Analysis of Amyloid Fibrillation of Two Family 1 Glycoside Hydrolases
Abstract
1. Introduction
2. Results and Discussion
2.1. Amylogenic Regions in BglA and BglB Structures
2.2. Fluorometric and Microscopic Analysis of BglA and BglB Solutions at a Wide Range of pH and Temperature Conditions
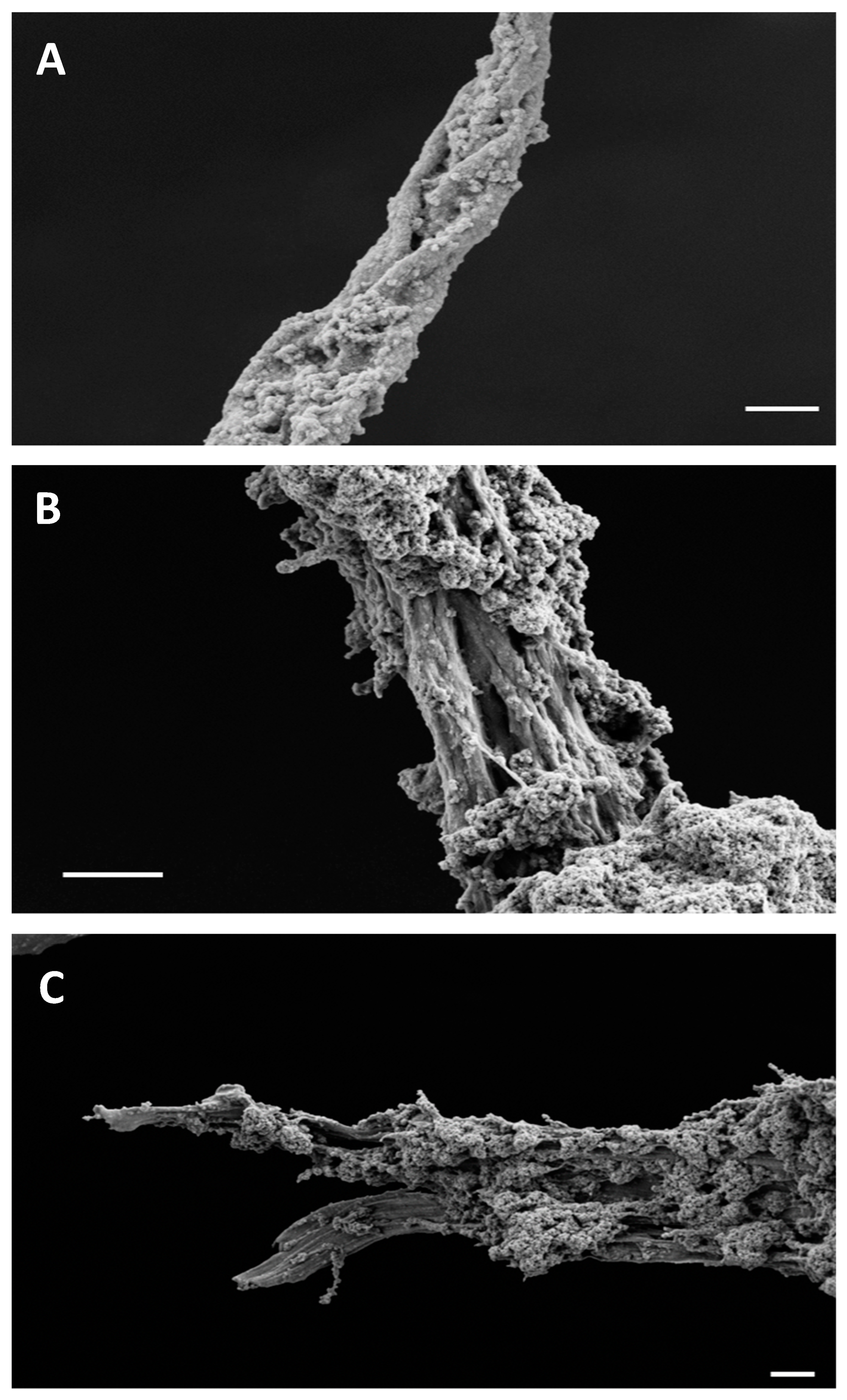
2.3. FESEM and Confocal Microscopy Analysis of Amyloid Fibers
2.4. Circular Dichroism Spectroscopy Analysis
3. Materials and Methods
3.1. Enzyme Production and Purification
3.2. Extrinsic Fluorescence
3.3. Activity Assays
3.4. Intrinsic Fluorescence
3.5. Microscopy
3.6. Circular Dichroism
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurung, R.; Li, T. Renal Amyloidosis: Presentation, Diagnosis, and Management. Am. J. Med. 2022, 135, S38–S43. [Google Scholar] [CrossRef] [PubMed]
- Sinnige, T. Molecular Mechanisms of Amyloid Formation in Living Systems. Chem. Sci. 2022, 13, 7080–7097. [Google Scholar] [CrossRef] [PubMed]
- Picken, M.M. The Pathology of Amyloidosis in Classification: A Review. Acta Haematol. 2020, 143, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Escamilla, A.M.; Rousseau, F.; Schymkowitz, J.; Serrano, L. Prediction of Sequence-Dependent and Mutational Effects on the Aggregation of Peptides and Proteins. Nat. Biotechnol. 2004, 22, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the Cross-β Spine of Amyloid-like Fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Ladanza, M.G.; Jackson, M.P.; Hewitt, E.W.; Ranson, N.A.; Radford, S.E. A New Era for Understanding Amyloid Structures and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Penke, B.; Szucs, M.; Bogár, F. Oligomerization and Conformational Change Turn Monomeric β-Amyloid and Tau Proteins Toxic: Their Role in Alzheimer’s Pathogenesis. Molecules 2020, 25, 1659. [Google Scholar] [CrossRef]
- Dear, A.J.; Michaels, T.C.; Meisl, G.; Klenerman, D.; Wu, S.; Perrett, S.; Linse, S.; Dobson, C.M.; Knowles, T.P. Kinetic Diversity of Amyloid Oligomers. Proc. Natl. Acad. Sci. USA 2020, 117, 12087–12094. [Google Scholar] [CrossRef]
- Taylor, A.I.P.; Staniforth, R.A. General Principles Underpinning Amyloid Structure. Front. Neurosci. 2022, 16, 878869. [Google Scholar] [CrossRef]
- Wang, L.Q.; Ma, Y.; Yuan, H.Y.; Zhao, K.; Zhang, M.Y.; Wang, Q.; Huang, X.; Xu, W.C.; Dai, B.; Chen, J.; et al. Cryo-EM Structure of an Amyloid Fibril Formed by Full-Length Human SOD1 Reveals Its Conformational Conversion. Nat. Commun. 2022, 13, 3491. [Google Scholar] [CrossRef] [PubMed]
- Heerde, T.; Rennegarbe, M.; Biedermann, A.; Savran, D.; Pfeiffer, P.B.; Hitzenberger, M.; Baur, J.; Puscalau-Girtu, I.; Zacharias, M.; Schwierz, N.; et al. Cryo-EM Demonstrates the in Vitro Proliferation of an Ex Vivo Amyloid Fibril Morphology by Seeding. Nat. Commun. 2022, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Seidler, P.M.; Murray, K.A.; Boyer, D.R.; Ge, P.; Sawaya, M.R.; Hu, C.J.; Cheng, X.; Abskharon, R.; Pan, H.; DeTure, M.A.; et al. Structure-Based Discovery of Small Molecules That Disaggregate Alzheimer’s Disease Tissue Derived Tau Fibrils in Vitro. Nat. Commun. 2022, 13, 5451. [Google Scholar] [CrossRef] [PubMed]
- Wierenga, R.K. The TIM-Barrel Fold: A Versatile Framework for Efficient Enzymes. FEBS Lett. 2001, 492, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Physiology: Suppression of Aging in Mice by the Hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, Y.; Goetz, R.; Fu, L.; Jayaraman, S.; Hu, M.C.; Moe, O.W.; Liang, G.; Li, X.; Mohammadi, M. α-Klotho Is a Non-Enzymatic Molecular Scaffold for FGF23 Hormone Signalling. Nature 2018, 553, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, J.; Mohanty, J.; Sousa, L.P.; Tome, F.; Pardon, E.; Steyaert, J.; Lemmon, M.A.; Lax, I.; Schlessinger, J. Structures of β-Klotho Reveal a ‘Zip Code’-like Mechanism for Endocrine FGF Signalling. Nature 2018, 553, 501–505. [Google Scholar] [CrossRef]
- Behrendt, M.; Polaina, J.; Naim, H.Y. Structural Hierarchy of Regulatory Elements in the Folding and Transport of an Intestinal Multidomain Protein. J. Biol. Chem. 2010, 285, 4143–4152. [Google Scholar] [CrossRef]
- Sanz-Aparicio, J.; Hermoso, J.A.; Martínez-Ripoll, M.; Lequerica, J.L.; Polaina, J. Crystal Structure of Beta-Glucosidase A from Bacillus Polymyxa: Insights into the Catalytic Activity in Family 1 Glycosyl Hydrolases. J. Mol. Biol. 1998, 275, 491–502. [Google Scholar] [CrossRef]
- Isorna, P.; Polaina, J.; Latorre-García, L.; Cañada, F.J.; González, B.; Sanz-Aparicio, J. Crystal Structures of Paenibacillus Polymyxa β-Glucosidase B Complexes Reveal the Molecular Basis of Substrate Specificity and Give New Insights into the Catalytic Machinery of Family I Glycosidases. J. Mol. Biol. 2007, 371, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- González-Blasco, G.; Sanz-Aparicio, J.; González, B.; Hermoso, J.A.; Polaina, J. Directed Evolution of β-Glucosidase A from Paenibacillus Polymyxa to Thermal Resistance. J. Biol. Chem. 2000, 275, 13708–13712. [Google Scholar] [CrossRef] [PubMed]
- Arrizubieta, M.J.; Polaina, J. Increased Thermal Resistance and Modification of the Catalytic Properties of a β-Glucosidase by Random Mutagenesis and in Vitro Recombination. J. Biol. Chem. 2000, 275, 28843–28848. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Ventura, S. Computational Methods to Predict Protein Aggregation. Curr. Opin. Struct. Biol. 2022, 73, 102343. [Google Scholar] [CrossRef]
- Louros, N.; Konstantoulea, K.; De Vleeschouwer, M.; Ramakers, M.; Schymkowitz, J.; Rousseau, F. WALTZ-DB 2.0: An Updated Database Containing Structural Information of Experimentally Determined Amyloid-Forming Peptides. Nucleic Acids Res. 2020, 48, D389–D393. [Google Scholar] [CrossRef] [PubMed]
- López De La Paz, M.; Serrano, L. Sequence Determinants of Amyloid Fibril Formation. Proc. Natl. Acad. Sci. USA 2004, 101, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.K.; Eisenberg, D. Short Protein Segments Can Drive a Non-Fibrillizing Protein into the Amyloid State. Protein Eng. Des. Sel. 2009, 22, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Tsiolaki, P.L.; Louros, N.N.; Iconomidou, V.A. Hexapeptide Tandem Repeats Dictate the Formation of Silkmoth Chorion, a Natural Protective Amyloid. J. Mol. Biol. 2018, 430, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
- Louros, N.N.; Bolas, G.M.P.; Tsiolaki, P.L.; Hamodrakas, S.J.; Iconomidou, V.A. Intrinsic Aggregation Propensity of the CsgB Nucleator Protein Is Crucial for Curli Fiber Formation. J. Struct. Biol. 2016, 195, 179–189. [Google Scholar] [CrossRef]
- Tian, Y.; Viles, J.H. PH Dependence of Amyloid-β Fibril Assembly Kinetics: Unravelling the Microscopic Molecular Processes. Angew. Chem. Int. Ed. 2022, 61, e202210675. [Google Scholar] [CrossRef]
- Biancalana, M.; Koide, S. Molecular Mechanism of Thioflavin-T Binding to Amyloid Fibrils. Biochim. Biophys. Acta 2010, 1804, 1405–1412. [Google Scholar] [CrossRef]
- Sulatsky, M.I.; Sulatskaya, A.I.; Povarova, O.I.; Antifeeva, I.A.; Kuznetsova, I.M.; Turoverov, K.K. Effect of the Fluorescent Probes ThT and ANS on the Mature Amyloid Fibrils. Prion 2020, 14, 67–75. [Google Scholar] [CrossRef]
- Younan, N.D.; Viles, J.H. A Comparison of Three Fluorophores for the Detection of Amyloid Fibers and Prefibrillar Oligomeric Assemblies. ThT (Thioflavin T); ANS (1-Anilinonaphthalene-8-Sulfonic Acid); and BisANS (4,4′-Dianilino-1,1′-Binaphthyl-5,5′-Disulfonic Acid). Biochemistry 2015, 54, 4297–4306. [Google Scholar] [CrossRef]
- Ábrahám, Á.; Massignan, F.; Gyulai, G.; Katona, M.; Taricska, N.; Kiss, É. Comparative Study of the Solid-Liquid Interfacial Adsorption of Proteins in Their Native and Amyloid Forms. Int. J. Mol. Sci. 2022, 23, 13219. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Yang, F.; Yu, H.; Xie, Y.; Yao, W. Lysozyme Amyloid Fibril: Regulation, Application, Hazard Analysis, and Future Perspectives. Int. J. Biol. Macromol. 2022, 200, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Howie, A.J.; Brewer, D.B.; Howell, D.; Jones, A.P. Physical Basis of Colors Seen in Congo Red-Stained Amyloid in Polarized Light. Lab. Investig. 2008, 88, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Howie, A.J.; Brewer, D.B. Optical Properties of Amyloid Stained by Congo Red: History and Mechanisms. Micron 2009, 40, 285–301. [Google Scholar] [CrossRef]
- Howie, A.J. Origins of a Pervasive, Erroneous Idea: The “Green Birefringence” of Congo Red-Stained Amyloid. Int. J. Exp. Pathol. 2019, 100, 208–221. [Google Scholar] [CrossRef]
- Brännström, K.; Gharibyan, A.L.; Islam, T.; Iakovleva, I.; Nilsson, L.; Lee, C.C.; Sandblad, L.; Pamren, A.; Olofsson, A. Scanning Electron Microscopy as a Tool for Evaluating Morphology of Amyloid Structures Formed on Surface Plasmon Resonance Chips. Data Brief 2018, 19, 1166–1170. [Google Scholar] [CrossRef]
- Chamberlain, A.K.; MacPhee, C.E.; Zurdo, J.; Morozova-Roche, L.A.; Hill, H.A.O.; Dobson, C.M.; Davis, J.J. Ultrastructural Organization of Amyloid Fibrils by Atomic Force Microscopy. Biophys. J. 2000, 79, 3282–3293. [Google Scholar] [CrossRef]
- Surmacz-Chwedoruk, W.; Babenko, V.; Dec, R.; Szymczak, P.; Dzwolak, W. The Emergence of Superstructural Order in Insulin Amyloid Fibrils upon Multiple Rounds of Self-Seeding. Sci. Rep. 2016, 6, 32022. [Google Scholar] [CrossRef] [PubMed]
- Lövestam, S.; Scheres, S.H.W. High-Throughput Cryo-EM Structure Determination of Amyloids. Faraday Discuss. 2022, 240, 243–260. [Google Scholar] [CrossRef] [PubMed]







| BglA | |||||||||
| Temperature | IF 280 nm | IF 295 nm | ThT | ANS | Residual Activity | ||||
| °C | pH | 1 h | 1 h | 1 h | 16 h | 1 h | 16 h | 1 h | 16 h |
| 25 | 3 | 0.6 | 0.5 | 0.9 | 1.4 | 1 | 4 | 0.6 | 0.9 |
| 7 | 1 | 1 | 1 | 1.1 | 1 | 1.4 | 1 | 1 | |
| 12 | 0.7 | 0.9 | 0.6 | 1 | 1.5 | 3 | 0.9 | 0.9 | |
| 45 | 3 | 0.5 | 0.8 | 0.4 | 0.1 | 1 | 1 | 0.5 | 0.8 |
| 7 | 1.2 | 0.6 | 0.6 | 0.5 | 0.7 | 0.8 | 1.2 | 1 | |
| 12 | 0.2 | 0.7 | 0 | 0 | 1.5 | 2.3 | 0.7 | 1 | |
| 65 | 3 | 0.4 | 0.5 | 0.3 | 1.5 | 0.6 | 1 | 0 | 0 |
| 7 | 0.9 | 0.3 | 0.3 | 1 | 0.5 | 0.6 | 1.1 | 0 | |
| 12 | 0.15 | 0.2 | 0.9 | 0.8 | 0.9 | 0.8 | 0 | 0 | |
| BglB | |||||||||
| Temperature | IF 280 nm | IF 295 nm | ThT | ANS | Residual Activity | ||||
| °C | pH | 1 h | 1 h | 1 h | 16 h | 1 h | 16 h | 1 h | 16 h |
| 25 | 3 | 1.5 | 0.5 | 0.9 | 1.3 | 1 | 1.4 | 0.8 | 0.7 |
| 7 | 1 | 1 | 1 | 0.8 | 1 | 1 | 1 | 1 | |
| 12 | 0.9 | 0.7 | 0.6 | 0 | 1.5 | 7.8 | 0.8 | 0.6 | |
| 45 | 3 | 0.4 | 0.8 | 6.5 | 24.5 | 3.8 | 19.8 | 0.2 | 0 |
| 7 | 1.5 | 0.6 | 2.4 | 10.6 | 1.2 | 10.3 | 0.9 | 0.6 | |
| 12 | 0.4 | 0.6 | 0 | 0 | 0.8 | 2 | 0 | 0 | |
| 65 | 3 | 4.2 | 3.7 | 3.9 | 7.9 | 2.5 | 4.8 | 0 | 0 |
| 7 | 0.6 | 0.6 | 1.2 | 4.1 | 1 | 3.4 | 0 | 0 | |
| 12 | 0.6 | 1.1 | 0 | 0 | 0.3 | 0.3 | 0 | 0 | |
| Protein | pH | α-Helix a | β-Sheet a | Turns a | Unordered a | NRMSD b |
|---|---|---|---|---|---|---|
| BglA | 7 | 28 | 16 | 15 | 25 | 0.015 |
| 4 | 3 | 34 | 26 | 31 | 0.032 | |
| BglB | 7 | 31 | 14 | 14 | 24 | 0.018 |
| 4 | 7 | 34 | 23 | 30 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-López, M.A.; Coll-Marqués, J.M.; Talens-Perales, D.; Marín-Navarro, J.; Polaina, J.; Vázquez-Contreras, E. Analysis of Amyloid Fibrillation of Two Family 1 Glycoside Hydrolases. Int. J. Mol. Sci. 2024, 25, 8536. https://doi.org/10.3390/ijms25158536
Rodríguez-López MA, Coll-Marqués JM, Talens-Perales D, Marín-Navarro J, Polaina J, Vázquez-Contreras E. Analysis of Amyloid Fibrillation of Two Family 1 Glycoside Hydrolases. International Journal of Molecular Sciences. 2024; 25(15):8536. https://doi.org/10.3390/ijms25158536
Chicago/Turabian StyleRodríguez-López, Miguel A., José María Coll-Marqués, David Talens-Perales, Julia Marín-Navarro, Julio Polaina, and Edgar Vázquez-Contreras. 2024. "Analysis of Amyloid Fibrillation of Two Family 1 Glycoside Hydrolases" International Journal of Molecular Sciences 25, no. 15: 8536. https://doi.org/10.3390/ijms25158536
APA StyleRodríguez-López, M. A., Coll-Marqués, J. M., Talens-Perales, D., Marín-Navarro, J., Polaina, J., & Vázquez-Contreras, E. (2024). Analysis of Amyloid Fibrillation of Two Family 1 Glycoside Hydrolases. International Journal of Molecular Sciences, 25(15), 8536. https://doi.org/10.3390/ijms25158536





