martino, Francesco Addabbo, Annagrazia Cecere, Rocco Tritto, Gianluigi Napoli, Palma Luisa Nestola, Plinio Cirillo, Giuseppe Biondi-Zoccai, Salvatore Giordano, and Marco Matteo Ciccone. 2024. "Acute Hyperglycemia-Induced Injury in Myocardial Infarction" International Journal of Molecular Sciences 25, no. 15: 8504. https://doi.org/10.3390/ijms25158504
APA StylePepe, M., Addabbo, F., Cecere, A., Tritto, R., Napoli, G., Nestola, P. L., Cirillo, P., Biondi-Zoccai, G., Giordano, S., & Ciccone, M. M. (2024). Acute Hyperglycemia-Induced Injury in Myocardial Infarction. International Journal of Molecular Sciences, 25(15), 8504. https://doi.org/10.3390/ijms25158504







