Impact of a 12-Week Dietary Intervention on Adipose Tissue Metabolic Markers in Overweight Women of Reproductive Age
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Glucagon
- Role in Glucose Homeostasis: Glucagon, a hormone produced by pancreatic alpha cells, counterbalances insulin effects by stimulating hepatic glucose production through glycogenolysis and gluconeogenesis, raising blood glucose levels during fasting states or hypoglycemia [20].
- Relevance in Dietary Studies: Monitoring glucagon levels elucidates how different diets impact hepatic glucose production and overall glucose homeostasis. Diets that modulate glucagon secretion could influence fasting glucose levels and glucose variability, crucial components of metabolic health [21].
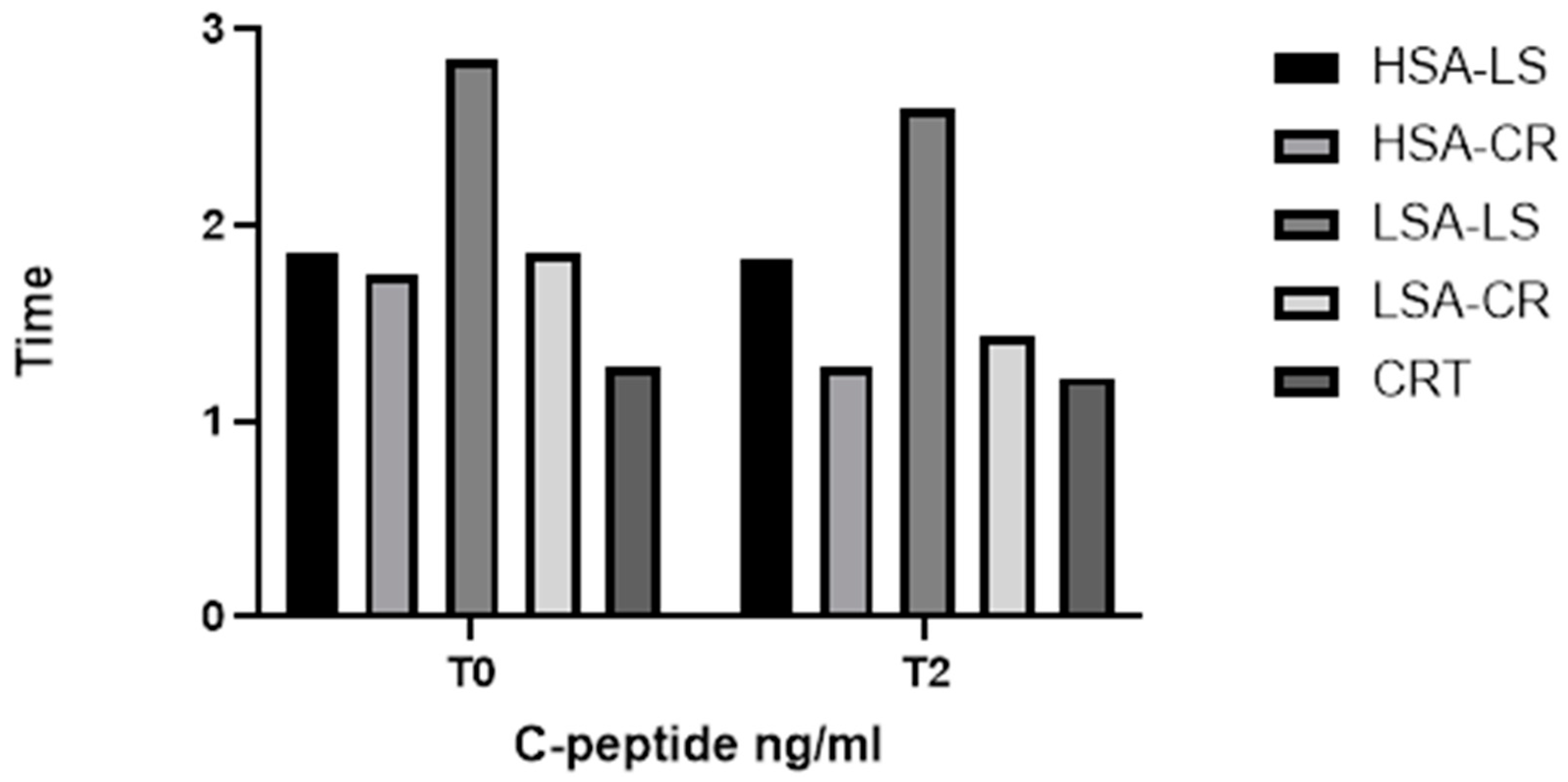
3.2. C-Peptide
- Role in Glucose Homeostasis: C-peptide, co-secreted with insulin from pancreatic beta cells in equimolar amounts, serves as a marker of endogenous insulin production [23]. Unlike insulin, the C-peptide is not extracted by the liver, making it a reliable measure of beta-cell function.
- Relevance in Dietary Studies: Assessing C-peptide levels helps determine how dietary interventions affect insulin secretion and beta-cell function. This information is essential for understanding the long-term impacts of diets on insulin production and the risk of developing insulin resistance or type 2 diabetes.
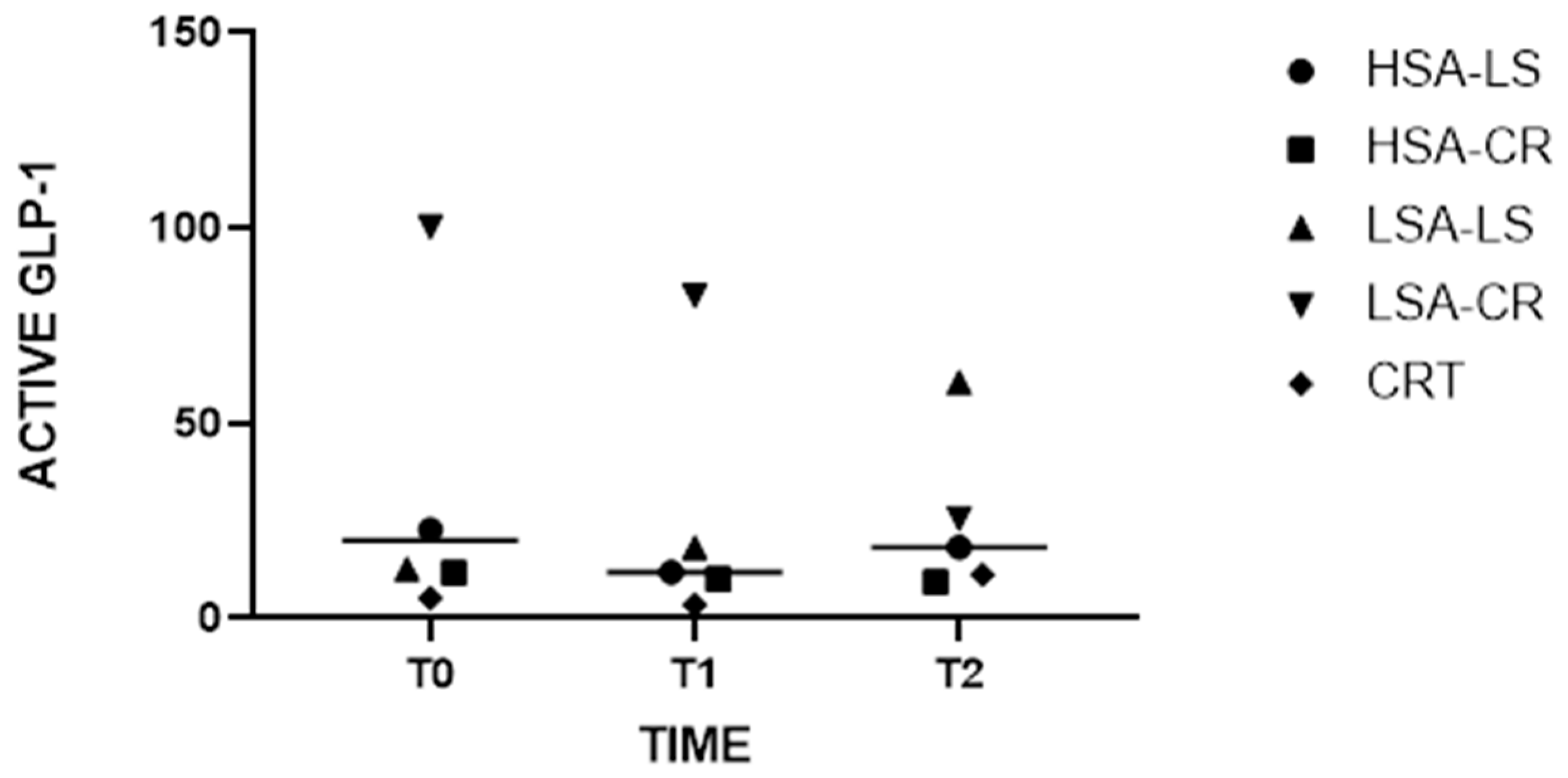
3.3. GLP-1
- Role in Glucose Homeostasis: Glucagon-like peptide-1 (GLP-1) is an incretin hormone secreted by enteroendocrine L-cells in the intestine in response to food intake. It enhances glucose-dependent insulin secretion, inhibits glucagon release, and slows gastric emptying, collectively contributing to post-prandial glucose control [24,25].
- Relevance in Dietary Studies: Diet-induced changes in GLP-1 levels significantly affect post-prandial glucose metabolism. Measuring GLP-1 provides insights into how different dietary patterns influence incretin response, satiety, and insulin sensitivity. This is particularly relevant for developing dietary strategies to improve glucose regulation and manage diabetes.
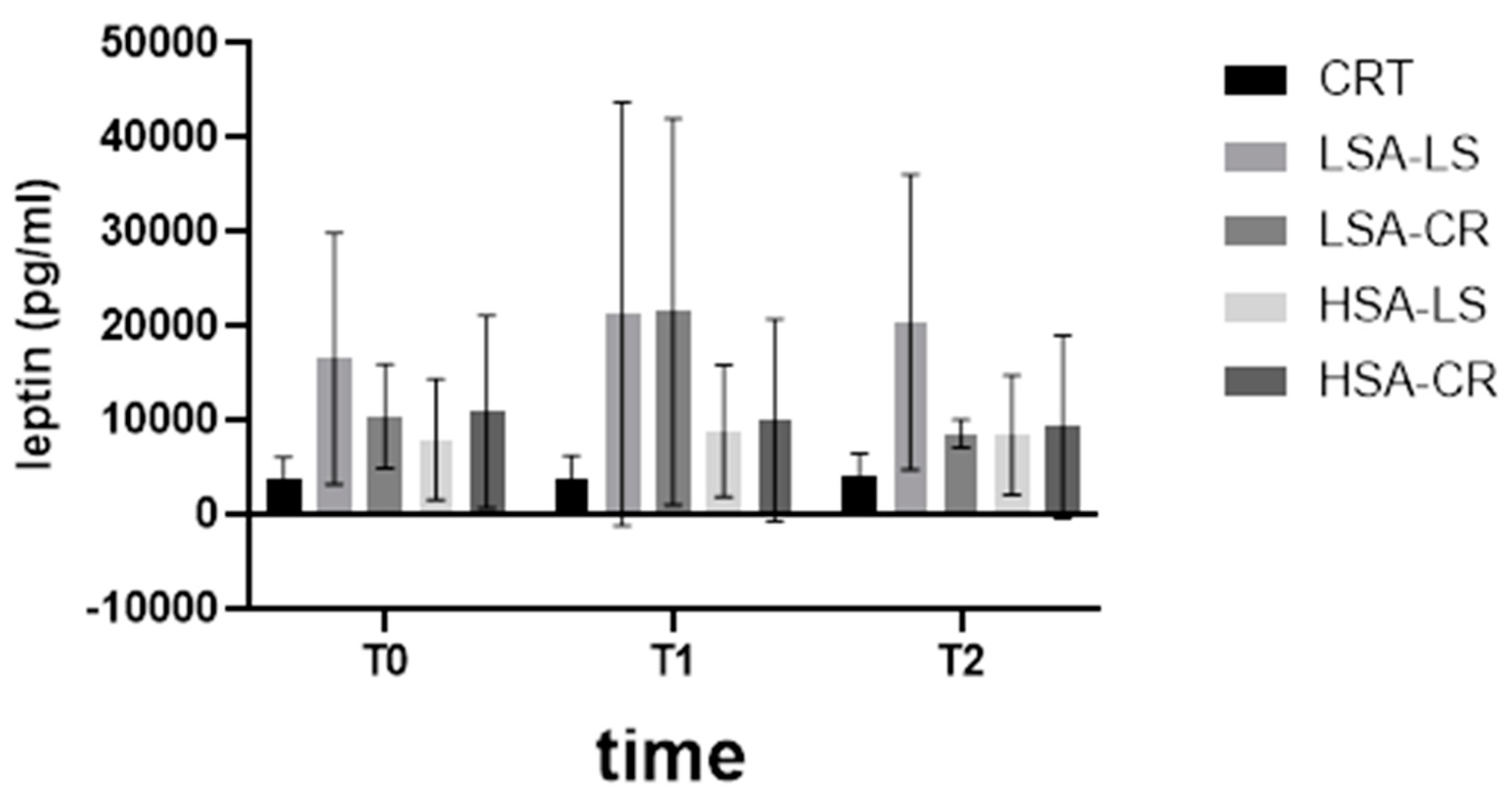
3.4. Leptin Levels
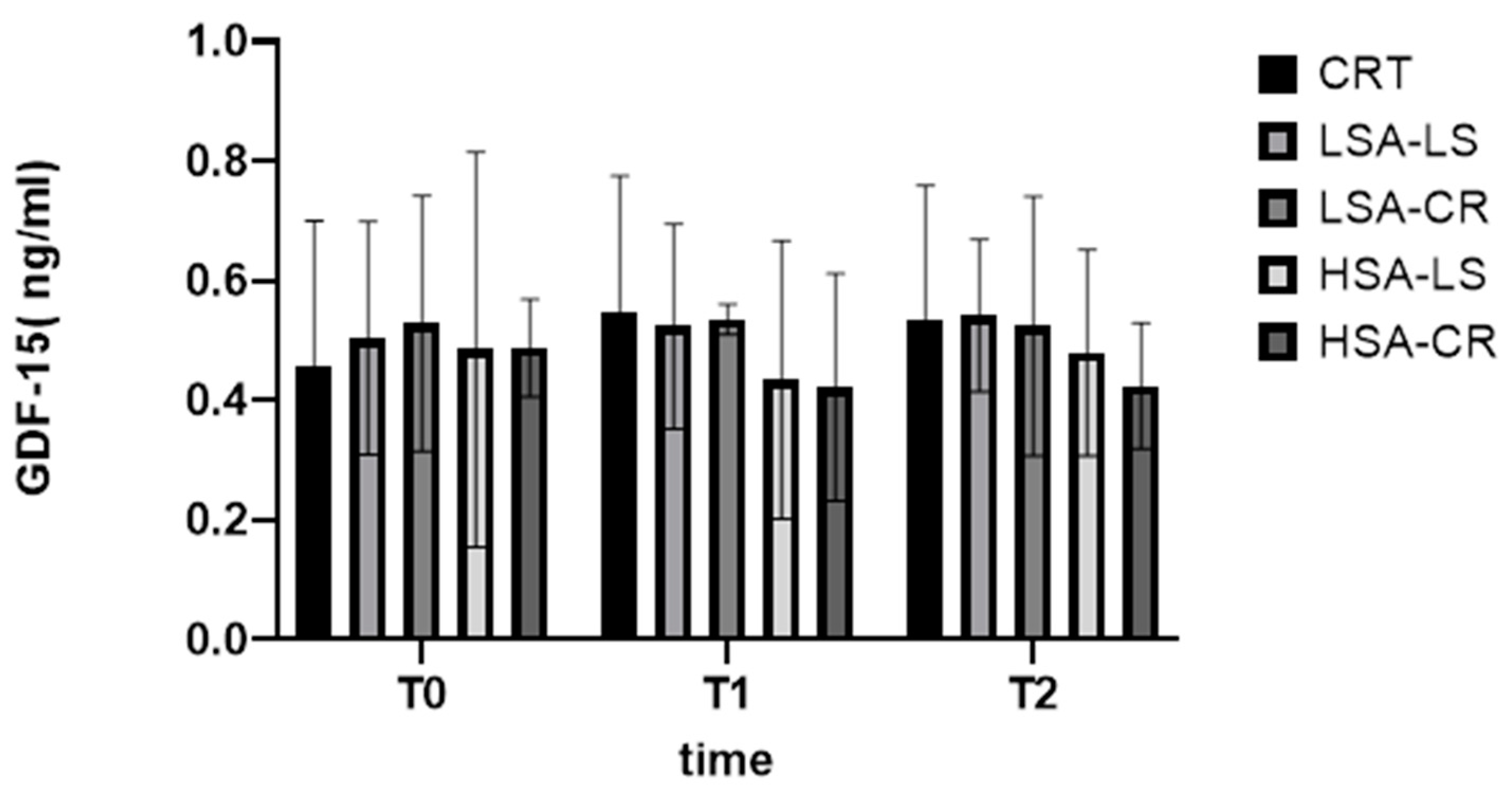
3.5. GDF-15 Levels
3.6. Implications and Future Directions
- Long-term Effects and Sustainability: Future studies should investigate the long-term effects and sustainability of different diet interventions. This could include reviewing the maintenance of improvements of metabolic health and adherence to dietary regimens for extended periods.
- Personalized Nutrition Approaches: Given the variability in individual responses based on salivary amylase activity, future research should focus on developing personalized nutrition strategies. This includes exploring how genetic and phenotypic differences can guide personalized diet recommendations to improve metabolic health.
- Mechanistic Studies: More mechanistic studies are needed to elucidate the underlying pathways through which diet interventions affect metabolic markers. This involves exploring the molecular and cellular mechanisms in adipose tissue that respond to different dietary components.
3.7. Limitations
3.8. Key Findings
- Improvements in Metabolic Markers: Both calorie-restricted (CR) and low-starch (LS) diets led to significant improvements in metabolic markers, although of different kinds.
- Impact of Salivary Amylase Activity: Participants with a higher baseline salivary amylase activity (HSA) showed better responses to the CR diet in terms of insulin sensitivity, although this finding should be interpreted with caution due to variability in individual responses.
3.9. Personalized Nutrition
3.10. Detailed Findings
- Leptin Levels: Significant reductions in leptin levels were observed in the LSA-CR group from T0 to T2, indicating the effectiveness of calorie restriction diets in decreasing leptin levels in participants with low salivary amylase activity. Interestingly, a more pronounced increase in leptin levels was observed after consuming starch-containing muesli (T1) in both LSA groups compared to those with high salivary amylase activity.
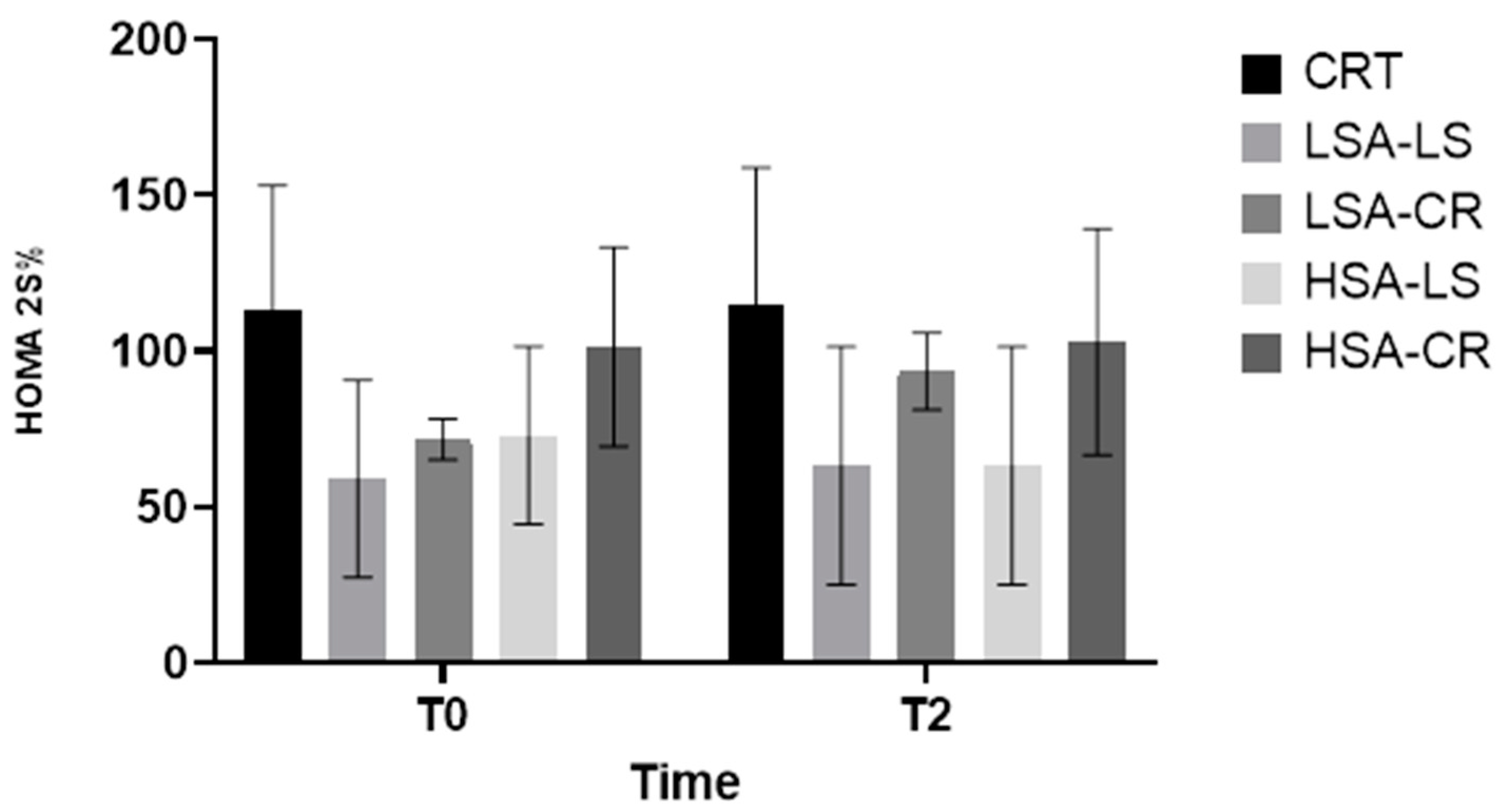
3.11. Insulin Sensitivity (HOMA2-%S)
- Physiological Mechanism: Calorie restriction can result in a more effectively reduced fat mass and enhanced insulin signaling pathways.
- Overall Metabolic Improvements: The 12-week dietary intervention resulted in significant metabolic improvements in overweight women of reproductive age, highlighting the importance of considering salivary amylase activity.
3.12. Mechanistic Insights
- Caloric Restriction: It likely reduces adipocyte size and fat mass, leading to lower leptin production and enhanced insulin sensitivity [26].
3.13. Personalized Responses Based on Salivary Amylase Activity
3.14. Implications and Future Directions
- Personalized Nutrition Approaches: The findings highlight the potential of personalized nutrition strategies based on salivary amylase activity to enhance the efficacy of dietary interventions for metabolic health.
- Long-term Effects and Sustainability: Future studies should investigate the long-term effects and sustainability of different diet interventions, including gene expression profiling to understand changes in lipid metabolism, inflammation, and insulin signaling pathways.
- Larger, Diverse Cohorts: Studies with larger and more diverse cohorts are necessary to confirm these findings and determine their generalizability to broader populations.
4. Materials and Methods
4.1. Study Design and Participants
4.2. Dietary Interventions
- Low-starch Diet Group: This group followed a low-starch diet, emphasizing the consumption of low-glycemic-index vegetables, proteins, and healthy fats. Daily starch intake was limited to less than 50 g.
- Caloric Restriction Group: This group followed a caloric restriction diet, reducing their daily caloric intake by 500 kcal from their estimated energy requirement, calculated based on the Harris–Benedict equation.
4.3. Evaluation of Salivary Amylase Activity
4.4. Biochemical and Molecular Analysis
- Active GLP-1: Measured using a GLP-1 (Active) ELISA kit (Millipore, Billerica, MA, USA).
- C-peptide: Assessed using a chemiluminescent immunoassay kit (IMMULITE 2000, Siemens Healthineers, Erlangen, Germany).
- Glucagon: Measured using the Glucagon ELISA Kit (Cat. No. EZGLU-30K, Millipore Sigma, Burlington, MA, USA).
- HOMA2-%S: Calculated using fasting insulin and glucose levels with the HOMA calculator (version 2.2.3, Diabetes Trials Unit, University of Oxford).
- Leptin: Measured using a multiplex immunoassay (Luminex, Austin, TX, USA).
- GDF-15: Quantified using ELISA kits (R&D Systems, Minneapolis, MN, USA).
- Triglycerides: Measured using an enzymatic colorimetric method with a commercial kit (Roche Diagnostics, Basel, Switzerland).
- Visceral fat: Measured using a bioimpedance scale (Omron BF511, Omron Healthcare, Kyoto, Japan).
4.5. Study Groups and Interventions
- Calorie Restriction (CR) Group: Participants followed a calorie-restricted diet, reducing their daily caloric intake by 500 kcal.
- Low-Starch (LS) Diet Group: Participants followed a low-starch diet, limiting their daily intake of starch to less than 50 g.
4.6. Dietary Intervention
4.7. Sample Collection and Analysis
- Saliva Samples: Collected in the morning after an overnight fast. Salivary amylase activity was measured using an enzymatic assay kit (Salimetrics, State College, PA, USA) according to the manufacturer’s instructions.
- Blood Samples: Fasting blood samples were analyzed for metabolic markers as described above.
4.8. Ethical Approval
4.9. Statistical Analysis
Data Analysis
- Descriptive Statistics: Median and IQR were calculated for each variable (e.g., leptin, GDF-15, HOMA2-%B) at each time point (T0, T1, T2) across all groups (HSA-CR, HSA-LS, LSA-CR, LSA-LS, and control) (Table 1).
- Group Comparisons: The Kruskal–Wallis H test was used to assess differences between the five groups at each time point. For post hoc pairwise comparisons, Dunn’s multiple comparison test with Bonferroni correction was used to determine which specific groups differ from each other (Table 2)
- Within-group Comparisons: Changes within each group over time (T0 to T1, T1 to T2, T0 to T2) were analyzed using the Wilcoxon signed rank test.
- Effect of Dietary Interventions: To evaluate the impact of diet interventions on the primary outcomes, the Friedman test was used to compare values at T0, T1, and T2 within each subgroup, followed by post hoc analysis with Dunn’s test.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt (PKB) | Protein kinase B |
| AMY1 | Alpha amylase 1 |
| CTR | Control group |
| GLP-1 | Glucagon-like peptide-1 |
| HOMA2-%B | Homeostatic model assessment of beta-cell function |
| HOMA2-IR | Homeostatic model assessment of beta-cell function—insulin resistance |
| HOMA2-%S | Homeostatic model assessment of insulin sensitivity percentage |
| HSA-CR | High-salivary-amylase calorie restriction group |
| HSA-LS | High-salivary-amylase low-starch group |
| LSA-CR | Low-salivary-amylase calorie restriction group |
| LSA-LS | Low-salivary-amylase low-starch group |
| Quantitative PCR | Quantitative polymerase chain reaction |
| RCT | Randomized controlled trial |
| RNA sequencing | Ribonucleic acid sequencing |
| T0 | Baseline |
| T1 | 30 min after starch-containing muesli consumption |
| T2 | 12 weeks post-intervention |
References
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- An, S.M.; Cho, S.H.; Yoon, J.C. Adipose Tissue and Metabolic Health. Diabetes Metab. J. 2023, 47, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S. Adipose tissue as an endocrine organ. Obesity 2006, 14 (Suppl. 5), 242S–249S. [Google Scholar] [CrossRef] [PubMed]
- Santillana, N.; Astudillo-Guerrero, C.; D’Espessailles, A.; Cruz, G. White Adipose Tissue Dysfunction: Pathophysiology and Emergent Measurements. Nutrients 2023, 15, 1722. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long-term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef] [PubMed]
- Kc, K.; Shakya, S.; Zhang, H. Gestational diabetes mellitus and macrosomia: A literature review. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 14–20. [Google Scholar] [CrossRef] [PubMed]
- Broughton, D.E.; Moley, K.H. Obesity and female infertility: Potential mediators of obesity’s impact. Fertil. Steril. 2017, 107, 840–847. [Google Scholar] [CrossRef]
- George, A.B.; Samara, J.N.; George, P.C. Diets That Reduce Calories Lead to Weight Loss Regardless of Carbohydrate Protein or Fat Content; Harvard, T.H., Ed.; Chan School of Public Health: Boston, MA, USA, 2008. [Google Scholar] [CrossRef]
- Hu, F.B. Diet strategies for promoting healthy aging and longevity: An epidemiological perspective. J. Intern. Med. 2024, 295, 508–531. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef]
- Sievenpiper, J.L. Low-carbohydrate diets and cardiometabolic health: The importance of carbohydrate quality over quantity. Nutr. Rev. 2020, 78 (Suppl. 1), 69–77. [Google Scholar] [CrossRef]
- Chavda, V.P.; Balar, P.C.; Vaghela, D.A.; Dodiya, P. Unlocking longevity with GLP-1: A key to turn back the clock? Maturitas 2024, 186, 108028. [Google Scholar] [CrossRef] [PubMed]
- Gandasi, N.R.; Gao, R.; Kothegala, L.; Pearce, A.; Santos, C.; Acreman, S.; Basco, D.; Benrick, A.; Chibalina, M.V.; Clark, A.; et al. GLP-1 metabolite GLP-1(9-36) is a systemic inhibitor of mouse and human pancreatic islet glucagon secretion. Diabetologia 2024, 67, 528–546. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Hollingsworth, K.G.; Small, P.K.; Woodcock, S.A.; Pucci, A.; Aribasala, B.; Al-Mrabeh, A.; Batterham, R.L.; Taylor, R. Calorie restriction and not glucagon-like peptide-1 explains the acute improvement in glucose control after gastric bypass in Type 2 diabetes. Diabet. Med. J. Br. Diabet. Assoc. 2016, 33, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Al-Akl, N.; Thompson, R.I.; Arredouani, A. Elevated levels of salivary α-amylase activity in saliva associated with reduced odds of obesity in adult Qatari citizens: A cross-sectional study. PLoS ONE 2022, 17, e0264692. [Google Scholar] [CrossRef] [PubMed]
- Goh, A.T.; Yao, J.; Chua, X.H.; Whitton, C.; van Dam, R.M.; Forde, C.G. Associations between oral processing, saliva, and bolus properties on daily glucose excursions amongst people at risk of type-2 diabetes. Food Funct. 2023, 14, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Bonnefond, A.; Yengo, L.; Dechaume, A.; Canouil, M.; Castelain, M.; Roger, E.; Allegaert, F.; Caiazzo, R.; Raverdy, V.; Pigeyre, M.; et al. Relationship between salivary/pancreatic amylase and body mass index: A systems biology approach. BMC Med. 2017, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Woolnough, J.W.; Bird, A.R.; Monro, J.A.; Brennan, C.S. The Effect of a Brief Salivary α-Amylase Exposure During Chewing on Subsequent in Vitro Starch Digestion Curve Profiles. Int. J. Mol. Sci. 2010, 11, 2780–2790. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.; Dhar, S.; Mitchell, L.M.; Fu, B.; Tyson, J.; Shwan, N.A.; Yang, F.; Thomas, M.G.; Armour, J.A. Obesity, starch digestion and amylase: Association between copy number variants at human salivary (AMY1) and pancreatic (AMY2) amylase genes. Hum. Mol. Genet. 2015, 24, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Klempel, N.; Thomas, K.; Conlon, J.M.; Flatt, P.R.; Irwin, N. Alpha-cells and therapy of diabetes: Inhibition, antagonism or death? Peptides 2022, 157, 170877. [Google Scholar] [CrossRef]
- Suppli, M.P.; Høgedal, A.; Bagger, J.I.; Chabanova, E.; van Hall, G.; Forman, J.L.; Christensen, M.B.; Albrechtsen NJ, W.; Holst, J.J.; Knop, F.K. Signs of Glucagon Resistance After a 2-Week Hypercaloric Diet Intervention. J. Clin. Endocrinol. Metab. 2024, 109, 955–967. [Google Scholar] [CrossRef]
- Kajani, S.; Laker, R.C.; Ratkova, E.; Will, S.; Rhodes, C.J. Hepatic glucagon action: Beyond glucose mobilization. Physiol. Rev. 2024, 104, 1021–1060. [Google Scholar] [CrossRef] [PubMed]
- Bonnet-Serrano, F.; Devin-Genteuil, C.; Thomeret, L.; Laguillier-Morizot, C.; Leguy, M.C.; Vaczlavik, A.; Bouys, L.; Zientek, C.; Bricaire, L.; Bessiène, L.; et al. C-peptide level concomitant with hypoglycemia gives better performances than insulin for the diagnosis of endogenous hyperinsulinism: A single-center study of 159 fasting trials. Eur. J. Endocrinol. 2023, 188, lvad012. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Gasbjerg, L.S.; Rosenkilde, M.M. The Role of Incretins on Insulin Function and Glucose Homeostasis. Endocrinology 2021, 162, bqab065. [Google Scholar] [CrossRef]
- Yang, S.; Cao, J.; Sun, C.; Yuan, L. The Regulation Role of the Gut-Islets Axis in Diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2024, 17, 1415–1423. [Google Scholar] [CrossRef]
- Cabeza de Baca, T.; Parrington, S.; Votruba, S.; Piaggi, P.; Krakoff, J.; Chang, D.C. Adipocyte size, adipose tissue calories, and circulating adipokines, before and after diet-induced weight loss in humans. Endocrine 2024, 84, 490–499. [Google Scholar] [CrossRef]
- Richter, M.M.; Thomsen, M.N.; Skytte, M.J.; Kjeldsen SA, S.; Samkani, A.; Frystyk, J.; Magkos, F.; Holst, J.J.; Madsbad, S.; Krarup, T.; et al. Effect of a 6-Week Carbohydrate-Reduced High-Protein Diet on Levels of FGF21 and GDF15 in People With Type 2 Diabetes. J. Endocr. Soc. 2024, 8, bvae008. [Google Scholar] [CrossRef]
- Fiorenza, M.; Checa, A.; Sandsdal, R.M.; Jensen, S.B.K.; Juhl, C.R.; Noer, M.H.; Bogh, N.P.; Lundgren, J.R.; Janus, C.; Stallknecht, B.M.; et al. Weight-loss maintenance is accompanied by interconnected alterations in circulating FGF21-adiponectin-leptin and bioactive sphingolipids. Cell Rep. Med. 2024, 5, 101629. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, S.K.; Argyrakopoulou, G.; Simati, S.; Stefanakis, K.; Kokkinos, A.; Analitis, A.; Mantzoros, C.S. Total and H-specific growth/differentiation factor 15 levels are unaffected by liraglutide or naltrexone/bupropion administration. Diabetes Obes. Metab. 2024, 26, 3147–3154. [Google Scholar] [CrossRef]
- Barr, V.A.; Malide, D.; Zarnowski, M.J.; Taylor, S.I.; Cushman, S.W. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology 1997, 138, 4463–4472. [Google Scholar] [CrossRef]
- Tsubai, T.; Noda, Y.; Ito, K.; Nakao, M.; Seino, Y.; Oiso, Y.; Hamada, Y. Insulin elevates leptin secretion and mRNA levels via cyclic AMP in 3T3-L1 adipocytes deprived of glucose. Heliyon 2016, 2, e00194. [Google Scholar] [CrossRef]



| Biomarker | Group | Time Point | Median (IQR) |
|---|---|---|---|
| Active GLP-1 | LSA-LS | T0 | 12.65 pg/mL (3.428–128.0) |
| Active GLP-1 | LSA-LS | T2 | 60.38 pg/mL (7.93–224.3) |
| Glucagon | HSA-CR | T0 | 17.85 pg/mL (12.02–31.75) |
| Glucagon | HSA-CR | T2 | 14.40 pg/mL (8.33–34.28) |
| C-peptide | LSA-LS | T0 | 2.31 ng/mL (1.26–3.3) |
| C-peptide | LSA-LS | T2 | 2.1 ng/mL (0.9–2.6) |
| Leptin | HSA-CR | T0 | 7146 pg/mL (4879–13,123) |
| Leptin | HSA-CR | T2 | 5607 pg/mL (4452–10,536) |
| Statistic/Analysis | Method | Result |
|---|---|---|
| Group comparisons | Kruskal–Wallis H test | Differences in glucagon levels between groups (H = 10.97, p = 0.0035). Glucagon levels significantly lower in high-amylase calorie restriction group |
| Kruskal–Wallis H test | Differences in C-peptide levels (H = 5.0, p = 0.0222) | |
| Kruskal–Wallis H test | Differences in leptin levels (H = 38.42, p = 0.0004) | |
| Within-group Comparisons | Wilcoxon signed rank test | Significant increase in active GLP-1 levels in LSA-LS group from 12.65 to 60.38 (W = 45, p = 0.001) |
| Dietary interventions | Friedman test followed by Dunn’s test with Bonferroni correction | Post hoc analysis revealed a significant increase in active GLP-1 levels from T0 (median: 12.65) to T2 (median: 60.38), with a significant p-value (p = 0.001) |
| Characteristic | |
|---|---|
| Age (years) | |
| Mean (SD) | 29.5 ± 6.2 |
| Range | 18–45 |
| BMI (kg/m2) | |
| Mean (SD) | 27.8 ± 2.1 |
| Range | 25–29.9 |
| Adherence (%) | |
| Mean (SD) | 85 ± 10 |
| Range | 60–100 |
| Group | Number of Participants |
|---|---|
| Control Group | 7 |
| HSA-CR | 15 |
| HSA-LS | 15 |
| LSA-CR | 15 |
| LSA-LS | 15 |
| Total | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erta, G.; Gersone, G.; Jurka, A.; Tretjakovs, P. Impact of a 12-Week Dietary Intervention on Adipose Tissue Metabolic Markers in Overweight Women of Reproductive Age. Int. J. Mol. Sci. 2024, 25, 8512. https://doi.org/10.3390/ijms25158512
Erta G, Gersone G, Jurka A, Tretjakovs P. Impact of a 12-Week Dietary Intervention on Adipose Tissue Metabolic Markers in Overweight Women of Reproductive Age. International Journal of Molecular Sciences. 2024; 25(15):8512. https://doi.org/10.3390/ijms25158512
Chicago/Turabian StyleErta, Gita, Gita Gersone, Antra Jurka, and Peteris Tretjakovs. 2024. "Impact of a 12-Week Dietary Intervention on Adipose Tissue Metabolic Markers in Overweight Women of Reproductive Age" International Journal of Molecular Sciences 25, no. 15: 8512. https://doi.org/10.3390/ijms25158512








