Strigolactones Negatively Regulate Tobacco Mosaic Virus Resistance in Nicotiana benthamiana
Abstract
1. Introduction
2. Results
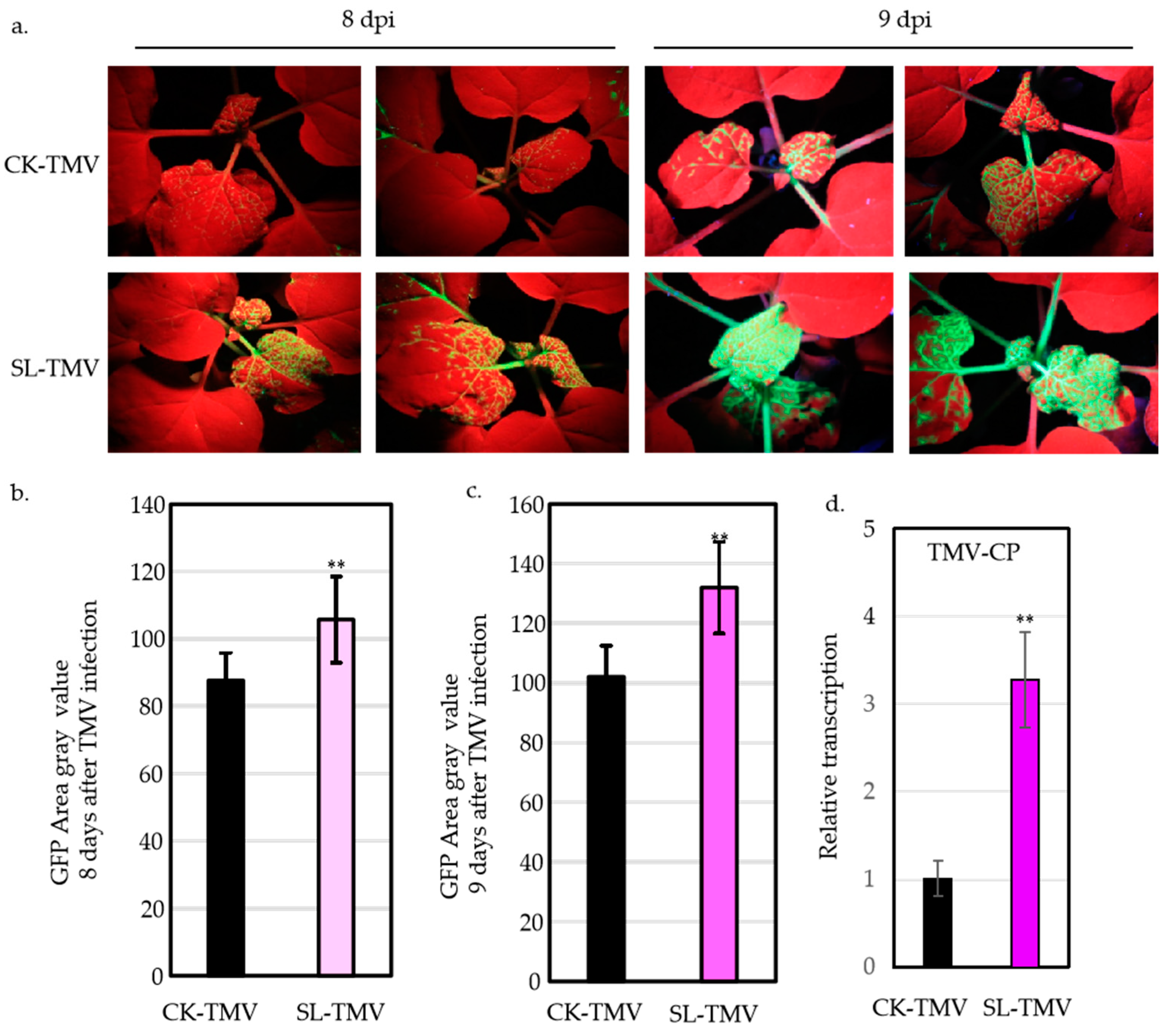
2.1. SLs Facilitating TMV Infection in N. benthamiana
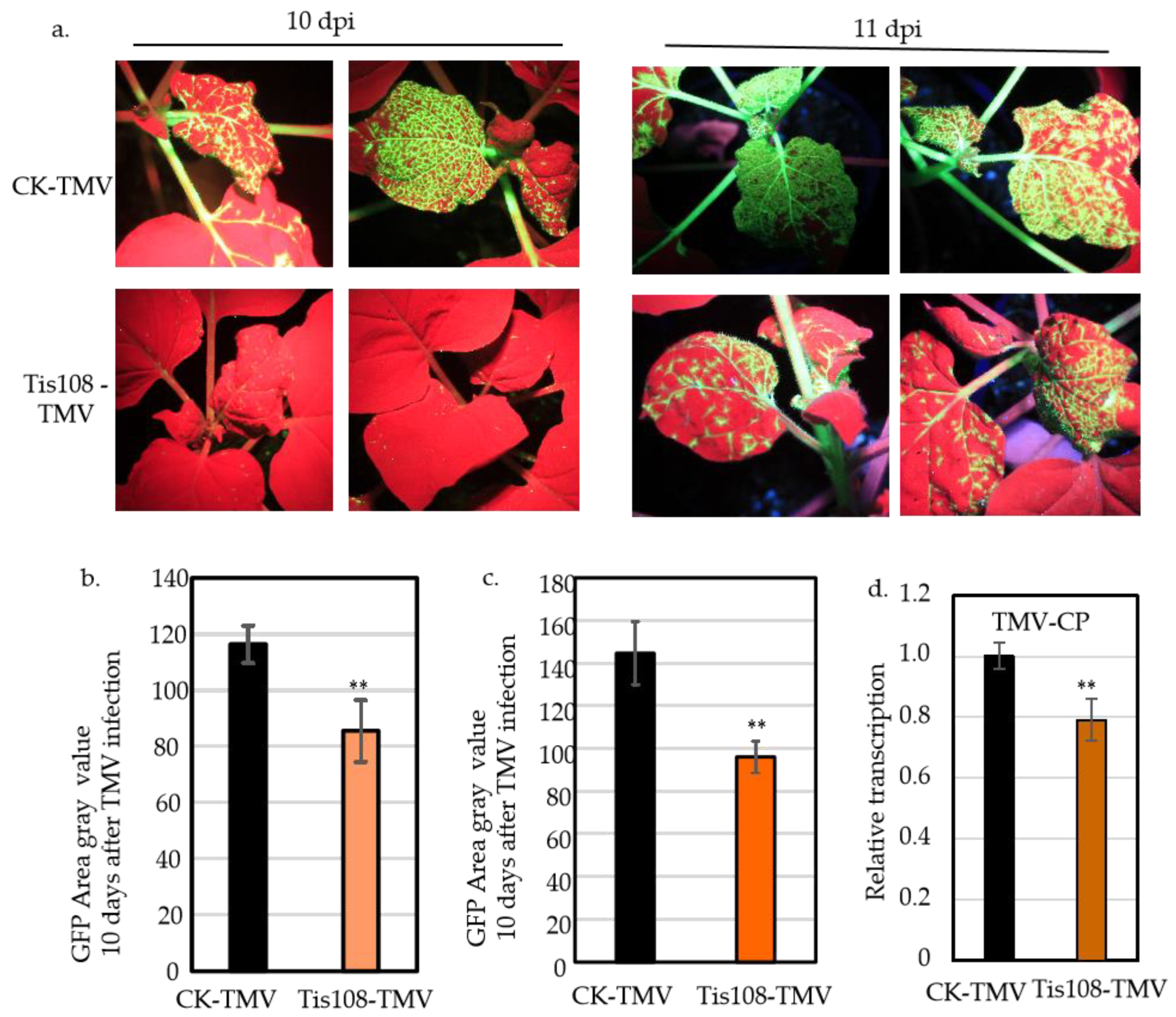
2.2. SL Inhibitor Tis108 Suppresses TMV Infection in N. benthamiana
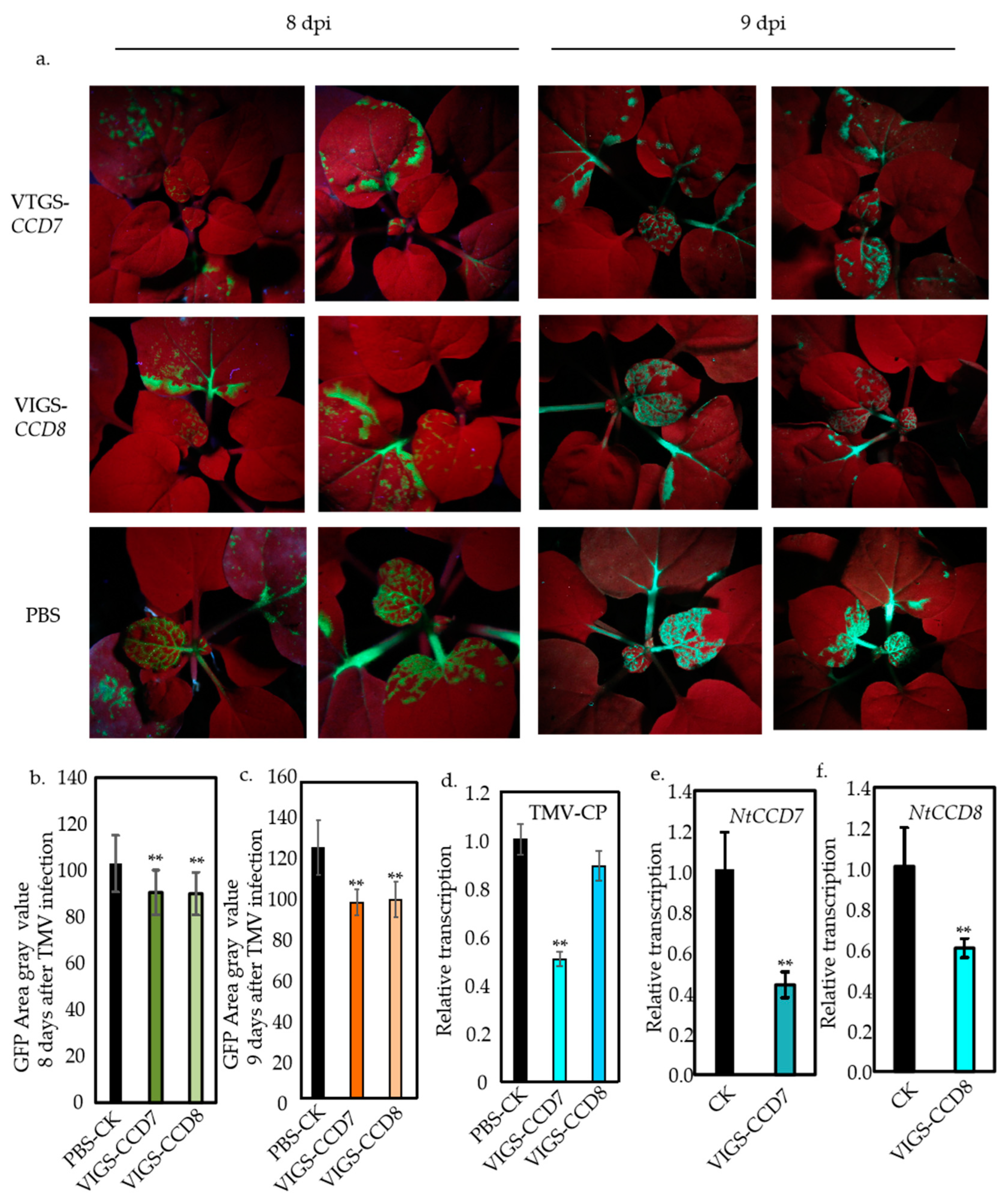
2.3. Suppression of NtCCD7 and NtCCD8 Expression in N. benthamiana Plants Dampens TMV Infection
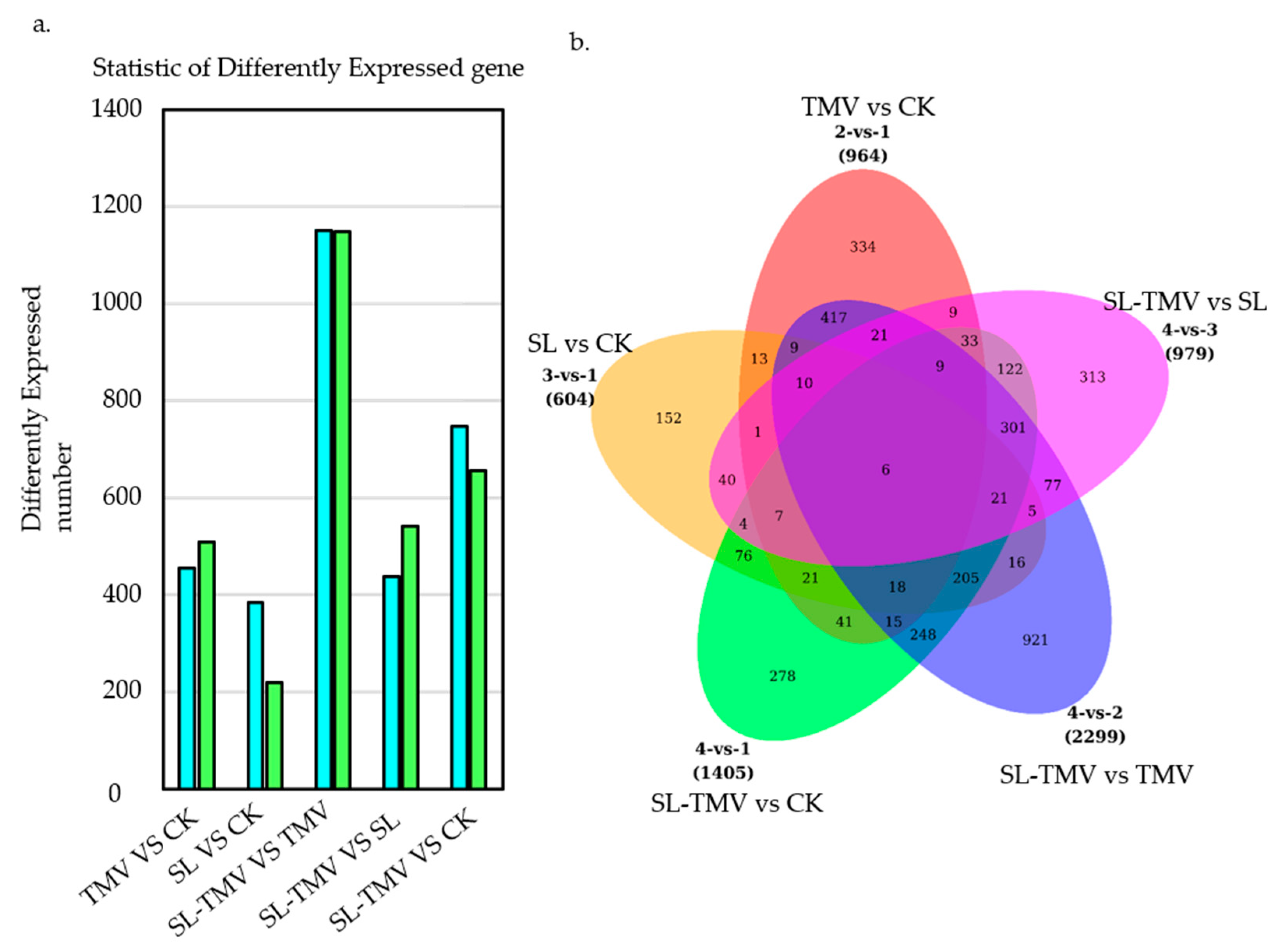
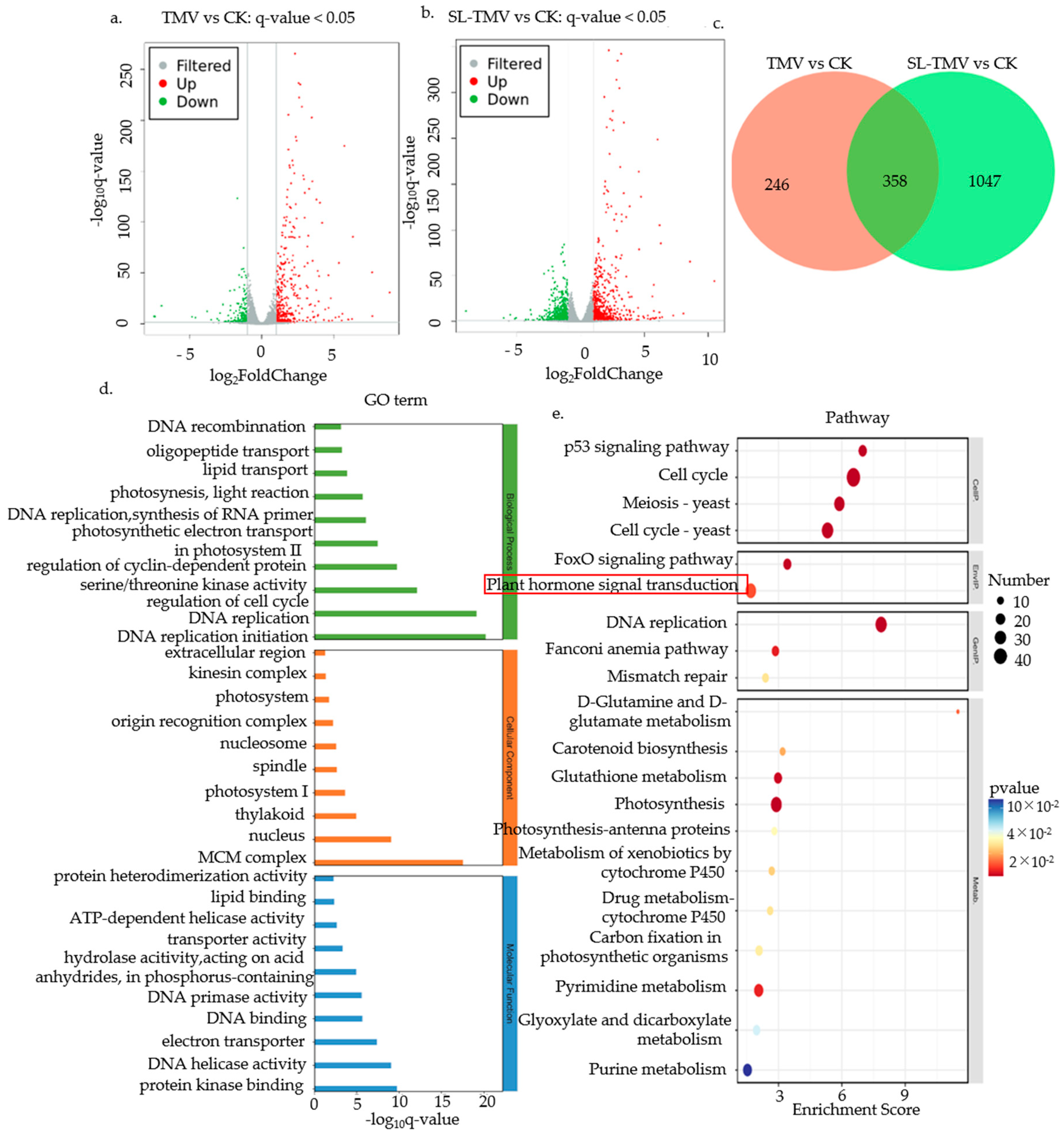
2.4. Identification of the Influence of DEGs of SLs on TMV Infection
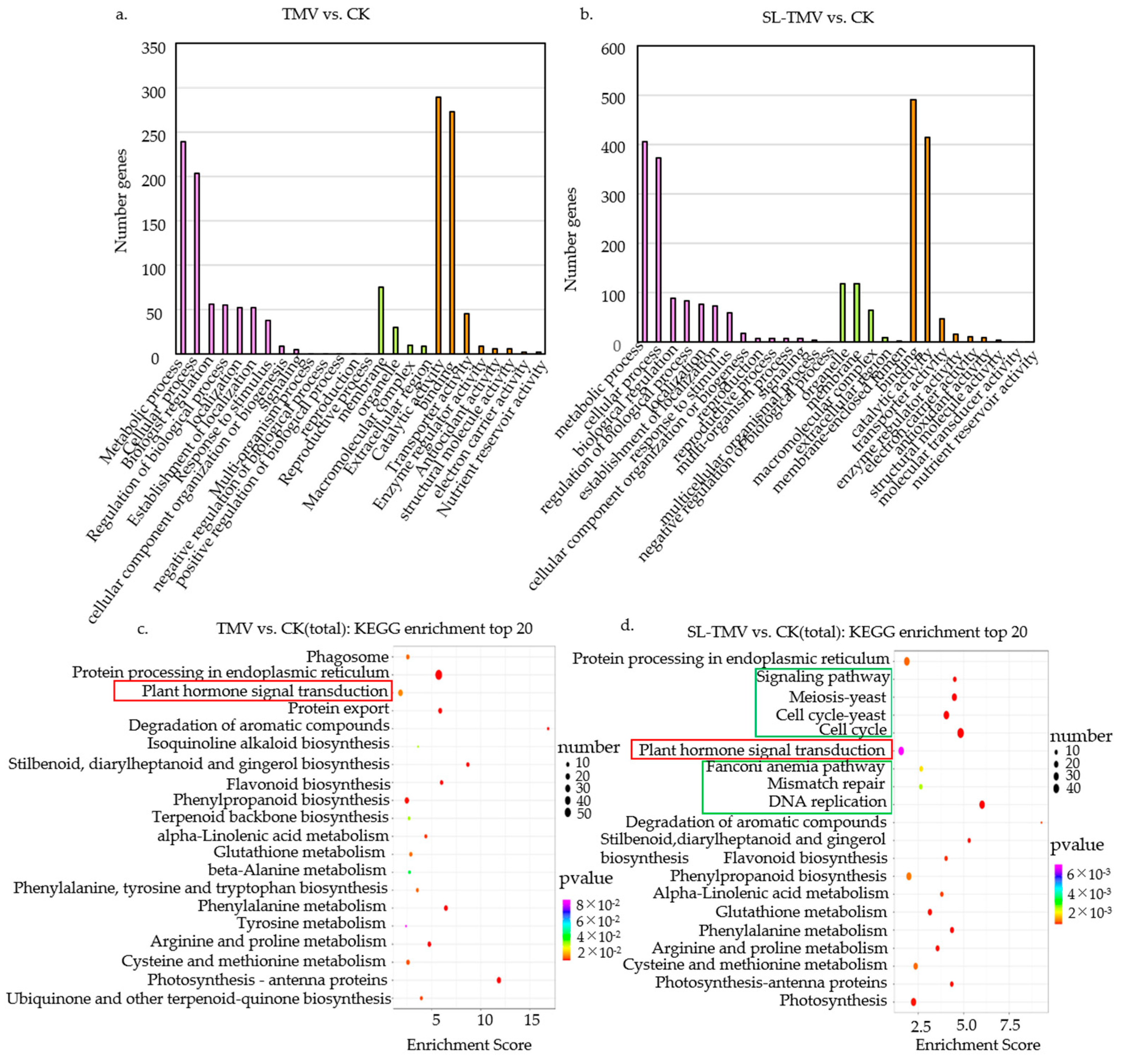
2.5. GO Functional Annotation and KEGG Pathway Analysis of SLs’ Influence on TMV Infection
2.6. Venn Diagram Analysis of DEGs in the TMV vs. CK and SL-TMV vs. CK Groups
2.7. Gene Co-Expression Network Analysis of SLs’ Influence on TMV Infection
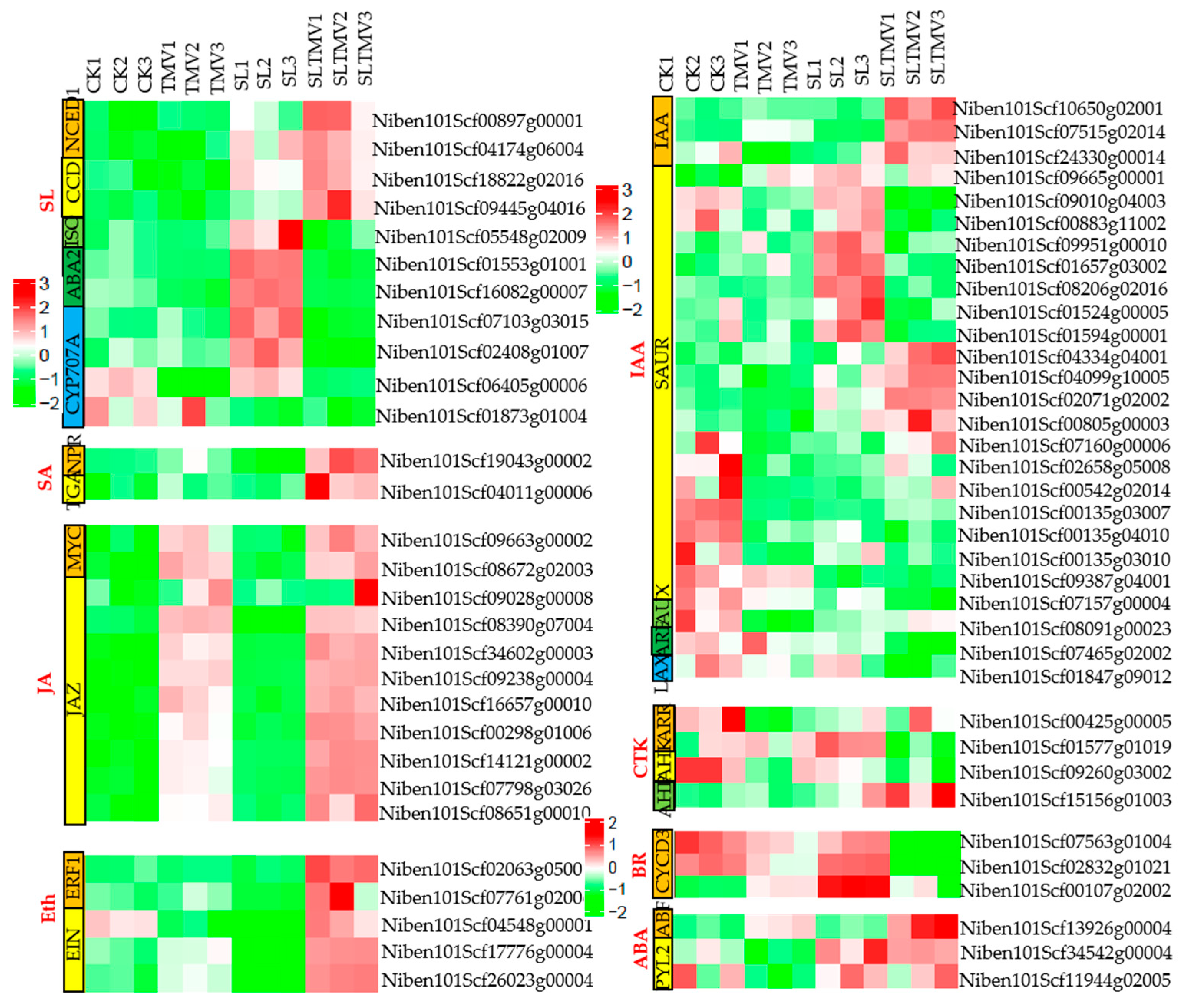
2.8. DEGs Related to Plant Hormone Signal Transduction
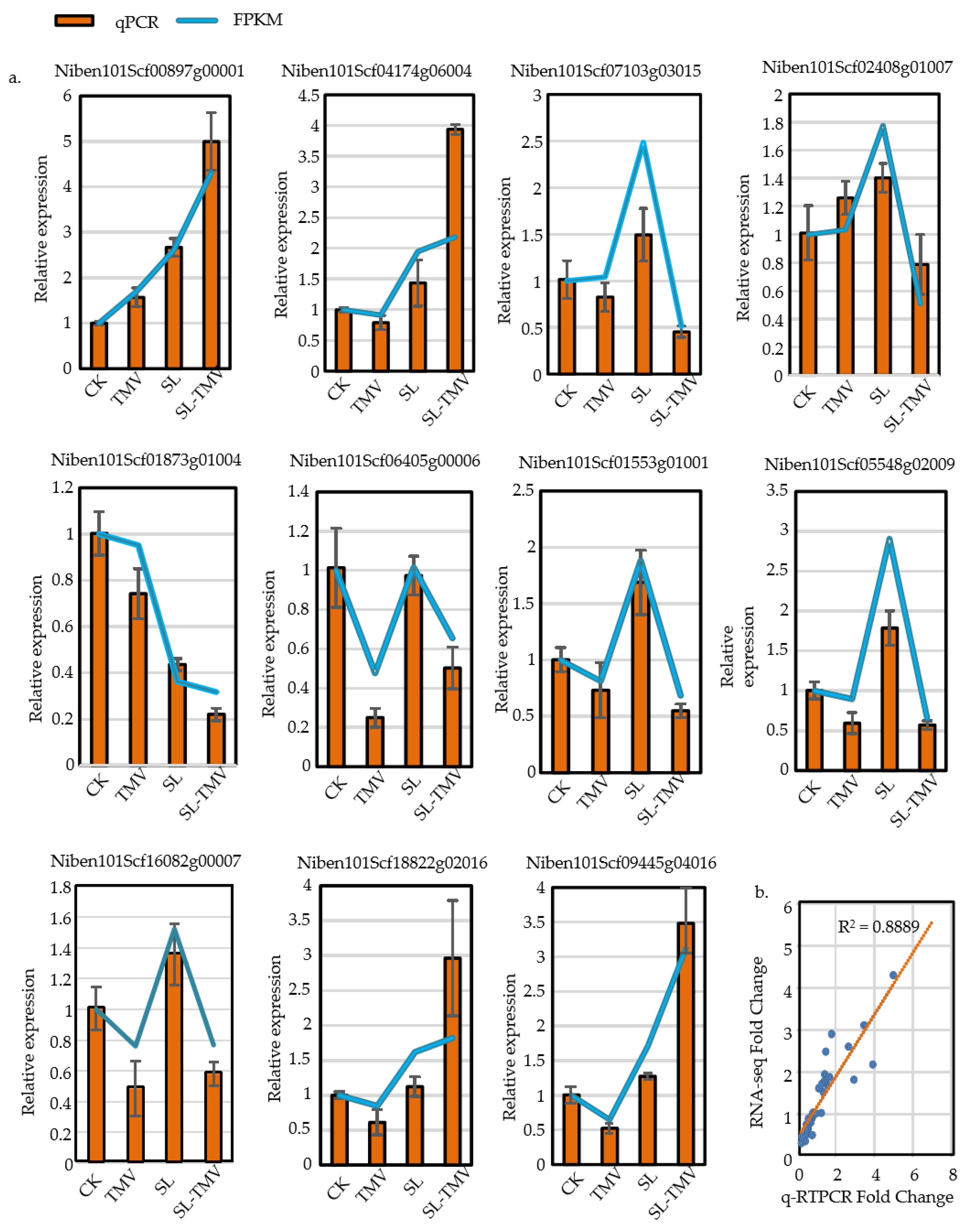
2.9. qRT-PCR Validation of Strigolactone-Signaling-Related Genes
3. Discussion
4. Materials and Methods
4.1. Virus Sources, Inoculation, and SLs/Tis108 Treatment
4.2. VIGS Vector Construction and TRV-Mediated VIGS Assay
4.3. RNA Extraction, cDNA Library Construction, and Sequencing
4.4. RNA Sequencing and Analysis of Differentially Expressed Genes
4.5. Gene Co-Expression Network Analysis
4.6. qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, R.; Qian, Q.; Yan, M.; Meng, X.; Fu, Z.; Yan, C.; Jiang, B.; Su, Z.; Li, J.; et al. DWArf27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 2009, 21, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Brewer, P.B.; Bussell, J.D.; Smith, S.M.; Beveridge, C.A. The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by Strigolactones. Plant Physiol. 2012, 159, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Stirnberg, P.; De, S.K.; Leyser, H.M. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 2002, 129, 1131–1141. [Google Scholar] [CrossRef]
- Tomotsugu, A.; Hirotaka, I.; Kenji, O.; Masahiko, M.; Masatoshi, N.; Mikiko, K.; Hitoshi, S.; Junko, K. DWARF10, an RMS1/MAX4/DAD1 ortholog, control lateral bud outgrowth in rice. Plant J. 2007, 51, 1019–1029. [Google Scholar]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Xu, J.; Zha, M.; Li, Y.; Chen, L.; Ding, C.; Wang, S. The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L.). Plant Cell Rep. 2015, 34, 1647–1662. [Google Scholar] [CrossRef]
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Piisila, M.; Keceli, M.A.; Brader, G.; Jakobson, L.; Joesaar, I.; Sipari, N.; Kollist, H.; Palva, E.T.; Kariola, T. The F-box protein MAX2 contributes to resistance to bacterial phytopathogens in Arabidopsis thaliana. BMC Plant Biol. 2015, 15, 53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, X.; Fang, P.; Zhang, H.; Chi, C.; Song, L.; Xia, X.; Shi, K.; Zhou, Y.; Zhou, J.; Yu, J. Strigolactones positively regulate defense against root-knot nematodes in tomato. J. Exp. Bot. 2019, 70, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Song, A.; Cheng, K.; Liu, J.; Wang, C.; Shao, L.; Wu, S.; Wang, P.; Zhu, J.; Liang, Z.; et al. Strigolactones positively regulate Verticillium wilt resistance in cotton via crosstalk with other hormones. Plant Physiol. 2023, 192, 945–966. [Google Scholar] [CrossRef] [PubMed]
- Loake, G.; Grant, M. Salicylic acid in plant defence—The players and protagonists. Curr. Opin. Plant Biol. 2007, 10, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Goetz, S.; Hellwege, A.; Forner, S.; Strnad, M.; Hause, B. Another JA/COI1-independent role of OPDA detected in tomato embryo development. Plant Signal. Behav. 2012, 7, 1349–1353. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; Gallei, M.; Kornienko, A.E.; Saado, I.; Khan, M.; Chia, K.S.; Darino, M.A.; Bindics, J.; Djamei, A. TOPLESS promotes plant immunity by repressing Auxin signaling and is targeted by the fungal effector Naked1. Plant Commun. 2021, 3, 100269. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Yin, Z.; Li, Y.; Lu, C.; Wang, Q.; Ding, X.A. Novel Guanine Elicitor Stimulates Immunity in Arabidopsis and Rice by Ethylene and Jasmonic Acid Signaling Pathways. Front. Plant Sci. 2022, 13, 841228. [Google Scholar] [CrossRef]
- Yao, T.; Xie, R.; Zhou, C.; Wu, X.; Li, D. Roles of Brossinosteroids Signaling in Biotic and Abiotic Stresses. J. Agric. Food Chem. 2023, 71, 7947–7960. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y. Current understanding of the interplays between host hormones and lant viral infections. PLoS Pathog. 2021, 17, e1009242. [Google Scholar] [CrossRef]
- Saito, T.; Meshi, T.; Takamatsu, N.; Okada, Y. Coat protein gene sequence of tobacco mosaic-virus encodes a host response determinant. Proc. Natl. Acad. Sci. USA 1987, 84, 6074–6077. [Google Scholar] [CrossRef]
- Yamaya, J.; Yoshioka, M.; Meshi, T.; Okada, Y.; Ohno, T. Expression of tobacco mosaic-virus rna RNA in transgenic plants. Mol. Gen. Genet. 1988, 211, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Britt, D.W.; Buijs, J.; Hlady, V. Tobacco mosaic virus adsorption on self-assembled and Langmuir-Blodgett monolayers studied by TIRF and SFM. Thin Solid Films 1988, 327, 824–828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ummers, W.C. The life of a virus: Tobacco mosaic virus as an experimental model, 1930–1965. J. Hist. Med. Allied Sci. 2003, 58, 105–106. [Google Scholar] [CrossRef]
- Adeel, M.; Farooq, T.; White, J.C.; Hao, Y.; He, Z.; Rui, Y. Carbon- based nanomaterials suppress tobacco mosaic virus (TMV) infection and induce resistance in Nicotiana benthamiana. J. Hazard. Mater. 2021, 404, 124167. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.D.; Hoak, J.M.; Ellis, B.W.; Brown, J.A.; Sit, T.L.; Wilkinson, C.A.; Reed, T.D.; Welbaum, G.E. Quantitative Real-Time PCR Analysis of Individual Flue-Cured Tobacco Seeds and Seedlings Reveals Seed Transmission of Tobacco MosaicVirus. Phytopathology 2020, 110, 194–205. [Google Scholar] [CrossRef]
- Lv, X.; Xiang, S.; Wang, X.; Wu, L.; Liu, C.; Yuan, M.; Gong, W.; Win, H.; Hao, C.; Xue, Y.; et al. Synthetic chloroinconazide compound exhibits highly efficient antiviral activity against tobacco mosaic virus. Pest Manag. Sci. 2020, 76, 3636–3648. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Miao, J.; Kang, W.; Shi, S. Exogenous application of salicylic acid improves freezing stress tolerance in alfalfa. Front. Plant Sci. 2023, 14, 1091077. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Chen, Y.; Wu, D.; Du, Y.; Wang, M.; Liu, C.; Chu, J.; Yao, X. Transcriptome Analysis Reveals an Essential Role of Exogenous Brassinolide on the Alkaloid Biosynthesis Pathway in Pinellia Ternata. Int. J. Mol. Sci. 2022, 23, 10898. [Google Scholar] [CrossRef]
- Luo, J.; Xia, W.; Cao, P.; Xiao, Z.; Zhang, Y.; Liu, M.; Zhan, C.; Wang, N. Integrated Transcriptome Analysis Reveals Plant Hormones Jasmonic Acid and Salicylic Acid Coordinate Growth and Defense Responses upon Fungal Infection in Poplar. Biomolecules 2019, 9, 12. [Google Scholar] [CrossRef]
- Koo, M.B.; Lee, S.W.; Lee, J.M.; Kim, K.T. Iterative Convergent Synthesis of Large Cyclic Polymers and Block Copolymers with Discrete Molecular Weights. J. Am. Chem. Soc. 2020, 142, 14028–14032. [Google Scholar] [CrossRef]
- Oka, K.; Kobayashi, M.; Mitsuhara, I.; Seo, S. Jasmonic acid negatively regulates resistance to Tobacco mosaic virus in tobacco. Plant Cell Physiol. 2013, 54, 1999–2010. [Google Scholar] [CrossRef]
- Deng, X.G.; Zhu, T.; Peng, X.J.; Xi, D.H.; Guo, H.; Yin, Y.; Zhang, D.W.; Lin, H.H. Role of brassinosteroid signaling in modulating Tobacco mosaic virus resistance in Nicotiana benthamiana. Sci. Rep. 2016, 6, 20579. [Google Scholar] [CrossRef] [PubMed]
- Kusajima, M.; Fujita, M.; Soudthedlath, K.; Nakamura, H.; Yoneyama, K.; Nomura, T.; Akiyama, K.; Maruyama-Nakashita, A.; Asami, T.; Nakashita, H. Strigolactones Modulate Salicylic Acid- Mediated Disease Resistance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 5246. [Google Scholar] [CrossRef] [PubMed]
- Visentin, I.; Vitali, M.; Ferrero, M.; Zhang, Y.; Ruyter-Spira, C.; Novák, O.; Strnad, M.; Lovisolo, C.; Schubert, A.; Cardinale, F. Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytol. 2016, 212, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, X.; Zhu, X.; Gu, P.; Zhang, W.; Tao, W.; Wang, D.; Wu, Y.; Zhao, Q.; Xu, G.; et al. Strigolactone and gibberellin signaling coordinately regulate metabolic adaptations to changes in nitrogen availability in rice. Mol. Plant 2023, 16, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhang, P.; Meng, Y.F.; Xu, F.; Zhang, D.W.; Cheng, J.; Lin, H.H.; Xi, D.H. Alpha-momorcharin, a RIP produced by bitter melon, enhances defense response in tobacco plants against diverse plant viruses and shows antifungal acitivity in vitro. Planta 2013, 237, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xi, D.H.; Yuan, S.; Xu, F.; Zhang, D.W.; Lin, H.H. Salicylic acid and jasmonic acid are essential for systemic resistance against tobacco mosaic virus in Nicotiana benthamiana. Mol. Plant Microbe Interact. 2014, 27, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Zou, W.S.; Wu, G.; Lin, H.H.; Xi, D.H. Tobacco alpha-expansin EXPA4 plays a role in Nicotiana benthamiana defence against Tobacco mosaic virus. Planta 2018, 247, 355–368. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Bie, S.; Li, S.; Yuan, B.; Zhang, L.; Zhang, Z.; Chen, J.; Ning, W.; Peng, J.; Zhang, Y.; et al. Strigolactones Negatively Regulate Tobacco Mosaic Virus Resistance in Nicotiana benthamiana. Int. J. Mol. Sci. 2024, 25, 8518. https://doi.org/10.3390/ijms25158518
Huang R, Bie S, Li S, Yuan B, Zhang L, Zhang Z, Chen J, Ning W, Peng J, Zhang Y, et al. Strigolactones Negatively Regulate Tobacco Mosaic Virus Resistance in Nicotiana benthamiana. International Journal of Molecular Sciences. 2024; 25(15):8518. https://doi.org/10.3390/ijms25158518
Chicago/Turabian StyleHuang, Renyan, Shuaijun Bie, Shan Li, Bin Yuan, Li Zhang, Zhuo Zhang, Jianbin Chen, Weimin Ning, Jing Peng, Yu Zhang, and et al. 2024. "Strigolactones Negatively Regulate Tobacco Mosaic Virus Resistance in Nicotiana benthamiana" International Journal of Molecular Sciences 25, no. 15: 8518. https://doi.org/10.3390/ijms25158518
APA StyleHuang, R., Bie, S., Li, S., Yuan, B., Zhang, L., Zhang, Z., Chen, J., Ning, W., Peng, J., Zhang, Y., Zhang, S., Liu, Y., & Zhang, D. (2024). Strigolactones Negatively Regulate Tobacco Mosaic Virus Resistance in Nicotiana benthamiana. International Journal of Molecular Sciences, 25(15), 8518. https://doi.org/10.3390/ijms25158518





