Aging, Cancer, and Inflammation: The Telomerase Connection
Abstract
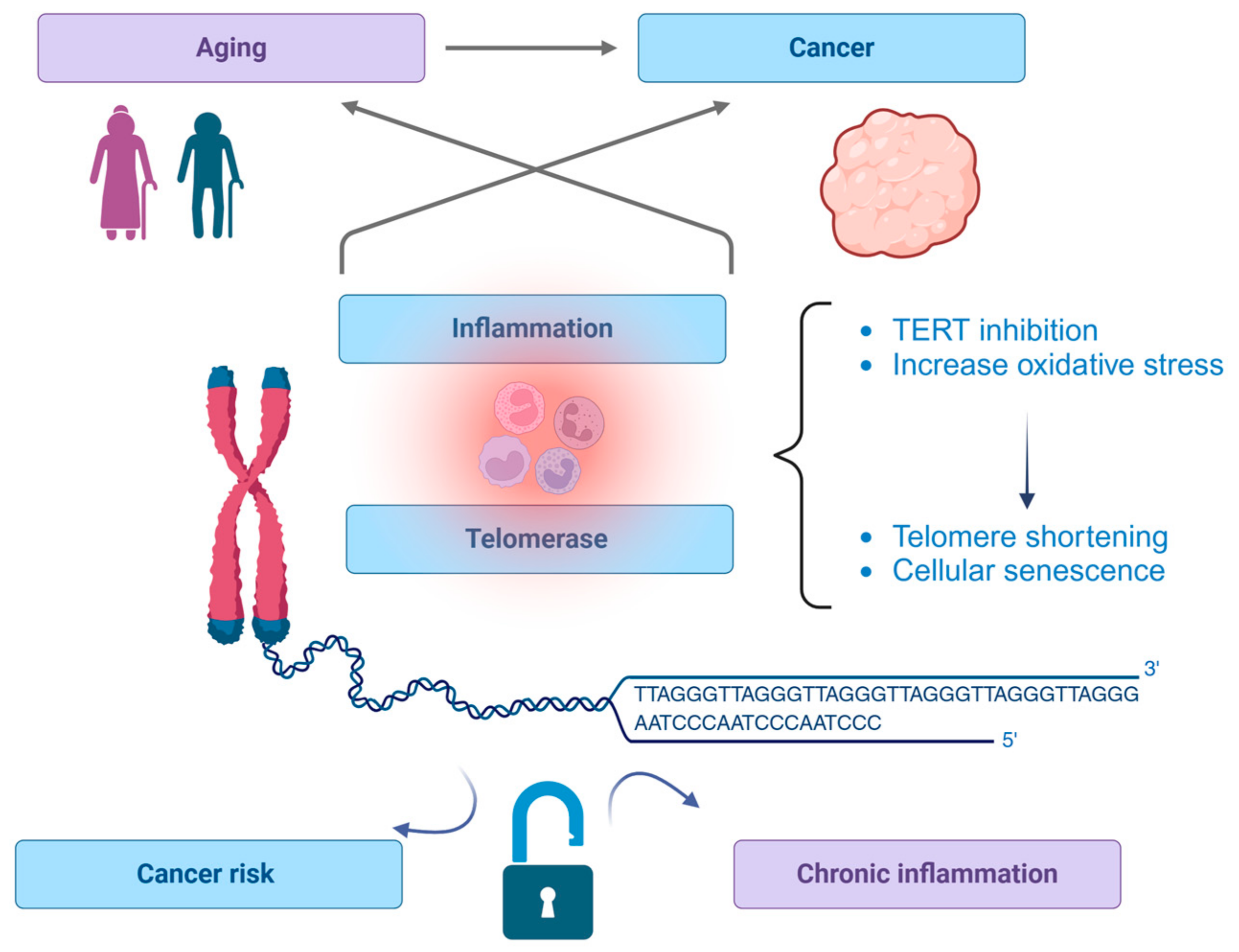
:1. Introduction
2. Methodology
3. The Telomere/Telomerase Biology: Implication in Aging and Cancer
3.1. Telomerase and Aging: Insight from Age-Related Diseases and Progeroid Syndromes
3.2. Telomerase and Cancer
4. Chronic Inflammation and Telomerase Activity
The Impact of Chronic Inflammation on Telomerase Activity
- Telomerase inhibition: Proinflammatory cytokines such as IL-6 and TNF-α can inhibit telomerase activity, particularly the catalytic subunit TERT. This inhibition accelerates telomere shortening, leading to premature cellular senescence [80].
- Oxidative stress: Chronic inflammation increases oxidative stress by generating ROS. ROS directly damage telomeres, further contributing to their attrition and the aging process [33].
- Senescence and SASP: Cells with critically short telomeres often enter a state of senescence, characterized by the secretion of SASP. SASP factors perpetuate local and systemic inflammation, creating a feedback loop that exacerbates telomere dysfunction and cellular aging [81].
- Mitochondrial dysfunction: The telomere–mitochondrial axis is crucial in understanding aging processes. Telomere shortening disrupts mitochondrial function, leading to increased ROS production and further oxidative damage. Dysfunctional mitochondria exacerbate cellular aging and promote the inflammatory response [82,83].
5. Therapeutic Strategies: A Comprehensive Overview of the Key Studies and Findings
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Pietrocola, F.; Roiz-Valle, D.; Galluzzi, L.; Kroemer, G. Meta-Hallmarks of Aging and Cancer. Cell Metab. 2023, 35, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2021, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and Aging: Signaling Pathways and Intervention Therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Blasco, M.A. Telomere-Driven Diseases and Telomere-Targeting Therapies. J. Cell Biol. 2017, 216, 875–887. [Google Scholar] [CrossRef]
- Boccardi, V.; Paolisso, G. Telomerase Activation: A Potential Key Modulator for Human Healthspan and Longevity. Ageing Res. Rev. 2014, 15, 1–5. [Google Scholar] [CrossRef]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.P.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the Telomere Connection: An Intimate Relationship with Inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef]
- Robinson, N.J.; Schiemann, W.P. Telomerase in Cancer: Function, Regulation, and Clinical Translation. Cancers 2022, 14, 808. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Genome Assembly in the Telomere-to-Telomere Era. Nat. Rev. Genet. 2024, 2024, 135. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, B.B.; Blasco, M.A. Potential of Telomerase Activation in Extending Health Span and Longevity. Curr. Opin. Cell Biol. 2012, 24, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Yamamoto, S.; Chikenji, T.S. Role of cellular senescence in inflammation and regeneration. Inflamm. Regener. 2024, 44, 28. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and Cancer—Role and Therapeutic Opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2020, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.; Skordalakes, E. Structure and Function of the Telomeric CST Complex. Comput. Struct. Biotechnol. J. 2016, 14, 161–167. [Google Scholar] [CrossRef]
- Lyu, X.; Sang, P.B.; Chai, W. CST in Maintaining Genome Stability: Beyond Telomeres. DNA Repair. 2021, 102, 103104. [Google Scholar] [CrossRef]
- Artandi, S.E.; DePinho, R.A. Telomeres and Telomerase in Cancer. Carcinogenesis 2010, 31, 9. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. Shelterin: The Protein Complex That Shapes and Safeguards Human Telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Khan, P.; Queen, A.; Dohare, R.; Alajmi, M.F.; Hussain, A.; Islam, A.; Ahmad, F.; Hassan, I. Structural Features of Nucleoprotein CST/Shelterin Complex Involved in the Telomere Maintenance and Its Association with Disease Mutations. Cells 2020, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, K.; Tong, J.; Zhang, H.; Xiong, M.; Liu, J.; Jia, S. The Altered Functions of Shelterin Components in ALT Cells. Int. J. Mol. Sci. 2023, 24, 16830. [Google Scholar] [CrossRef] [PubMed]
- Bandaria, J.N.; Qin, P.; Berk, V.; Chu, S.; Yildiz, A. Shelterin Protects Chromosome Ends by Compacting Telomeric Chromatin. Cell 2016, 164, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Bisht, K.; Savage, S.A.; Nandakumar, J.; Keegan, C.E.; Maillard, I. The Shelterin Complex and Hematopoiesis. J. Clin. Investig. 2016, 126, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Yamamoto, I.; Tarumoto, Y.; Tashiro, S.; Runge, K.W.; Ishikawa, F. Telomere-Binding Proteins Taz1 and Rap1 Regulate DSB Repair and Suppress Gross Chromosomal Rearrangements in Fission Yeast. PLoS Genet. 2019, 15, e1008335. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Liu, J.; Zhang, Z.; Zhang, H.; Liu, H.; Gao, S.; Rong, Y.S.; Zhao, Y. Homologous Recombination-Dependent Repair of Telomeric DSBs in Proliferating Human Cells. Nat. Commun. 2016, 7, 12154. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, R.J.; Karlseder, J. Telomeres: Protecting Chromosomes against Genome Instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef]
- Palamarchuk, A.I.; Kovalenko, E.I.; Streltsova, M.A. Multiple Actions of Telomerase Reverse Transcriptase in Cell Death Regulation. Biomedicines 2023, 11, 1091. [Google Scholar] [CrossRef]
- Denham, J. Canonical and Extra-telomeric Functions of Telomerase: Implications for Healthy Ageing Conferred by Endurance Training. Aging Cell 2023, 22, e13836. [Google Scholar] [CrossRef] [PubMed]
- Guidarelli, A.; Clementi, E.; Sciorati, C.; Cantoni, O. The Mechanism of the Nitric Oxide-Mediated Enhancement of Tert-Butylhydroperoxide-Induced DNA Single Strand Breakage. Br. J. Pharmacol. 1998, 125, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The Role of Cellular Reactive Oxygen Species in Cancer Chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.M.; Hong, S.M.; Lee, Y.K.; Min, S.; Yoon, G. Metabolic features and regulation in cell senescence. BMB Rep. 2019, 52, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Trybek, T.; Kowalik, A.; Góźdź, S.; Kowalska, A. Telomeres and Telomerase in Oncogenesis. Oncol. Lett. 2020, 20, 1015. [Google Scholar] [CrossRef] [PubMed]
- Van Nhieu, J.T.; Renard, C.A.; Wei, Y.; Cherqui, D.; Zafrani, E.S.; Buendia, M.A. Nuclear Accumulation of Mutated Beta-Catenin in Hepatocellular Carcinoma Is Associated with Increased Cell Proliferation. Am. J. Pathol. 1999, 155, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A.; Lee, H.W.; Rizen, M.; Hanahan, D.; DePinho, R.; Greider, C.W. Mouse Models for the Study of Telomerase. In Ciba Foundation Symposium; John Wiley & Sons: Hoboken, NJ, USA, 1997; Volume 211, pp. 160–176. [Google Scholar] [CrossRef]
- Sahin, E.; Depinho, R.A. Linking Functional Decline of Telomeres, Mitochondria and Stem Cells during Ageing. Nature 2010, 464, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the Cells: Insight into Cellular Senescence and Detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Passos, J.F. Telomeres and Cell Senescence—Size Matters Not. eBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef]
- Armanios, M. Syndromes of Telomere Shortening. Annu. Rev. Genom. Hum. Genet. 2009, 10, 45–61. [Google Scholar] [CrossRef]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic Pulmonary Fibrosis. Nat. Rev. Dis. Primers 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Young, N.S. Telomere Maintenance and Human Bone Marrow Failure. Blood 2008, 111, 4446. [Google Scholar] [CrossRef] [PubMed]
- Handley, T.P.B.; McCaul, J.A.; Ogden, G.R. Dyskeratosis Congenita. Oral Oncol. 2006, 42, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, M.; Dokal, I. Dyskeratosis Congenita, Stem Cells and Telomeres. Biochim. Biophys. Acta 2009, 1792, 371. [Google Scholar] [CrossRef] [PubMed]
- Lebel, M.; Monnat, R.J. Werner Syndrome (WRN) Gene Variants and Their Association with Altered Function and Age-Associated Diseases. Ageing Res. Rev. 2018, 41, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, C.; Bassetti, J.A.; Ellis, N.A. Bloom’s Syndrome: Clinical Spectrum, Molecular Pathogenesis, and Cancer Predisposition. Mol. Syndromol. 2017, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska, K.H.; Gregorek, H.; Dembowska-Bagińska, B.; Kalina, M.A.; Digweed, M. Nijmegen Breakage Syndrome (NBS). Orphanet. J. Rare Dis. 2012, 7, 13. [Google Scholar] [CrossRef]
- Moreno, O.M.; Paredes, A.C.; Suarez-Obando, F.; Rojas, A. An Update on Fanconi Anemia: Clinical, Cytogenetic and Molecular Approaches (Review). Biomed. Rep. 2021, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Rothblum-Oviatt, C.; Wright, J.; Lefton-Greif, M.A.; McGrath-Morrow, S.A.; Crawford, T.O.; Lederman, H.M. Ataxia Telangiectasia: A Review. Orphanet. J. Rare Dis. 2016, 11, 159. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Gensous, N.; Bacalini, M.G.; Conte, M.; Salvioli, S. Accelerated Bio-cognitive Aging in Down Syndrome: State of the Art and Possible Deceleration Strategies. Aging Cell 2019, 18, e12903. [Google Scholar] [CrossRef]
- Blasco, M.A.; Lee, H.W.; Hande, M.P.; Samper, E.; Lansdorp, P.M.; DePinho, R.A.; Greider, C.W. Telomere Shortening and Tumor Formation by Mouse Cells Lacking Telomerase RNA. Cell 1997, 91, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hornsby, P.J. Telomerase and the Aging Process. Exp. Gerontol. 2007, 42, 575. [Google Scholar] [CrossRef] [PubMed]
- Zigman, W.B. Atypical Aging in down Syndrome. Dev. Disabil. Res. Rev. 2013, 18, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142. [Google Scholar] [CrossRef] [PubMed]
- Yaswen, P.; MacKenzie, K.L.; Keith, W.N.; Hentosh, P.; Rodier, F.; Zhu, J.; Firestone, G.L.; Matheu, A.; Carnero, A.; Bilsland, A.; et al. Therapeutic Targeting of Replicative Immortality. Semin. Cancer Biol. 2015, 35, S104. [Google Scholar] [CrossRef] [PubMed]
- Ciernikova, S.; Sevcikova, A.; Stevurkova, V.; Mego, M. Tumor Microbiome—An Integral Part of the Tumor Microenvironment. Front. Oncol. 2022, 12, 1063100. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of Life-Span by Introduction of Telomerase into Normal Human Cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.C.; Stewart, S.A.; Brooks, M.W.; York, S.G.; Eaton, E.; Kurachi, A.; Beijersbergen, R.L.; Knoll, J.H.M.; Meyerson, M.; Weinberg, R.A. Inhibition of Telomerase Limits the Growth of Human Cancer Cells. Nat. Med. 1999, 5, 1164–1170. [Google Scholar] [CrossRef]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef]
- Yi, X.; Shay, J.W.; Wright, W.E. Quantitation of Telomerase Components and HTERT MRNA Splicing Patterns in Immortal Human Cells. Nucleic Acids Res. 2001, 29, 4818. [Google Scholar] [CrossRef]
- Rasouli, S.; Dakic, A.; Wang, Q.E.; Mitchell, D.; Blakaj, D.M.; Putluri, N.; Li, J.; Liu, X. Noncanonical Functions of Telomerase and Telomeres in Viruses-Associated Cancer. J. Med. Virol. 2024, 96, e29665. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, G.; Shan, Y.; Hartmann, C.; Von Deimling, A.; Xing, M. Highly Prevalent TERT Promoter Mutations in Bladder Cancer and Glioblastoma. Cell Cycle 2013, 12, 1637. [Google Scholar] [CrossRef]
- Jang, J.W.; Kim, J.S.; Kim, H.S.; Tak, K.Y.; Lee, S.K.; Nam, H.C.; Sung, P.S.; Kim, C.M.; Park, J.Y.; Bae, S.H.; et al. Significance of TERT Genetic Alterations and Telomere Length in Hepatocellular Carcinoma. Cancers 2021, 13, 2160. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, W.; Yu, H.; Xu, Y.; Bai, C.; Cong, Q.; Zhu, Y. Research Progress on the Role of Epigenetic Methylation Modification in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2024, 11, 1143. [Google Scholar] [CrossRef]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms Underlying the Activation of TERT Transcription and Telomerase Activity in Human Cancer: Old Actors and New Players. Oncogene 2019, 38, 6172. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, K.; Kim, K.; Yi, S.J. The Role of Histone Modifications: From Neurodevelopment to Neurodiseases. Signal Transduct. Target. Ther. 2022, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Grant, P.A. The Role of DNA Methylation and Histone Modifications in Transcriptional Regulation in Humans. Subcell. Biochem. 2013, 61, 289. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Jian, Y.; Gu, L.; Zhang, D.; Zhou, H.; Wang, Y.; Xu, Z.X. The Regulations of Telomerase Reverse Transcriptase (TERT) in Cancer. Cell Death Dis. 2024, 15, 90. [Google Scholar] [CrossRef]
- Akincilar, S.C.; Unal, B.; Tergaonkar, V. Reactivation of Telomerase in Cancer. Cell. Mol. Life Sci. 2016, 73, 1659. [Google Scholar] [CrossRef]
- Fleming-de-Moraes, C.D.; Rocha, M.R.; Tessmann, J.W.; de Araujo, W.M.; Morgado-Diaz, J.A. Crosstalk between PI3K/Akt and Wnt/β-Catenin Pathways Promote Colorectal Cancer Progression Regardless of Mutational Status. Cancer Biol. Ther. 2022, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, A.; Krauss, S. Wnt/Beta-Catenin Signaling and Small Molecule Inhibitors. Curr. Pharm. Des. 2012, 19, 634. [Google Scholar] [CrossRef]
- Akbari, M.; Kirkwood, T.B.L.; Bohr, V.A. Mitochondria in the Signaling Pathways That Control Longevity and Health Span. Ageing Res. Rev. 2019, 54, 100940. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human Telomere Biology: A Contributory and Interactive Factor in Aging, Disease Risks, and Protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Gil, J. Mechanisms and Functions of Cellular Senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef]
- Jurk, D.; Wilson, C.; Passos, J.F.; Oakley, F.; Correia-Melo, C.; Greaves, L.; Saretzki, G.; Fox, C.; Lawless, C.; Anderson, R.; et al. Chronic Inflammation Induces Telomere Dysfunction and Accelerates Ageing in Mice. Nat. Commun. 2014, 5, 4172. [Google Scholar] [CrossRef]
- Sahin, E.; DePinho, R.A. Axis of Ageing: Telomeres, P53 and Mitochondria. Nat. Rev. Mol. Cell Biol. 2012, 13, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef]
- Bajaj, S.; Kumar, M.S.; Peters, G.J.; Mayur, Y.C. Targeting Telomerase for Its Advent in Cancer Therapeutics. Med. Res. Rev. 2020, 40, 1871–1919. [Google Scholar] [CrossRef]
- Sławińska, N.; Krupa, R. Molecular Aspects of Senescence and Organismal Ageing—DNA Damage Response, Telomeres, Inflammation and Chromatin. Int. J. Mol. Sci. 2021, 22, 590. [Google Scholar] [CrossRef]
- Shadel, G.S. Expression and Maintenance of Mitochondrial DNA: New Insights into Human Disease Pathology. Am. J. Pathol. 2008, 172, 1445. [Google Scholar] [CrossRef] [PubMed]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Sędek, E.; Pyrżak, B. Chronic Inflammation and the Growth Hormone/Insulin-like Growth Factor-1 Axis. Cent. Eur. J. Immunol. 2021, 45, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.; Dhillon, V.S.; Lim, W.M.; Jaunay, E.L.; Donnellan, L.; Peake, B.; McCullough, C.; Fenech, M. Advanced Glycation End-Products Accelerate Telomere Attrition and Increase pro-Inflammatory Mediators in Human WIL2-NS Cells. Mutagenesis 2020, 35, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Correia-Melo, C.; Hewitt, G.; Passos, J.F. Telomeres, Oxidative Stress and Inflammatory Factors: Partners in Cellular Senescence? Longev. Healthspan. 2014, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A.; Bär, C. Telomeres and Telomerase as Therapeutic Targets to Prevent and Treat Age-Related Diseases. F1000Res 2016, 5, 89. [Google Scholar] [CrossRef]
- Galiè, S.; Canudas, S.; Muralidharan, J.; García-Gavilán, J.; Bulló, M.; Salas-Salvadó, J. Impact of Nutrition on Telomere Health: Systematic Review of Observational Cohort Studies and Randomized Clinical Trials. Adv. Nutr. 2020, 11, 576. [Google Scholar] [CrossRef]
- He, M.; Wang, K.; Che, H.; Wang, H.; Yang, K.; Zhang, G.; Yao, J.; Wang, J. A Comprehensive Review of Cycloastragenol: Biological Activity, Mechanism of Action and Structural Modifications. Eur. J. Med. Chem. Rep. 2022, 5, 100060. [Google Scholar] [CrossRef]
- Lagah, S.; Tan, I.L.; Radhakrishnan, P.; Hirst, R.A.; Ward, J.H.; O’Callaghan, C.; Smith, S.J.; Stevens, M.F.G.; Grundy, R.G.; Rahman, R. RHPS4 G-Quadruplex Ligand Induces Anti-Proliferative Effects in Brain Tumor Cells. PLoS ONE 2014, 9, e86187. [Google Scholar] [CrossRef]
- Gutlapalli, S.D.; Kondapaneni, V.; Toulassi, I.A.; Poudel, S.; Zeb, M.; Choudhari, J.; Cancarevic, I. The Effects of Resveratrol on Telomeres and Post Myocardial Infarction Remodeling. Cureus 2020, 12, e11482. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Fonseca, H.B.; Jhabvala, P.; Escalon, E.A.; Melnick, S.J. Curcumin Inhibits Telomerase Activity through Human Telomerase Reverse Transcritpase in MCF-7 Breast Cancer Cell Line. Cancer Lett. 2002, 184, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wei, S.; Savani, M.; Lin, X.; Du, K.; Mender, I.; Siteni, S.; Vasilopoulos, T.; Reitman, Z.J.; Ku, Y.; et al. A Modified Nucleoside 6-Thio-2′-Deoxyguanosine Exhibits Anti-Tumor Activity in Gliomas. Clin. Cancer Res. 2021, 27, 6800. [Google Scholar] [CrossRef] [PubMed]
- Altamura, G.; degli Uberti, B.; Galiero, G.; De Luca, G.; Power, K.; Licenziato, L.; Maiolino, P.; Borzacchiello, G. The Small Molecule BIBR1532 Exerts Potential Anti-Cancer Activities in Preclinical Models of Feline Oral Squamous Cell Carcinoma Through Inhibition of Telomerase Activity and Down-Regulation of TERT. Front. Vet. Sci. 2021, 7, 620776. [Google Scholar] [CrossRef] [PubMed]
- Frink, R.E.; Peyton, M.; Schiller, J.H.; Gazdar, A.F.; Shay, J.W.; Minna, J.D. Telomerase Inhibitor Imetelstat Has Preclinical Activity across the Spectrum of Non-Small Cell Lung Cancer Oncogenotypes in a Telomere Length Dependent Manner. Oncotarget 2016, 7, 31639. [Google Scholar] [CrossRef] [PubMed]
- Chiappori, A.A.; Kolevska, T.; Spigel, D.R.; Hager, S.; Rarick, M.; Gadgeel, S.; Blais, N.; Von Pawel, J.; Hart, L.; Reck, M.; et al. Editor’s Choice: A Randomized Phase II Study of the Telomerase Inhibitor Imetelstat as Maintenance Therapy for Advanced Non-Small-Cell Lung Cancer. Ann. Oncol. 2015, 26, 354. [Google Scholar] [CrossRef]
- Bartoszewska, E.; Molik, K.; Woźniak, M.; Choromańska, A. Telomerase Inhibition in the Treatment of Leukemia: A Comprehensive Review. Antioxidants 2024, 13, 427. [Google Scholar] [CrossRef]
- Tao, H.Y.; Zhao, C.Y.; Wang, Y.; Sheng, W.J.; Zhen, Y.S. Targeting Telomere Dynamics as an Effective Approach for the Development of Cancer Therapeutics. Int. J. Nanomed. 2024, 19, 3805–3825. [Google Scholar] [CrossRef] [PubMed]
- Benameur, T.; Soleti, R.; Panaro, M.A.; La Torre, M.E.; Monda, V.; Messina, G.; Porro, C. Curcumin as Prospective Anti-Aging Natural Compound: Focus on Brain. Molecules 2021, 26, 4794. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Farjana, M.; Moni, A.; Hossain, K.S.; Hannan, M.A.; Ha, H. Prospective Pharmacological Potential of Resveratrol in Delaying Kidney Aging. Int. J. Mol. Sci. 2021, 22, 8258. [Google Scholar] [CrossRef]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H. Bin Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA Damage Is Irreparable and Causes Persistent DNA-Damage-Response Activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, E.; Kaneko, S. Telomerase-Targeted Cancer Immunotherapy. Int. J. Mol. Sci. 2019, 20, 1823. [Google Scholar] [CrossRef] [PubMed]
- Khoury, H.J.; Collins, R.H.; Blum, W.; Stiff, P.S.; Elias, L.; Lebkowski, J.S.; Reddy, A.; Nishimoto, K.P.; Sen, D.; Wirth, E.D.; et al. Immune Responses and Long-Term Disease Recurrence Status after Telomerase-Based Dendritic Cell Immunotherapy in Patients with Acute Myeloid Leukemia. Cancer 2017, 123, 3061–3072. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, L.; Yang, Y.; Liu, Y. Cycloastragenol: An Exciting Novel Candidate for Age-Associated Diseases. Exp. Ther. Med. 2018, 16, 2175. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Jo, M.H.; Choe, K.; Khan, A.; Ahmad, S.; Saeed, K.; Kim, M.W.; Kim, M.O. Cycloastragenol, a Triterpenoid Saponin, Regulates Oxidative Stress, Neurotrophic Dysfunctions, Neuroinflammation and Apoptotic Cell Death in Neurodegenerative Conditions. Cells 2021, 10, 2719. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, H.; Luo, Y. Anti-Aging Implications of Astragalus Membranaceus (Huangqi): A Well-Known Chinese Tonic. Aging Dis. 2017, 8, 868. [Google Scholar] [CrossRef]
- Schellnegger, M.; Lin, A.C.; Hammer, N.; Kamolz, L.P. Physical Activity on Telomere Length as a Biomarker for Aging: A Systematic Review. Sports Med. Open 2022, 8, 308. [Google Scholar] [CrossRef]

| Modulator | Mechanism | Evidence in Animals | Evidence in Humans |
|---|---|---|---|
| Imetelstat (GRN163L) | Telomerase inhibitor. | Inhibited tumor growth and metastasis in mouse models of various cancers, including breast and lung cancer. | Phase II clinical trials showed partial responses and disease stabilization in patients with myelofibrosis and certain solid tumors. |
| BIBR1532 | Telomerase inhibitor. | Suppressed tumor growth in xenograft models of glioblastoma and leukemia. | Preclinical studies only, no clinical trials yet. |
| 6-thio-2′-deoxyguanosine (6-thio-dG) | Telomerase substrate that induces telomere dysfunction. | Reduced tumor growth and improved survival in mouse models of glioblastoma and melanoma. | Preclinical studies only, showing promise for future clinical trials. |
| Curcumin | Natural compound, inhibits telomerase activity. | Decreased telomerase activity and tumor growth in mouse models of prostate cancer. | Limited clinical studies suggest potential benefits, but more research is needed. |
| Resveratrol | Natural compound, modulates telomerase and telomeres. | Extended lifespan and reduced tumor incidence in animal models; effects on telomerase activity are mixed. | Some clinical trials indicate potential anticancer effects, though data on telomerase modulation in humans is limited. |
| RHPS4 | G-quadruplex stabilizer, inhibits telomerase. | Reduced tumor growth and telomere shortening in mouse models of melanoma and glioblastoma. | Preclinical studies only, showing potential for clinical development. |
| TA-65 | Telomerase activator, derived from Astragalus membranaceus. | Improved health span and telomere length in aged mice; data on cancer effects are mixed. | Some human studies suggest telomere lengthening and improved markers of aging, but data on cancer effects is limited and controversial. |
| Tert promoter mutations | Genetic modifications to increase telomerase expression. | Extended lifespan and delayed cancer onset in some transgenic mouse models; increased cancer risk in others. | Observed in various cancers; some patients show increased telomerase activity, contributing to tumor progression. |
| GRNVAC1 | Telomerase-based dendritic cell vaccine. | Induced immune response and reduced tumor burden in mouse models of prostate cancer. | Phase II clinical trials showed immune activation and potential clinical benefits in patients with acute myeloid leukemia. |
| Cycloastragenol | Telomerase activator, another Astragalus extract. | Improved telomere length and reduced oxidative stress in aged mice; effects on cancer are unclear. | Some small human studies indicate telomere lengthening, but more research is needed to determine cancer-related effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccardi, V.; Marano, L. Aging, Cancer, and Inflammation: The Telomerase Connection. Int. J. Mol. Sci. 2024, 25, 8542. https://doi.org/10.3390/ijms25158542
Boccardi V, Marano L. Aging, Cancer, and Inflammation: The Telomerase Connection. International Journal of Molecular Sciences. 2024; 25(15):8542. https://doi.org/10.3390/ijms25158542
Chicago/Turabian StyleBoccardi, Virginia, and Luigi Marano. 2024. "Aging, Cancer, and Inflammation: The Telomerase Connection" International Journal of Molecular Sciences 25, no. 15: 8542. https://doi.org/10.3390/ijms25158542
APA StyleBoccardi, V., & Marano, L. (2024). Aging, Cancer, and Inflammation: The Telomerase Connection. International Journal of Molecular Sciences, 25(15), 8542. https://doi.org/10.3390/ijms25158542







